Abstract
Neotropical forests are being increasingly replaced by a mosaic of patches of different successional stages, agricultural fields and pasture lands. Consequently, the identification of factors shaping the performance of taxa in anthropogenic landscapes is gaining importance, especially for taxa playing critical roles in ecosystem functioning. As phyllostomid bats provide important ecological services through seed dispersal, pollination and control of animal populations, in this study we assessed the relationships between phyllostomid occurrence and the variation in local and landscape level habitat attributes caused by disturbance. We mist-netted phyllostomids in 12 sites representing 4 successional stages of a tropical dry forest (initial, early, intermediate and late). We also quantitatively characterized the habitat attributes at the local (vegetation structure complexity) and the landscape level (forest cover, area and diversity of patches). Two focal scales were considered for landscape characterization: 500 and 1000 m. During 142 sampling nights, we captured 606 individuals representing 15 species and 4 broad guilds. Variation in phyllostomid assemblages, ensembles and populations was associated with variation in local and landscape habitat attributes, and this association was scale-dependent. Specifically, we found a marked guild-specific response, where the abundance of nectarivores tended to be negatively associated with the mean area of dry forest patches, while the abundance of frugivores was positively associated with the percentage of riparian forest. These results are explained by the prevalence of chiropterophilic species in the dry forest and of chiropterochorous species in the riparian forest. Our results indicate that different vegetation classes, as well as a multi-spatial scale approach must be considered for evaluating bat response to variation in landscape attributes. Moreover, for the long-term conservation of phyllostomids in anthropogenic landscapes, we must realize that the management of the habitat at the landscape level is as important as the conservation of particular forest fragments.
Introduction
Tropical landscapes have been increasingly modified by human activities, altering their natural structure and the course of ecological processes. In the Neotropics, cattle raising, agriculture and logging, have severely modified the natural vegetation, causing their replacement by a mosaic of patches of different successional stages, agricultural fields, and pasture lands [1], [2], 3. Under this scenario, considered by some authors the predominant habitat for wildlife in the near future [4], [5], the identification of factors that shape the taxa distribution and performance is gaining importance [6], [7]. Special attention must be paid to taxa playing critical roles in ecosystem functioning, which will help to maintain ecosystem structure and key ecological processes [8], [9].
Bats are considered an important component of biodiversity in the Neotropics, as well as a keystone group [10], [11], [12]. Due to their dramatic ecological and evolutionary radiation, they occupy virtually every trophic level, from primary to tertiary consumers, interacting with a large spectrum of organisms and regulating complex ecological processes [11], [13], [14], [15], [16]. They play an important role in ecosystem functioning by providing ecological services such as seed dispersal, pollination, and control of invertebrate and small vertebrate populations; they also contribute to the recycling and translocation of nutrients and energy in the ecosystem [11], [16], [17], [18], [19], [20], [21], [22], [23], [24]. In the Neotropics, bats visit and presumably pollinate approximately 573 species and disperse seeds from 549 species [25], [26], contributing to the maintaining of plant diversity, connecting distant plant populations via pollen and seed movement, and promoting forest regeneration in degraded lands via seed dispersal. In some Neotropical regions, nearly half of the most abundant pioneer plant species are bat-dispersed (i.e. Solanum, Cecropia, Piper, Vismia) [17].
The response of bats to anthropogenic disturbance in Neotropical regions has received increasing attention during the last twenty years but still remains poorly understood as studies have reported contradictory results (Table 1 in [27]). Some studies suggest that bats are more tolerant to habitat modification than other animals attributing this to: (1) their capacity to fly, crossing habitat boundaries and open areas (including physical barriers for other species), (2) their ability to exploit resources that are patchy in space and time, and (3) their capacity to shift diets or adapt their behavior to resource availability [13], [28], [29], [30], [31], [32], [33], [34], [35], [36]. Other studies, in contrast, suggest that bats are sensitive to habitat loss or modification and to the resulting variation in habitat structure, food and shelter. Bat responses to habitat alteration reported at the assemblage, ensemble, population and individuals levels include: (1) changes in species composition, (2) reductions in species diversity, (3) reductions in abundance, (4) strong deviations in sex ratios, (6) changes in their foraging patterns, and (5) the presence of more physiologically stressed individuals in fragments [8], [37], [38], [39], [40], [41], [42], [43], [44], 45,46,47,48,49,50,51,52,53. We use the terms assemblage and ensemble sensu Fauth et al. [54], who recognized as assemblage a phylogenetically bounded group of species inhabiting a given local habitat (i.e. phyllostomid assemblage) and as ensemble a set of species within an assemblage that use a similar set of resources (i.e. phyllostomid frugivores).
Table 1. Summary statistics of parameters at population, ensemble and assemblage-level.
| Parameter | Mean | SD | Range |
| Population-level | |||
| Micronycteri microtis (GI) | 0.00 | 0.02 | 0–0.06 |
| Glossophaga soricina (N) | 0.62 | 0.68 | 0–2.00 |
| Glossophaga commissarisi (N) | 0.17 | 0.32 | 0–1.14 |
| Leptonycteris yerbabuenae (N) | 0.20 | 0.37 | 0–1.07 |
| Choeroniscus godmani (N) | 0.00 | 0.02 | 0–0.06 |
| Musonycteris harrisoni (N) | 0.01 | 0.02 | 0–0.06 |
| Carollia sp. (F) | 0.03 | 0.11 | 0–0.39 |
| Artibeus jamaicensis (F) | 1.26 | 1.72 | 0–6.22 |
| Artibeus watsoni (F) | 0.09 | 0.14 | 0–0.47 |
| Artibeus phaeotis (F) | 0.14 | 0.23 | 0–0.72 |
| Artibeus lituratus (F) | 0.24 | 0.36 | 0–1.28 |
| Sturnira lilium (F) | 0.07 | 0.12 | 0–0.29 |
| Centurio senex (F) | 0.01 | 0.03 | 0–0.11 |
| Chiroderma salvini (F) | 0.01 | 0.03 | 0–0.11 |
| Desmodus rotundus (S) | 0.60 | 1.08 | 0–3.78 |
| Ensemble-level | |||
| S8N* | 1.65 | 1.06 | 0–2.98 |
| AbN | 1.00 | 1.29 | 0–4.21 |
| S8F* | 2.65 | 1.18 | 1–5.14 |
| AbF | 2.52 | 3.15 | 0.38–14.22 |
| Assemblage-level | |||
| SC1 | 0 | 0.50 | −0.99–0.58 |
| SC2 | 0 | 0.55 | −0.75–1.17 |
| S8P* | 4.61 | 1.97 | 1.00–8.04 |
| AbP | 3.46 | 3.89 | 0.38–14.22 |
Parameters at population-level: capture rate (individuals/night) as indicator of species local abundance. Parameters at ensemble-level: rarified number of nectarivorous (S8N) and frugivorous species (S8F), capture rate of nectarivores (AbN) and frugivores (AbF). Parameters at assemblage-level: scores of the first (SC1) and second (SC2) ordination axis reflecting assemblage's dissimilarities in species composition and structure, rarified number of phyllostomid species (S8P) and capture rate of phyllostomids (AbP). Mean: mean per site of the parameters at population, ensemble and assemblage-level. SD: standard deviation. Species in bold are those analyzed at the population-level. Species ensemble assignations are shown between parentheses: gleaning insectivores (GI), nectarivores (N), frugivores (F) and sanguivores (S). The number of sites sampled was 12 for all parameters except for three, which are marked with an asterisk. In these three parameters the site P1 was excluded from the analyses due to its low number of sampling nights.
A better understanding of bat response to habitat disturbance demands us to move from the dichotomous and qualitative description of habitat (i.e. fragmented vs. continuous forest, disturbed vs. undisturbed habitat) to a quantitative characterization of habitat attributes [55], [56]. Additionally, because the occurrence of vagile species in a particular fragment is greatly determined by the landscapes attributes, we need to evaluate the effect of variation not only on local habitat attributes (i.e. tree density, canopy cover, and fragment shape and area), but also on the spatial configuration and composition of the landscape at different focal scales [14], [32], [39], [46], [56], [57].
The main objective of this study was to identify potential explanatory relationships between changes in phyllostomid bat assemblages, ensembles and populations and the variation in habitat attributes, at local and landscape levels, caused by the two most common anthropogenic disturbances in the Neotropics: agriculture and cattle raising [1]. We focus on phyllostomid bats because this is the most diverse bat family in the Neotropics, in both taxonomic and functional terms, containing most of the foraging guilds and all the nectarivorous and frugivorous species [15]. The study was carried out in a tropical dry forest as this is one of the most widespread and disturbed neotropical systems [58], [59]. In order to adequately evaluate bat response to habitat disturbance we quantitatively characterized the habitat attributes that could influence bat occurrence at the local and landscape levels and at different focal scales. We finally discuss the implications of our results for the study and conservation of phyllostomid bats in anthropogenic landscapes.
To our knowledge, this is the first detailed investigation evaluating, at the landscape level, how variation in habitat attributes determines the occurrence of bats in an anthropogenic dry forest landscape. Our study is also one of the few in the Neotropics comparing the importance of local vs landscape level habitat attributes on bat presence and abundance [31], [46].
Based on results from previous studies we made the following predictions: (1) variation in phyllostomid assemblage composition, which includes a significant portion of species tightly associated with mature forest (6 of 15 species in the study region, [27]), will be mainly explained by variation in vegetation structural complexity and in vegetation cover; and (2) because of their contrasting ecological requirements (i.e. food resources), the abundance of nectarivores and frugivores will respond in a guild-specific way to changes in local and landscape habitat attributes [56]. Specifically, we expect to find the highest abundance of frugivores in sites surrounded by the highest amount of riparian vegetation, which hosts most of the chiropterochoric species of the region [60]. In contrast, the highest nectarivore abundance is expected to occur in sites surrounded by a reduced amount of dry forest, as the most abundant nectarivorous species in the region tend to occur in higher abundance in the early successional stages of the tropical dry forest [27].
Materials and Methods
Ethics Statement
Bat captures and handling were in accordance with the laws of the Mexican Government and with the authorization of the Oficina de Fauna Silvestre, Mexico (SGPA/DGVS Permit 3644 to KES). This study was also approved by the Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), and the Consejo Nacional de Ciencia y Tecnología (CONACYT) from Mexico (Projects 2002-C01-0597 and CB-2005-51043).
Study area and sampling sites
The study was conducted in and surrounding the Chamela-Cuixmala Biosphere Reserve (CCBR, Fig. 1), located in the central western coast of Mexico in the state of Jalisco (19°22′–19°35′N, 104°56′–105°03′W). The CCBR has an extension of 13,200 ha and is covered by a well preserved tropical dry forest and small areas of riparian forest, among other vegetation types [60]. Its precipitation regime follows a markedly seasonal pattern as most of the rainfall occurs during June–October. Average annual precipitation is 763±258 (SD) mm and average annual temperature is 24.6°C (Chamela Biological Station website: www.ibiologia.unam.mx/ebchamela/, accessed 2008 Feb 1; [27]).
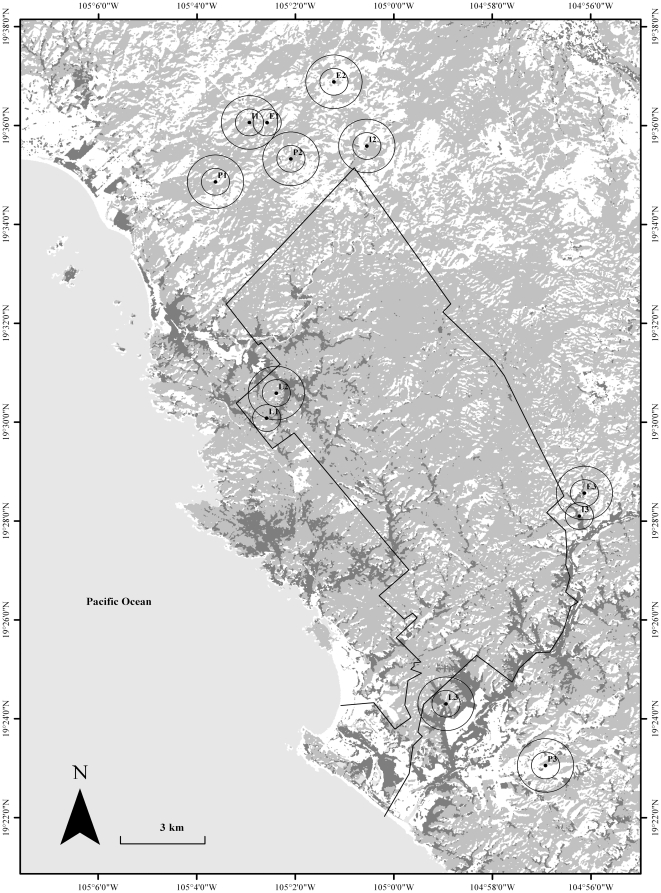
Figure 1. Classified image showing sampling sites and concentric focal scales.
Circles around sampling sites represent the focal scales of 500 and 1000 m radii. Successional stages: pasture (P), early (E), intermediate (I) and late (L). Dry forest is colored light gray, whereas small areas of riparian forest are colored dark gray. The polygon encloses the area of the Chamela-Cuixmala Biosphere Reserve.
We selected twelve sampling sites (Fig. 1) representing a dry forest successional gradient (chronosequence) of four successional stages differing in their age of abandonment: 3 pastures (0 years), 3 early stage sites (3–5 years), 3 intermediate stage sites (8–12 years) and 3 late stage sites located in the CCBR (at least 50 years old). These sites were selected based on: (1) their slope (ranging from 15° to 25°), allowing the nets to be erected; (2) their aspect, avoiding north facing slopes because of the higher heterogeneity of plant communities occurring on these slopes [61]; (3) their accessibility through trails; (4) their distribution around and distance from the CCBR (pastures, early and intermediate sites were distributed around the reserve in order to generate a research design reasonably balanced; their distance from such reserve was equal or higher than 1000 m). All secondary sites were constituted by plots of 120 * 90 m embedded within the same vegetation type. The land use history of secondary sites is quite similar. First, forest was removed through slash and burn. Second, lands were subsequently used for maize and bean production. Finally, lands were converted into cattle pastures, being burned approximately every two years before rainfall. For more detailed information about the land use history of sampling sites, including pictures, see [27].
Bat sampling
Bats were captured following a standardized sampling unit consisting of a set of five mist nets covering an area of 109 m2 each sampling night. Mist nets were located within and surrounding each plot, crossing natural corridors representing flyways for bats. In pastures, mist nets were located in temporary creeks or in trails delimited by shrubs and treelets. Distance between nets was not shorter than 30 m. Sampling was performed for 5 hours after sunset, a period of time that coincides with the foraging peak for most phyllostomid bats [62], avoiding windy, rainy and full moon nights to reduce variation in capture success. We also avoided biases due to trap-shy behavior by sampling each site for only one night during a census period.
Mist netting remains the single most effective method to sample phyllostomid bat assemblages and to evaluate their response to habitat modification, being widely used in pastures, agricultural fields, secondary vegetation and mature forests [7], [14], [41], [44], [45], [63], [64]. Acoustic sampling was not used as a complementary sampling technique because: (1) phyllostomid bats produce low-intensity calls that can not be clearly detected, and (2) their calls show low inter-specific variation, which precludes species identification [65], [66]. Biases associated with the use of mist nets have been addressed [67], [68], [69].
From June 2004 to August 2006, most sites were sampled every 46±15 (SD) days. In pastures we reduced the number of sampling nights due to the paucity of captures. During each census period we randomized the order in which sites were sampled. Nets were checked approximately every 30 min and captured bats were stored temporarily in cloth bags. Bats were identified to the species level based on the dichotomous keys of Medellín et al. [70] and Timm and Laval [71]. Excluding juveniles and non-healthy individuals, all bats were marked on their forearm using numbered aluminum bands. Individuals were released where they were originally captured. We also collected and identified the seeds found in bat feces inside the cloth bags. In addition, the chiropterophilic and chiropterochoric plants occurring in the region were identified based on literature (see Table S3). We used this information as ancillary data for the discussion of our results, considering which resources are being used by nectarivores and frugivores and how they are distributed in the vegetation matrix. Bat nomenclature follows Simmons [72] and bat ensemble assignation follows Timm and Laval [71].
Habitat attributes at the local scale
In a quadrate of 50*20 m within each plot, we measured the following vegetation attributes considering all the woody plants with a diameter at breast height equal or greater than 2.5 cm: 1. Number of individuals (IN), 2. Species number (SP), 3. Basal area (BA), and 4. Average leaf area index per plot (LAI), which is the projected green leaf area per unit of a horizontal plane [73]. This index is considered a useful indicator of the biophysical characteristics of vegetation and allows for discrimination of successional stages as its value increases toward sites with higher vegetation complexity (i.e. higher number of strata, woody species, and basal area, [74]). LAI was measured during the rainy season by using an LAI-2000 Plant Canopy Analyzer (LI-COR, USA). Detailed information on how measurements were recorded can be found in Nassar et al. [75].
To obtain a continuous synthetic variable summarizing sampling site variation in vegetation parameters, we performed a principal component analysis (PCA) of the variables described above. All variables were positively correlated with axis 1 of the PCA and their eigenvector values were: IN (0.48), SP (0.52), BA (0.52) and LAI (0.48). Based on this, we assumed that axis 1 represented a successional gradient where the sites with higher vegetation structural complexity (late and intermediate stages) presented higher scores. Axes 1 and 2 explained 83% and 11% of the variation respectively (Fig. S1). Consequently, axis 1 scores were considered as a new variable reflecting the vegetation structural complexity of each site. This new variable was used as an explanatory variable for evaluating bat responses to local scale variation in the habitat.
Habitat attributes at the landscape scale
The estimation of the landscape metrics used as explanatory variables were performed on a classified image comprised of 4 ASTER, cloud free, satellite images acquired for the Pacific coast of Mexico on December 28, 2005. This date represents an intermediate moment along the bat sampling period and corresponds to the dry season, when the highest differentiation between pastures, dry and riparian forest occurs [76], [77]. For image classification, we analyzed the first three bands of the ASTER sensor as well as two other bands produced by the calculation of two spectral indices: the normalized difference vegetation index (NDVI) and the single ratio (SR), all with a nominal spatial resolution of 15 m. Finally, the image was classified into 9 land-cover classes: (1) dry forest initial successional stages (including pastures and the early successional stage), (2) dry forest advanced successional stages (DF, including intermediate and late successional stages,), (3) riparian forest (RF), including both gallery forest located along large rivers and gallery forest located along temporary creeks, (4) mangroves, (5) oak forest, (6) seasonal growing field (i.e. corn, tomato, hot pepper, watermelon), (7) long term growing field (i.e. mango, papaya, coconut, citrus), (8) bare soil (including dirt and paved roads), and (9) water. The final accuracy of the classified image was 0.86 and 0.84 according to the overall accuracy and Tau coefficient statistics, respectively. The image was processed with ERDAS Imagine v.9.2 (Leica Geosystems, Georgia, USA). More detailed information about image processing can be found in the supporting information (Methods S1).
Landscape metrics were measured at two different focal scales within two nested concentric circles of 500 and 1000 m radius and centered on the centroid of the mist net distributions for each sampling site (Fig. 1). These focal scales allowed us: (1) to encompass the expected home range of small and medium size phyllostomids inhabiting the region (i.e. Glossophaga soricina: 500 m radius [78]; Sturnira lilium: 636 m radius [79]); (2) to minimize spatial overlap among neighboring circles (sites E1, I3 and L1 were not considered in the 1000 m radius analysis as they were partially overlapped), and (3) to compare our results with other studies [32], [46], [56]. A high degree of overlapping among neighboring circles was avoided in order to minimize the likelihood of making a Type I error (reject a true null hypothesis) due to the effect of pseudoreplication [80], [81]. Pseudoreplication likely would occur if the values of the predictor variables from nearly the same concentric circles were considered as multiple and independent observations in the dataset [82].
Five landscape metrics were selected as explanatory variables based on metrics found in other studies to be associated with the occurrence or abundance of phyllostomids [32], [46], [56]: (1) percentage of dry forest cover (estimated for the DF class, defined above), (2) percentage of riparian forest cover (estimated for the RF class), (3) mean area of dry forest patches (estimated for the DF class), (4) mean area of riparian forest patches (estimated for the RF class) and (5) diversity of patch types. Percentage of forest cover was defined as the sum of the areas (m2) of all patches of a given type (DF or RF) divided by the total area of the plot (circle) and multiplied by 100. Mean patch area was defined as the sum of the areas (m2) of all patches of a given type (DF or RF) divided by the number of patches of such type. Diversity of patch types was defined as the probability that any two cells selected at random would represent different patch types (Simpson's diversity index). All coverage classes considered in the image classification were used in the estimation of patch type diversity. Calculation of landscape metrics was performed using Fragstat v.3.3 [83].
Statistical Analyses
The completeness of bat surveys in all sampling sites was assessed by calculating the percentage of the total estimated species richness that effectively was covered by samples. Total species richness was estimated by computing the mean of the first and second order Jackknife indices [84]. These indexes deal properly with small sample sizes (<100 individuals per site), producing a low biased estimation of species richness [85]. Ninety percent of completeness was considered an appropriate level of sampling efficiency [86].
We tested our data for spatial autocorrelation using a Mantel test (999 permutations) based on Spearman rank correlation coefficients for ecological and geographic distance matrices. We assessed significance of spatial structure at an alpha = 0.05 level. The Bray-Curtis coefficient [84] was used for the construction of the ecological distance matrix, and the Euclidian distance between sites for the construction of the geographic distance matrix.
The response of phyllostomids to habitat variation at local and landscape scales was evaluated at the population, ensemble and assemblage-level. In all cases we used capture rate (individuals/night) as an indicator of local abundance. Only the seven most common species (n≥25) were analyzed at the population-level. We also considered as a response variable, at ensemble and assemblage-level, the number of captured species, which was rarified at 8 sampling nights (EstimateS software v 8: http://viceroy.eeb.uconn.edu/estimates). Rarefaction allows the comparison of indices by eliminating biases related to differences in sampling effort [87].
Dissimilarities between assemblages in terms of species identity and abundance were quantified using the Bray-Curtis coefficient, which is one of the preferred dissimilarity measures as it adequately reflects the intuitive ordering of sites [88]. However, the value of this coefficient is more influenced by the species with the largest difference in abundance and consequently, in a dataset dominated by a few species, this coefficient will mainly reflect differences for those species [88]. To avoid this bias, the matrix of sampling sites by species abundance was standardized using the square-root. All phyllostomids were considered in the analysis.
The distance matrix produced was used as an input matrix for a non-metric multidimensional scaling ordination (NMDS), which maps the observed assemblage's dissimilarities in species composition. This iterative method of ordination has the advantage of properly handling nonlinear species response of any shape [89] and has a good performance even when beta diversity is high [90]. The scores of the resulting axes were employed as a response variable to evaluate the relationship between the variation in assemblage species composition and the variation in habitat attributes.
We evaluated the relationship between the phyllostomid response and all the explanatory variables, at the 500 and 1000 m radius scales, using generalized linear models (GLMs). The NMDS scores and the rarified number of species at the ensemble and assemblage-level were modeled using a Gaussian error distribution with the identity link function, as this is the error distribution that best describes the structure of the data. The abundance data at the population, ensemble and assemblage-level were modeled using a Poisson error distribution with the log link function.
As in previous studies, we found multicollinearity problems because the metrics of landscape structure are correlated with the percentage of cover of the corresponding land-cover class [32], [55]. In order to avoid this problem, in all subsequent analyses we substituted the estimated values of mean patch area by the residual values of the regression between this variable and the percentage of cover [32].
The explanatory variables most likely to causally influence the response variables at the two focal scales were identified by performing a hierarchical partitioning analysis (HPA) [91]. In this analysis, all possible GLMs combining the explanatory variables are jointly considered and the increase in model fit generated by certain variables (measured by the log-likelihood) is estimated by averaging the variables influence over all models in which such variables appear [91], [92]. At the end, we obtained a measure of the independent effect of each explanatory variable over the response variable. This procedure alleviates problems of multicollinearity between the explanatory variables, which can not be properly handled by statistical sequential methods (i.e. stepwise selection). Sequential methods would tend to select spurious models due to a high type-I error [92]. Significance of relationships (α = 0.05) between explanatory and response variables was evaluated by the randomization test suggested by Mac Nally [93]. We compared the HPA outcomes using the small-corrected form of AICc for model selection [94] in order to validate their robustness [92]. The set of models considered included the null model and six other models considering each explanatory variable independently. More detailed information can be found in the supporting information (Table S2).
Finally, based on the results of HPA and model selection based on AIC, we classified the relationships between every explanatory variable and the corresponding response variables as: (1) robust: when a significant relationship was found (HPA) and when the explanatory variable was selected as part of the most plausible models (AIC), (2) those denoting a tendency: when the explanatory variables were associated with the greatest portion of the variation in the response variable, although no significant relationship was observed (HPA), and they were selected as part of the most plausible models (AIC), and (3) no relationship: when the explanatory variables were not associated with the greatest portion of the variation in the response variable (HPA), and/or they were not selected as part of the most plausible models (AIC).
All the statistical analyses were performed in R [95] using vegan [96], MASS [97] and hier.part packages [98].
Results
One hundred and forty-two sampling nights resulted in the capture of 606 phyllostomid individuals representing 15 species, 11 genera, 5 subfamilies and 4 broad guilds (Table 1). The most abundant species were Artibeus jamaicensis, G. soricina and Desmodus rotundus whereas the broad guilds best represented in terms of species richness were frugivores and nectarivores. The number of captures per species and the sampling effort per site can be found in Avila-Cabadilla et al. [27]. Individuals previously identified as A. intermedius (Table 2 on [27]), were re-classified as A. lituratus following Simmons [72] (Table 1).
Table 2. Relationships between population, ensemble and assemblage-level parameters and the habitat attributes.
| Type of relationships between variables | |||
| Parameter | Scale | Robust | Tendency |
| Population level | |||
| Nectarivore | |||
| G. soricina | 500 | DFarea(-); Div | |
| G. commissarisi | 500 | DFarea(-) | |
| 1000 | DFarea(-) | ||
| L. yerbabuenae | 1000 | DFarea(-) | |
| Frugivore | |||
| A. jamaicensis | 500 | Vstruct | RF% |
| 1000 | DFarea(-) | ||
| A. phaeotis | 500 | Vstruct; RF% | |
| 1000 | RF% | ||
| A. lituratus | 500 | RF% | |
| 1000 | DFarea; RF% | ||
| Sanguivore | |||
| D. rotundus | 500 | RF% | |
| 1000 | RFarea | ||
| Ensemble-level | |||
| Nectarivore | |||
| AbN | 500 | DFarea(-) | |
| 1000 | DFarea(-) | ||
| Frugivore | |||
| S8F | 500 | Vstruct | |
| 1000 | Vstruct | ||
| AbF | 500 | RF% | |
| 1000 | RF% | ||
| Assemblage-level | |||
| SC2 | 500 | Vstruct(-); RF%(-) | |
| 1000 | RF%(-) | ||
| AbP | 500 | RF% | |
| 1000 | RFarea | ||
Parameter at population-level: species abundance. Parameters at ensemble-level: rarified number of nectarivorous (S8N) and frugivorous species (S8F), abundance of nectarivores (AbN) and frugivores (AbF). Parameters at assemblage-level: scores of the first (SC1) and second ordination axis (SC2) reflecting assemblage dissimilarities in species composition and structure, rarified number of phyllostomid species (S8P) and abundance of phyllostomids (AbP). Habitat attributes: vegetation structural complexity (Vstruct), mean area of dry (DFarea) and riparian forest patches (RFarea), percentage of riparian forest cover (RF%) and diversity of patch types (Div). Negative relationships are shown in parentheses.
Sampling effort was considered sufficient to characterize phyllostomid assemblages occurring in each sampling site. Completeness reached 90% in all cases, ranging from 90 (P1, E2) to 96% (I2, I3). We found no evidence of spatial structure in our dataset. Ecological and Euclidian distance matrices were not significantly correlated (rs = −0.05, p = 0.59).
Phyllostomid response at the population-level
Summarizing the results of both statistical analyses (see Table S1 for HPA and Table S2 for AIC), we found that variation in abundance of the three nectarivorous species was not robustly associated with the variation of any explanatory variable at the two focal scales (Table 2), although it tended to be negatively associated with variation in the mean area of DF patches (at the two scales). We also identified a tendency for a positive association between G. soricina abundance and the diversity of land cover types (Table 2). On the other hand, while G. soricina only responded to changes in habitat attributes at the 500 m focal scale, Leptonycteris yerbabuenae only responded to such changes at the 1000 m focal scale.
Most of the variation in abundance of frugivorous species showed a robust, positive, association with variations in percentage of RF (all species) and complexity of vegetation structure (A. jamaicensis and A. phaeotis). We also detected, at the 1000 m focal scale, that the mean area of DF patches tended to be associated with the abundance of both frugivores, A. jamaicensis (negative association) and Artibeus lituratus (positive association) (Table 2).
The abundance of the sanguivorous D. rotundus, showed a robust, positive association with variations in the percentage of RF (at the 500 m focal scale) and tended to be positively associated to the mean area of RF patches (at the 1000 m focal scale) (Table 2).
Phyllostomid response at the ensemble-level
Similarly to what we observed at the population-level, the variation in the abundance of nectarivores tended to be negatively associated, at the two scales, with variations in the mean area of DF patches. No explanatory variable was associated with the variation in the number of nectarivorous species, whereas the variation in the number of frugivorous species was positively associated with variations in the complexity of vegetation structure. The abundance of frugivores was robustly and positively associated with the percentage of RF at the two scales.
Phyllostomid response at the assemblage-level
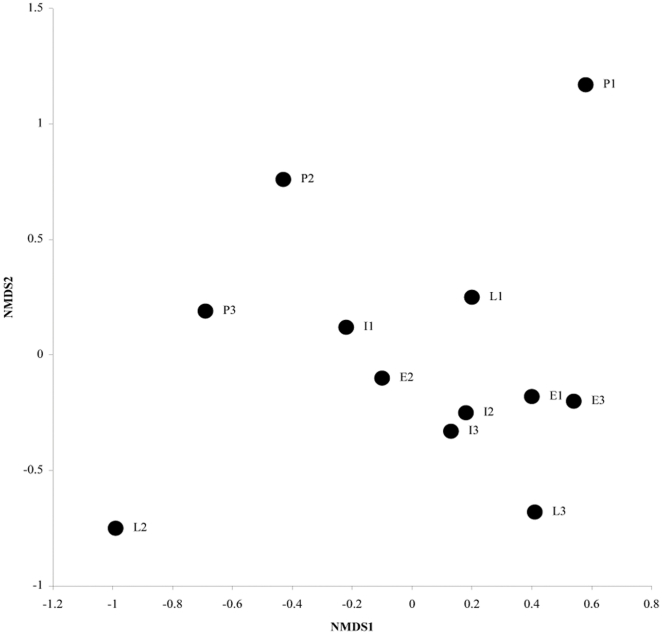
Only two axes were considered in the NMDS ordination of phyllostomid assemblages (Fig. 2, stress = 8.20), because additional dimensions did not substantially diminish the stress value. A successional gradient is represented along axis 2, where phyllostomid assemblages occurring in pastures tended to present higher scores and those occurring in late stages tended to present lower scores. Axis 1 did not show a clear gradient.
Figure 2. Ordination of sampling sites based on the species composition and structure of phyllostomid assemblages.
Successional stages: pasture (P), early (E), intermediate (I) and late (L). NMDS 1 and 2: axis 1 and 2 of the non-metric multidimensional scaling.
The assemblage dissimilarities represented by NMDS axis 2 (Fig. 2) were significantly associated with the gradient of vegetation structural complexity found along PCA axis 1 (Fig. S1) at the 500 m scale (Table 2). NMDS axis 2 was also negatively associated with the percentage of RF at the two scales (Table 2). None of the considered explanatory variables were associated with NMDS axis 1.
The variation in phyllostomid abundance was positively associated with the percentage of RF (at the 500 m scale) and with the mean area of RF patches (at the 1000 m scale). No explanatory variables were associated with the variation in the number of phyllostomid species.
Discussion
Phyllostomid response at the population and ensemble-level
As expected, we found a guild-specific response of bats to changes in local and landscape habitat attributes. First, the abundance of nectarivores, at both the population and ensemble-level, tended to decrease with the increase in the mean area of dry forest patches, while the number of species was not associated with any of the explanatory variables. On the other hand, frugivore abundance was tightly and positively associated with the percentage of riparian forest while the number of species and some species abundances (A. jamaicensis and A. phaeotis) were associated with variation in the vegetation structural complexity. Finally, the abundance of the unique sanguivore present in the region (D. rotundus) increased with the increase in the amount of riparian forest. Guild-specific bat responses previously have been found by Klingbeil and Willig [56] and Henry et al. [52] in different Amazonian rainforests. In both studies the authors consistently found that the frugivore abundance responded more to changes in landscape composition (i.e. percentage of forest cover), while gleaning animalivorous bats responded more to changes in landscape configuration (i.e. edge density). The guild-specific response of bats to variation in habitat attributes is likely due to the contrasting ecological requirements of bats from different ensembles.
The lack of relationship between the number of nectarivorous species and the considered habitat attributes probably occurs because three of the five nectarivores reported in this study are ubiquitous. These species (G. soricina, G. commissarisi and L. yerbabuenae) were present in most of the sampling sites and successional stages with the exception of pastures, where only 3 individuals of G. soricina were captured [27]. On the other hand, the two species most susceptible to variation in habitat attributes (C. godmani and M. harrisoni), which were strictly associated with the less disturbed areas, were scarcely represented (one individual) in two of the three sites of the late successional stage [27].
The negative association between nectarivore abundance and the mean area of dry forest patches, probably reflects the behavior of the three most abundant nectarivores (G. soricina, G. commissarisi and L. yerbabuenae) which are not so dependent on large dry forest patches. Naturally, these species are well adapted to inhabit regions where vegetation has a simple structure, like deserts, arid grasslands, scrublands, and lowland dry forest [99], [100], [101], [102], [103], being able to exploit the trophic resources available in these areas (i.e. plants of the family Cactaceae and Asparageceae, [25], [104]). In the study region, these three nectarivorous species occur at a higher abundance in the early successional stage, characterized by the presence of non-native grasses, shrubs and treelets, and where some potential chiropterophilic species (i.e. Acacia farnesiana, Cordia alliodora) are highly abundant [27], [105], [106], [107]. Other studies have reported that the abundance of nectarivores with a more generalist feeding and habitat preferences (i.e. G. soricina, G. commissarisi, Lonchophylla robusta, Monophyllus redmani and Phyllonycteris poeyi), appears unaffected or increases in disturbed and secondary vegetation [14], [30], [31], [37], [41], [44], [46], [108], [109], [110], [111].
In contrast to the nectarivores' response, the number of frugivorous species, as well as the abundance of some of these species, increased toward the more advanced successional stages, which present the highest vegetation structural complexity (Fig. S1, Table 2). Indeed, in pastures, the sites with the simplest vegetation structure, only three species of frugivores occurred (A. jamaicensis, A. watsoni, and A. lituratus) while in the late successional stage, 8 species occurred, three of them (Carollia sp., Centurio senex and Chiroderma salvini) exclusively in this stage [27]. These responses can be explained by an increase in the diversity and amount of resources (i.e. food and shelter) with the increase in the complexity of vegetation structure. The loss of trees due to clearing for grazing and agriculture can negatively influence frugivore abundance in the Neotropics by affecting their ability to locate suitable roosting sites, as they preferentially roost in hollow trees found in mature forest or in advanced successional stages [11], [112], [113]. In the tropical dry forest, the diversity and amount of trophic resources for frugivores decrease toward the early successional stage, dominated by anemochorous and autochorous plants which do not constitute food resources for these species [114] (P. Balvanera et al. unpublished data). This contrasts with the patterns found in tropical humid and rain forest, where chiropterochorous species such as Cecropia spp., Piper spp., Solanum spp., and Vismia spp., are dominant in the early stages of succession [11], [37].
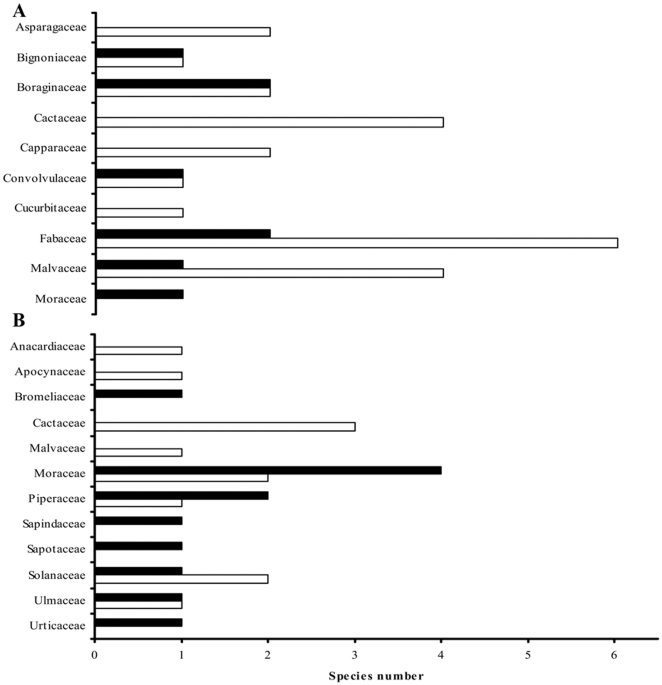
Nectarivores and frugivores showed a marked difference in their response to variations in landscape attributes. Variation in nectarivore abundance tended to be associated with variation in landscape attributes concerning dry forest vegetation while variation in frugivore abundance was tightly associated to variation in landscape attributes concerning riparian forest vegetation. This probably occurs because dry and riparian forests differ in terms of the availability of resources for both guilds. Although both forests host an equal number of chiropterochorous plant families and species (8 families, 12 species), the plants preferentially used by most of the frugivorous species (i.e. Moraceae and Piperaceae are heavily consumed by Artibeus and Carollia, respectively) are almost exclusively found in riparian forest (Fig. 3, Table S3, [26]). This explains why the abundance of the three frugivores analyzed, as well as the overall abundance of frugivorous bats, was higher in sites with a greater percentage of riparian forest. An analogous result has been reported for tropical rain forests, where the variation in the abundance of a chiropterocoric resource, Piper spp., was significantly and positively associated with variations in the abundance of shrub frugivorous bats [51]. In contrast, most chiropterophilic species (23 species from 9 families) as well as the chiropterochorous Cactaceae species, important food resources for nectarivorous bats, occur in the dry forest, within both the early and more advanced successional stages (Fig. 3, Table S3, [26], [115]). Only 8 chiropterophilic species, representing 6 plant families, occur in the riparian forest. Consequently, dry forest vegetation hosts more plant species that constitute resources for nectarivores than riparian forest vegetation (Fig. 3).
Figure 3. Number of chiropterophylic and chiropterochoric species per plant family occurring in dry and riparian forest.
A: chiropterophylic species, B: chiropterochoric species. Dry and riparian forests arerepresented by white and black bars respectively. The entire species' checklist of the Chamela-Cuixmala region, as well as detailed information on how it was generated, appear in Table S3.
The higher abundance of the sanguivorous D. rotundus toward sites with a greater amount of riparian vegetation, can occur because: 1) the riparian forest offers higher roost availability for this species [116], [117] and/or it uses this habitat as stepping stones when searching for food in the vegetation matrix, as suggested by Estrada and Coates-Estrada [40] for explaining how this species uses forested areas when searching for food in pasture-dominated landscapes; 2) there is a higher availability of sanguivores' native food sources (mammals) in the riparian vegetation. Riparian forests contain important resources for the maintenance and connectivity of the home ranges of medium and large-sized mammals [118], [119], [120]. Moreover, some mammal species concentrate their activities in riparian forest, especially during the dry season when most resources are limited in the region [77]; 3) the riparian forest also hosts a higher amount of non-native source of food (cattle), as it occurs on alluvial terraces along the channels of ephemeral and permanent streams. Farmers concentrate the cattle in these areas as they constitute the most important sources of water in the region; the cattle concentration in these areas reaches its highest point during the dry season (Pers. Obs).
Phyllostomid response at the assemblage-level
As expected, a portion of the variation among phyllostomid bat assemblages, in terms of species composition and structure (NMDS axis 2, Fig. 2), was tightly associated with the variation in vegetation structural complexity and vegetation cover (riparian forest). Moreover, phyllostomid abundance was significantly associated with the percentage of riparian forest (at 500 m scale) and with the mean area of riparian forest patches (at 1000 m scale). These results integrate the results obtained at the species and ensemble-level.
The association between assemblage characteristics and vegetation structural complexity has some potential explanations. First, a group of species is found exclusively in sites with a determined vegetation structure. This is the case for rare species (represented by one individual) and some species with an intermediate level of abundance (Micronycteris microtis, Musonycteris harrisoni, Choeroniscus godmani, Carollia sp., Centurio senex, Chiroderma salvini), which are strictly associated with the late successional stage [27]. In fact, M. microtis, M. harrisoni, C. godmani and C. senex already have been identified as forest dwelling species [14], [41], [49], [50], [106]. Second, variation of some species' abundance is tightly associated with variation in vegetation structure. This is the case for some of the most abundant frugivorous species. As discussed above, these responses could follow variations in availability of roosting sites and/or food as a consequence of structural changes.
The association between the amount of riparian forest and phyllostomid assemblage composition and overall abundance is mainly a consequence of frugivore response, as they constitute the best represented ensemble (in terms of species richness and number of captured individuals) and their abundance tends to increase towards sites with a greater amount of riparian forest.
Implications for the study of phyllostomid bats
In accordance with previous studies [32], [46], [52], [56], our results showed that bat responses to habitat modification are scale-dependent. Variation in some explanatory variables was associated with variation in some response variables at just one of the two analyzed scales (Table 2). Such dependence can be due to the species-specific degree of mobility, habitat requirements and life-history characteristics [56]. These results indicate that a single scale approach may be inadequate for understanding bat responses to habitat modifications and that a multi-spatial scale approach must be employed. Hence, the spatial scale must be taken into account when determining habitat attributes whose variation defines bat responses in a transformed landscape. The marked difference between the ensemble's response to landscape attributes also indicates the necessity of considering different land-cover classes when characterizing landscape attributes. With the exception of one study [121], a multi-land-cover class approach has not been considered in most of the related bat studies in the Neotropics.
The guild-specific association between bat abundance variation and variation in landscape features could be used for modeling the variation in the functional diversity of phyllostomid bat assemblages across anthropogenic landscapes [52]. This would represent an invaluable tool allowing us to identify the most important areas for preserving the ecological services provided by these bats (i.e. pollination, seed dispersal). The abundance-based modeling of variations in functional diversity can also be used as a surrogate for modeling variation in species richness, a widely used indicator for conservation planning. This approach would be highly useful especially when species diversity parameters cannot be accurately estimated in all sampled sites due to small sample sizes [52].
Our results also point out the importance of comparing outcomes from different statistical analyses in order to identify the most relevant associations among bat responses and variation in habitat attributes. The two statistical analyses we employed (HPA and AIC), for example, have recently been used for identifying habitat attributes whose variation may explain bat response to habitat modifications [46], [56], but the results we obtained from each statistical analysis were different in certain cases.
Implications for phyllostomids bats conservation
As in previous studies, our results reflect phyllostomid bats sensitivity to habitat loss or modification at both local and landscape levels [8], [32], [37], [39], [41], [44], [56]. Long-term conservation of phyllostomids in anthropogenic landscapes only can be achieved by realizing that management of the habitat at the landscape level (regarding its composition and configuration) is as important as the conservation of particular forest fragments. In this sense, to guarantee the conservation of phyllostomids in tropical dry forest landscapes the conservation and restoration of riparian forest should be prioritized. This habitat and its surrounding areas have been extensively affected by agricultural and cattle production, as they represent the main source of water and fertile soils in tropical dry forest regions [77], [122]. Bat abundance at the species, ensemble and assemblage-levels is negatively affected by reductions in the amount of riparian forest as this habitat represents an important source of trophic resources for a great number of phyllostomids, especially for the frugivores (Fig. 3). The amount of riparian forest can be even more critical for bat assemblages during the dry season, when availability of food is limited in dry forest and most resources are concentrated in riparian forest [77]. In addition, riparian forest can play an important role in the maintenance and connectivity of bats' home ranges functioning as a vegetation corridor across the agricultural landscape, providing physical connectivity among isolated forests or acting as stepping stones [45], [57].
Land-use policies must also focus on the maintenance of large areas of mature forest, as several species appear to be greatly associated with sites with a high vegetation structural complexity. The maintenance of mature forests appears to be critical for rare species which, due to their specialized foraging requirements and restricted mobility, are unable to move and use the resources available in other habitats [27], [40]. Finally, we suggest that policies must consider the inclusion of secondary vegetation in conservation areas/programs [123], as suggested by the “Red de Areas Ejidales Protegidas" proposed by Sanchez-Azofeifa et al. [77]. Several phyllostomids occur, and presumably exploit the resources available in such areas. In fact, some species of nectarivores show a higher abundance in secondary vegetation compared to mature forest. Consequently, secondary vegetation can be considered as an important habitat for preserving bat diversity.
Supporting Information
Ordination of sampling sites based on the structural attributes of vegetation.
(DOC)
Percentage of variation in population, ensemble and assemblage-level parameters, associated with the variation of the habitat attributes.
(DOC)
Most plausible models (95%) explaining the variation in population, ensemble and assemblage-level parameters.
(DOC)
Chiropterophylic and chiropterochoric species occurring in the Chamela-Cuixmala region.
(DOC)
Description of the image classification process.
(DOC)
Acknowledgments
We are grateful to M. Martinez-Ramos, J. Benitez-Malvido, P. Balvanera, M. Henry, A. Merenlender and Y. Duan for valuable information and ideas as well as to M. Chong, M. Lobato-García, M. Olvera-García, G. Sánchez-Montoya, H. Ferreira Medina, A. Valencia García, A. López Maldonado, G. Verduzco, and J. Mendez for technical assistance. Fieldwork was performed with the help of K.A. Oceguera-Salazar, C.I. García Leal, A. Jasso de la Rosa, J.A. Márquez Juárez, O. Sanchez-Lieja, A. Tovar, E. Zaragoza and R. Sayago. We especially thank M. Gargollo who allowed us to work on her property. We thank anonymous reviewers for valuable criticisms and suggestions that improved the manuscript. For logistical support, we thank the Programa de Posgrado en Ciencias Biológicas, the Dirección General de Estudios de Posgrado, the Centro de Investigaciones en Ecosistemas (CIEco), the Estación de Biología Chamela, and the Herbario Nacional MEXU from the Universidad Nacional Autónoma de México (UNAM) as well as the Center for Earth Observation Sciences (CEOS) from the University of Alberta.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Financial support was provided by the Inter American Institute for Global Change Research (Tropi-Dry-CRN2-21), SEMARNAT-CONACyT, Mexico (2002-C01-0597 and CB-2005-51043) and the Universidad Nacional Autónoma de México (Dirección General de Estudios de Posgrado and Programa de Posgrado en Ciencias Biológicas). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chazdon RL, Letcher SG, van Breugel M, Martínez-Ramos M, Bongers F, et al. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Philosophical Transactions of the Royal Society B. 2007;362:273–289. doi: 10.1098/rstb.2006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nepstad DC, Veríssimo A, Alencar A, Nobre CA, Lima E, et al. Large-scale impoverishment of Amazonian forests by logging and fire. Nature. 1999;398:505–508. [Google Scholar]
- 3.Primack R, Rozzi R, Massardo F, Feinsinger P. Destrucción y degradación del habitat. In: Primack R, Rozzi R, Feinsinger P, Dirzo R, Massardo F, editors. Fundamentos de Conservación Biológica: Perspectivas Latinoamericanas. FCE, D.F, Mexico; 2001. pp. 183–223. [Google Scholar]
- 4.Hilty JA, Lidicker WZ, Jr, Merenlender AM. Corridors Ecology: The Science and Practice Landscape for Biodiversity Conservation. Washinton, DC: Island Press; 2006. 323 [Google Scholar]
- 5.Quesada M, Sanchez-Azofeifa GA, Alvarez-Añorve M, Stoner KE, Avila-Cabadilla L, et al. Sucession and management of tropical dry forest in the Americas: review and new perspectives. Forest Ecology and Management. 2009;258:1014–1024. [Google Scholar]
- 6.Green RE, Cornell SJ, Scharlemann JPW, Balmford A. Farming and the fate of wild nature. Science. 2005;307:550–555. doi: 10.1126/science.1106049. [DOI] [PubMed] [Google Scholar]
- 7.Harvey CA, Medina A, Merlo D, Vílchez S, Hernández B, et al. Patterns of animal diversity in different forms of tree cover in agricultural landscapes. Ecological Applications. 2006;16:1986–1999. doi: 10.1890/1051-0761(2006)016[1986:poadid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Cosson J-F, Pons J-M, Masson D. Effects of forest fragmentation on frugivorous and nectarivorous bats in French Guiana. Journal of Tropical Ecology. 1999;15:515–534. [Google Scholar]
- 9.Jordán F. Keystone species and food webs. Philosophical Transactions of the Royal Society B. 2009;364:1733–1741. doi: 10.1098/rstb.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguirre LF, Lens L, van Damme R, Matthysen E. Consistency and variation in the bat assemblages inhabiting two forest islands within a Neotropical savanna in Bolivia. Journal of Tropical Ecology. 2003;19:367–374. [Google Scholar]
- 11.Fleming TH. The Short-Tailed Fruit Bat: A Study in Plant–Animal Interactions. Chicago: University of Chicago Press; 1988. 365 [Google Scholar]
- 12.Kalko EKV. Organization and diversity of tropical bat communities through space and time. Zoology: Analysis of Complex Systems. 1998;101:281–297. [Google Scholar]
- 13.Bernard E, Fenton MB. Species diversity of bats (Mammalia: Chiroptera) in forest fragments, primary forests, and savannas in central Amazonia, Brazil. Canadian Journal of Zoology. 2002;80:1124–1140. [Google Scholar]
- 14.Estrada A, Coates-Estrada R, Meritt D., Jr Bat species richness and abundance in tropical rain forest fragments and in agricultural habitats at Los Tuxtlas, Mexico. Ecography. 1993;16:309–318. [Google Scholar]
- 15.Hutson AM, Mickleburg SP, Racey PA. Microchiropteran Bats: Global Status Survey and Conservation Action Plan. 2001. IUCN/SSC, Chiroptera Specialist Group, IUCN, Gland, Switzerland and Cambridge, UK.
- 16.Kunz TH, de Torrez EB, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Annals of the New York Academy of Sciences. 2011;1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- 17.Charles-Dominique P. Inter-relations between frugivorous vertebrates and pioneers plants: Cecropia, birds and bats in French Guiana. In: Estrada A, Fleming TH, editors. Frugivores and Seed Dispersal. Dr W. Junk, Dordrecht; 1986. pp. 119–136. [Google Scholar]
- 18.Fleming TH. Foraging strategies of plant-visiting bats. In: Kuntz TH, editor. Ecology of Bats. Plenum Press; 1982. pp. 287–325. [Google Scholar]
- 19.Gorchov DL, Cornejo F, Ascorra C, Jaramillo M. The role of seed dispersal in the natural regeneration of rain forest after strip-cutting in the Peruvian Amazon. Vegetatio. 1993;107/108:339–349. [Google Scholar]
- 20.Kalka MB, Smith AR, Kalko EKV. Bats limit arthropods and herbivory in a tropical forest. Science. 2008;320:71. doi: 10.1126/science.1153352. [DOI] [PubMed] [Google Scholar]
- 21.Muscarella R, Fleming TH. The role of frugivorous bats in tropical forest succession. Biological Reviews. 2007;82:573–590. doi: 10.1111/j.1469-185X.2007.00026.x. [DOI] [PubMed] [Google Scholar]
- 22.Valiente-Banuet A, Molina-Freaner F, Torres A, Arizmendi M, Casas A. Geographic differentiation in the pollination system of the columnar cactus Pachycereus pecten-aboriginum. American Journal of Botany. 2004;91:850–855. doi: 10.3732/ajb.91.6.850. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker JO., Jr Bats, beetles and bugs. Bats. 1993;11:23. [Google Scholar]
- 24.Williams-Guillén K, Perfecto I, Vandermeer J. Bats limit insects in a Neotropical agroforestry system. Science. 2008;320:70. doi: 10.1126/science.1152944. [DOI] [PubMed] [Google Scholar]
- 25.Geiselman C, Mori SA, Lobova TA, Blanchard F. onward. Database of neotropical bat/plant interactions. 2002. Available: http://www.nybg.org/botany/tlobova/mori/batsplants/database/dbase_frameset.htm. Accessed 2008 Feb 1.
- 26.Lobova TA, Geiselman CK, Mori SA. Seed Dispersal by Bats in the Neotropics. New York: New York Botanical Garden; 2009. [Google Scholar]
- 27.Avila-Cabadilla LD, Stoner KE, Henry M, Alvarez-Añorve MY. Composition, structure and diversity of phyllostomid bat assemblages in different successional stages of a tropical dry forest. Forest Ecology and Management. 2009;258:986–996. [Google Scholar]
- 28.Bernard E, Fenton MB. Bat mobility and roosts in a fragmented landscape in Central Amazonia, Brazil. Biotropica. 2003;35:262–277. [Google Scholar]
- 29.Bernard E, Fenton MB. Bats in a fragmented landscape: species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biological Conservation. 2007;134:332–343. [Google Scholar]
- 30.Castro-Arellano I, Presley SJ, Saldaña LN, Willig MR, Wunderle JM., Jr Effects of reduced impact logging on bat biodiversity in terra firme forest of lowland Amazonia. Biological Conservation. 2007;138:269–285. [Google Scholar]
- 31.Castro-Luna AA, Sosa VJ, Castillo-Campos G. Bat diversity and abundance associated with the degree of secondary succession in a tropical forest mosaic in south-eastern Mexico. Animal Conservation. 2007;10:219–228. [Google Scholar]
- 32.Gorresen PM, Willig MR. Landscape responses of bats to habitat fragmentation in Atlantic forest of Paraguay. Journal of Mammalogy. 2004;85:688–697. [Google Scholar]
- 33.Lumsden LF, Bennett AF. Scattered trees in rural landscapes: foraging habitat for insectivorous bats in south- eastern Australia. Biological Conservation. 2005;122:205–222. [Google Scholar]
- 34.Montiel S, Estrada A, León P. Bat assemblages in a naturally fragmented ecosystem in the Yucatan Peninsula, Mexico: species richness, diversity and spatio-temporal dynamics. Journal of Tropical Ecology. 2006;22:267–276. [Google Scholar]
- 35.Offerman HL, Dale VN, Pearson SM, Bierregaard O, Jr, O'Neill RV. Effects of forest fragmentation on Neotropical fauna: current research and data availability. Environmental Review. 1995;3:190–211. [Google Scholar]
- 36.Turner IM. Species loss in fragments of tropical rain forest: a review of the evidence. Journal of Applied Ecology. 1996;33:200–209. [Google Scholar]
- 37.Brosset A, Charles-Dominique P, Cockle A, Cosson J-F, Masson D. Bat communities and deforestation in French Guiana. Canadian Journal of Zoology. 1996;74:1974–1982. [Google Scholar]
- 38.Bianconi GV, Mikich SB, Pedro WA. Diversidade de morcegos (Mammalia, Chiroptera) em remanescentes florestais do municipio de Fénix, noroeste do Paraná, Brasil. Revista Brasileira de Zoología. 2004;21:943–954. [Google Scholar]
- 39.Cosson JF, Ringuet S, Claessens O, de Massary JC, Dalecky A, et al. Ecological changes in recent land-bridge islands in French Guiana, with emphasis on vertebrate communities. Biological Conservation. 1999;91:213–222. [Google Scholar]
- 40.Estrada A, Coates-Estrada R. Bats in continuous forest, forest fragments and in an agricultural mosaic habitat-island at Los Tuxtlas, Mexico. Biological Conservation. 2002;103:237–245. [Google Scholar]
- 41.Fenton MB, Acharya L, Audet D, Hickey MB, Merriman C, et al. Phyllostomid bats (Chiroptera: Phyllostomidae) as indicator of habitat disruption in the Neotropics. Biotropica. 1992;243:440–446. [Google Scholar]
- 42.Henry M, Cosson JF, Pons JM. Abundance may be a misleading indicator of fragmentation-sensitivity: the case of fig-eating bats. Biological Conservation. 2007;139:462–467. [Google Scholar]
- 43.Medellín R, Gaona O. Seed dispersal by bats and birds in forest and disturbed habitats in Chiapas, México. Biotropica. 1999;31:432–441. [Google Scholar]
- 44.Medellín RA, Equihua M, Amin MA. Bat diversity and abundance as indicators of disturbance in neotropical rainforests. Conservation Biology. 2000;14:1666–1675. doi: 10.1111/j.1523-1739.2000.99068.x. [DOI] [PubMed] [Google Scholar]
- 45.Medina A, Harvey CA, Sánchez D, Vílchez S, Hernández B. Bat diversity and movement in an agricultural landscape in Matiguás, Nicaragua. Biotropica. 2007;39:120–128. [Google Scholar]
- 46.Meyer CFJ, Kalko EKV. Assemblage–level responses of phyllostomids bats to tropical forest fragmentation: land–bridge islands as a model system. Journal of Biogeography. 2008;35:1711–1726. [Google Scholar]
- 47.Quesada M, Stoner KE, Lobo JA, Herrerias-Diego Y, Palacios-Guevara C, et al. Effects of forest fragmentation on pollinator activity and consequences for plant reproductive success and mating patterns in bat-pollinated Bombacaceous trees. Biotropica. 2004;36:131–138. [Google Scholar]
- 48.dos Reis NR, Muller MF. Bat diversity of forest and open areas in a subtropical region of south Brazil. Ecología Austral. 1995;5:31–36. [Google Scholar]
- 49.Schulze MD, Seavy NE, Whitacre DF. A comparison of the phyllostomid bat assemblages in undisturbed Neotropical forest and in forest fragments of a slash-and-burn farming mosaic in Petén, Guatemala. Biotropica. 2000;32:174–184. [Google Scholar]
- 50.Stoner KE, Quesada M, Rosas-Guerrero V, Lobo JA. Effects of forest fragmentation on the Colima long-nosed bat (Musonycteris harrisoni) foraging in tropical dry forest of Jalisco, Mexico. Biotropica. 2002;34:462–467. [Google Scholar]
- 51.Henry M, Ponds J-M, Cosson J-F. Foraging behaviour of a frugivorous bat help bridge landscape connectivity and ecological processes in a fragmented rainforest. Journal of Animal Ecology. 2007;76:801–813. doi: 10.1111/j.1365-2656.2007.01258.x. [DOI] [PubMed] [Google Scholar]
- 52.Henry M, Cosson J-F, Pons J-M. Modelling multi-scale spatial variation in species richness from abundance data in a complex neotropical bat assemblage. Ecological Modelling. 2010;221:2018–2027. [Google Scholar]
- 53.de la Peña-Cuéllar E, Stoner KE, Avila-Cabadilla LD, Martínez-Ramos M, Estrada A. Phyllostomid bat assemblages in different successional stages of tropical rain forest in Chiapas, Mexico. Biodiversity and Conservation. 2012;20:1–17. [Google Scholar]
- 54.Fauth JE, Bernardo J, Camara M, Resetarits WJ, Van Buskirk J, Jr, et al. Simplifying the Jargon of Community Ecology: A Conceptual Approach. The American Naturalist. 1996;147:282–286. [Google Scholar]
- 55.Fahrig L. Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution and Systematics. 2003;34:487–515. [Google Scholar]
- 56.Klingbeil BT, Willig MR. Guild–specific response of bats to landscape composition and configuration in fragmented Amazonian rainforest. Journal of Applied Ecology. 2009;46:203–213. [Google Scholar]
- 57.Estrada A, Coates-Estrada R. Bat species richness in live fences and in corridors of residual rain forest vegetation at Los Tuxtlas, Mexico. Ecography. 2001;24:94–102. [Google Scholar]
- 58.Sanchez-Azofeifa GA, Quesada M, Rodríguez JP, Nassar JM, Stoner KE, et al. Research priorities for neotropical dry forest. Biotropica. 2005;37:477–485. [Google Scholar]
- 59.Portillo-Quintero CA, Sánchez-Azofeifa GA. Extent and conservation of tropical dry forests in the Americas. Biological Conservation. 2010;143:144–155. [Google Scholar]
- 60.Lott EJ. Lista anotada de la plantas vasculares de Chamela–Cuixmala. In: Noguera FA, Vega JH, García AN, Quesada M, editors. Historia Natural de Chamela. México: Instituto de Biología UNAM; 2002. pp. 99–136. [Google Scholar]
- 61.Balvanera P, Lott E, Segura G, Siebe C, Islas A. Patterns of β-diversity in a Mexican tropical dry forest. Journal of Vegetation Science. 2002;13:145–158. [Google Scholar]
- 62.Fenton MB, Kunz TH. Movements and behavior. In: Baker RJ, Jones JK Jr, Carter DC, editors. Biology of Bats of the New World family Phyllostomatidae. Part 2. Texas: Texas Tech. University Press; 1977. pp. 351–364. [Google Scholar]
- 63.Faria D, Baumgarten J. Shade cacao plantations (Theobroma cacao) and bat conservation in southern Bahia, Brazil. Biodiversity and Conservation. 2007;16:291–312. [Google Scholar]
- 64.Willig MR, Presley SJ, Bloch CP, Hice CL, Yanoviak SP, et al. Phyllostomid bats of lowland Amazonia: effects of habitat alteration on abundance. Biotropica. 2007;39:737–746. [Google Scholar]
- 65.Vaughan N, Parsons S, Barlow KE, Gannon MR. Echolocation calls and wing morphology of bats from the West Indies. Acta Chiropterologica. 2004;6:75–90. [Google Scholar]
- 66.MacSwiney MC, Clarke FM, Racey PA. What you see is not what you get: the role of ultrasonic detectors in increasing inventory completeness in Neotropical bat assemblages. Journal of Applied Ecology. 2008;45:1364–1371. [Google Scholar]
- 67.Kunz TH, Brock CE. A comparison of mist nets and ultrasonic detectors for monitoring the flight activity of bats. Journal of Mammalogy. 1975;56:907–911. [Google Scholar]
- 68.Kunz TH, Tidemann CR, Richards GC. Small volant mammals. In: Wilson DE, Cole FR, Nichols JD, Rudran R, Foster MS, editors. Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Washington DC: Smithsonian Institution Press; 1996. 409 [Google Scholar]
- 69.Larsen RJ, Boegler KA, Genoways HH, Masefield WP, Kirsch RA, et al. Mist netting bias, species accumulation curves, and the rediscovery of two bats on Montserrat (Lesser Antilles). Acta Chiropterologica. 2007;9:423–435. [Google Scholar]
- 70.Medellín RA, Arita HT, Sánchez O. Identificación de los Murciélagos de México: Clave de Campo. México: Asociación Mexicana de Mastozoología, A.C.; 1997. [Google Scholar]
- 71.Timm RM, Laval RK. A field key to the bats of Costa Rica. 1998. Occasional Publication Series, Center of Latin American Studies, The University of Kansas, vol. 22.
- 72.Simmons NB. Order Chiroptera. In: Wilson DE, Reeder DM, editors. Mammal Species of the World: A Taxonomic and Geographic Reference. Baltimore: Johns Hopkins University Press; 2005. pp. 312–529. [Google Scholar]
- 73.Fournier RA, Mailly D, Walter J-MN, Soudani K. Indirect measurement of forest canopy structure from in situ optical sensors. In: Wulder M, Franklin S, editors. Remote Sensing of Forest Environments: Concepts and Case studies. New York: Kluwer Academic Publishers; 2003. pp. 77–113. [Google Scholar]
- 74.Kalacska M, Sanchez-Azofeifa GA, Rivard B, Calvo-Alvarado JC, Journet ARP, et al. Leaf area index measurements in a tropical moist forest: a case study from costa Rica. Remote Sensing of Environment. 2004;91:134–152. [Google Scholar]
- 75.Nassar JM, Rodríguez JP, Sanchez-Azofeifa GA, Garvin T, Quesada M. Manual of Methods: Human, Ecological and Biophysical Dimensions of Tropical Dry Forests. Caracas: Instituto Venezolano de Investigaciones Científicas (IVIC); 2008. [Google Scholar]
- 76.Kalacska M, Calvo-Alvarado GAJC, Sanchez-Azofeifa GA. Calibration and assessment of seasonal changes in leaf area index of a tropical dry forest on different stages of succession. Tree Physiology. 2005;25:733–744. doi: 10.1093/treephys/25.6.733. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez-Azofeifa GA, Quesada M, Cuevas-Reyes P, Castillo A, Sanchez-Montoya G. Land cover and conservation in the area of influence of the Chamela-Cuixmala Biosphere Reserve, Mexico. Forest Ecology and Management. 2009;258:907–912. [Google Scholar]
- 78.Lemke TO. Foraging ecology of the long-nosed bat, Glossophaga soricina, with respect to resource availability. Ecology. 1984;65:538–548. [Google Scholar]
- 79.Fenton MB, Vonhof MJ, Bouchard S, Gill SA. Roosts used by Sturnira lilium (Chiroptera: Phyllostomidae) in Belize. Biotropica. 2000;32:729–733. [Google Scholar]
- 80.Hurlbert SH. Pseudoreplication and the design of ecological field experiments. Ecological Monograph. 1984;54:187–211. [Google Scholar]
- 81.Legendre P. Spatial autocorrelation: trouble or new paradigm? Ecology. 1993;74:1659–1673. [Google Scholar]
- 82.Eigenbrod F, Hecnar SJ, Fahrig L. Sub-optimal study design has major impacts on landscape-scale inference. Biological Conservation. 2011;144:298–305. [Google Scholar]
- 83.McGarigal K, Cushman SA, Neel MC, Ene E. FRAGSTATS: Spatial pattern analysis program for categorical maps. Computer software program produced by the authors at the University of Massachusetts, Amherst. UMass Landscape Ecology Lab website. 2002. Available: www.umass.edu/landeco/research/fragstats/fragstats.html. Accessed 2009 Jun 1.
- 84.Magurran AE. Measuring Biological Diversity. Oxford: Blackwell Publishing; 2004. [Google Scholar]
- 85.Colwell RK, Coddington JA. Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions: Biological Sciences. 1994;345:101–118. doi: 10.1098/rstb.1994.0091. [DOI] [PubMed] [Google Scholar]
- 86.Moreno CE, Halffter G. Assessing the completeness of bat biodiversity inventories using accumulation curves. Journal of Applied Ecology. 2001;37:149–158. [Google Scholar]
- 87.Gotelli NJ, Colwell RK. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters. 2001;4:79–391. [Google Scholar]
- 88.Kindt R, Coe R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies. Nairobi: World Agroforestry Centre (ICRAF); 2005. [Google Scholar]
- 89.Oksanen J. Multivariate analysis of ecological communities in R: vegan tutorial. Vegan: R functions for vegetation ecologists website; 2010. Available: http://cc.oulu.fi/~jarioksa/softhelp/vegan.html. Accessed 2010 Apr 7. [Google Scholar]
- 90.McCune B, Grace JB. Analysis of Ecological Communities. Gleneden Beach: MjM Software; 2002. [Google Scholar]
- 91.Chevan A, Sutherland M. Hierarchical partitioning. The American Statistician. 1991;45:90–96. [Google Scholar]
- 92.Mac Nally R. Regression and model–building in conservation biology, biogeography and ecology. The distinction between - and reconciliation of -‘predictive’ and ‘explanatory’ models. Biodiversity and Conservation. 2000;9:655–671. [Google Scholar]
- 93.Mac Nally R. Multiple regression and inference in ecology and conservation biology: further comments on identifying important predictor variables. Biodiversity and Conservation. 2002;11:1397–1401. [Google Scholar]
- 94.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach. New York: Springer–Verlag. 2 ed; 2002. [Google Scholar]
- 95.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. The R Project for Statistical Computing website. Available: http://www.R-project.org. Accessed 2009 Sep 15. [Google Scholar]
- 96.Oksanen J, Blanchet FG, Kindt R, Legendre P, O'Hara RG, et al. vegan: Community Ecology Package website. 2010. R package 1.17–0 ed. Available: http://cran.r-project.org/package=vegan. Accessed 2010 Jan 20.
- 97.Venables WN, Ripley BD. Modern Applied Statistics with S. New York: Springer; 2002. 4 ed. [Google Scholar]
- 98.Walsh C, Mac Nally R. hier.part: Hierarchical Partitioning. 2008. R package 1.0–3 ed.
- 99.Alvarez J, Willig MR, Jones K, Jr, Webster WmD. Glossophaga soricina. Mammalian Species. 1991;379:1–7. [Google Scholar]
- 100.Arita HT. Spatial segregation in long–nosed bats, Leptonycteris nivalis and Leptonycteris curasoae, in Mexico. Journal of Mammalogy. 1991;72:706–714. [Google Scholar]
- 101.Arita HT, Santos del Prado K. Conservation biology of nectar–feeding bats in Mexico. Journal of Mammalogy. 1999;80:31–41. [Google Scholar]
- 102.Cole FR Wilson DonE. Leptonycteris yerbabuenae. Mammalian Species. 2006;797:1–7. [Google Scholar]
- 103.Webster WmD, Jones JK., Jr Glossophaga commissarisi. Mammalian Species. 1993;446:1–4. [Google Scholar]
- 104.Valiente-Banuet A, Coro M, Rojas-Martínez A, Domínguez-Canseco L. Ecological relationships between columnar cactus and nectar–feeding bats in Mexico. Journal of Tropical Ecology. 1996;12:103–119. [Google Scholar]
- 105.Alvarez T, González-Quintero L. Análisis polínico del contenido gástrico de murciélagos Glossophaginae de México. México: Anales de la Escuela Nacional de Ciencias Biológicas. 1970;18:137–165. [Google Scholar]
- 106.Alvarez T, Sanchez-Casa N. Notas sobre la alimentación de Musonycteris y Choeroniscus (Mammalia: Phyllostomidae) en Mexico. Revista Mexicana de Mastozoología. 1997;2:113–115. [Google Scholar]
- 107.Tellez G, Ortega J. Musonycteris harrisoni. Mammalian Species. 1999;622:1–3. [Google Scholar]
- 108.Clarke FM, Rostant LV, Racey PA. Life after logging: post-logging recovery of a neotropical bat community. Journal of Applied Ecology. 2005;42:409–420. [Google Scholar]
- 109.Harvey CA, González JA. Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodiversity Conservation. 2007;16:2257–2292. [Google Scholar]
- 110.Mancina CA, García-Rivera L, Capote RT. Habitat use by phyllostomid bat assemblages in secondary forests of the ‘Sierra del Rosario’ Biosphere Reserve, Cuba. Acta Chiropterologica. 2007;9:203–218. [Google Scholar]
- 111.Peters SL, Malcolm JR, Zimmerman BL. Effects of selective logging on bat communities in the southeastern Amazon. Conservation Biology. 2006;20:1410–1421. doi: 10.1111/j.1523-1739.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 112.Evelyn MJ, Stiles DA. Roosting requirements of two frugivorous bats (Sturnira lilium and Arbiteus intermedius) in fragmented Neotropical forest. Biotropica. 2003;35:405–418. [Google Scholar]
- 113.Ortíz-Ramírez D, Lorenzo C, Naranjo E, León-Paniagua L. Selección de refugios por tres especies de murciélagos frugívoros (Chiroptera: Phyllostomidae) en la Selva Lacandona, Chiapas, México. Revista Mexicana de Biodiversidad. 2006;77:261–270. [Google Scholar]
- 114.Vieira DLM, Scariot A. Principles of natural regeneration of tropical dry forests for restoration. Restoration Ecology. 2006;14:11–20. [Google Scholar]
- 115.Stoner KE, O-Salazar KA, R-Fernández RC, Quesada M. Population dynamics, reproduction, and diet of the lesser long–nosed bat (Leptonycteris curasoae) in Jalisco, Mexico: implications for conservation. Biodiversity and Conservation. 2003;12:357–373. [Google Scholar]
- 116.Lord RD. Control of vampire bats. In: Greenhall AM, Schmidt U, editors. Natural History of Vampire Bats. Florida: CRC Press; 1988. pp. 215–226. [Google Scholar]
- 117.Taddei AV, Gonçalves CA, Pedro WA, Tadei WJ, Kotait I, et al. Distribuição do morcego vampiro Desmodus rotundus no Estado de São Paulo e a raiva dos animais domésticos. Brazil: CATI; 1991. 107 [Google Scholar]
- 118.Núñez R, Miller B, Lindzey F. Food habits of jaguars and pumas in Jalisco, Mexico. Journal of Zoology, London. 2000;252:373–379. [Google Scholar]
- 119.Saunders DA, Hobbs RJ, Margules CR. Biological consequences of ecosystem fragmentation: a review. Conservation Biology. 1991;5:18–32. [Google Scholar]
- 120.Valenzuela D, Ceballos G. Habitat selection, home range, and activity of the white-nosed coati (Nasua narica) in a mexican tropical dry forest. Journal of Mammalogy. 2000;81:810–819. [Google Scholar]
- 121.Bobrowiec PED, Gribel R. Effects of different secondary vegetation types on bat commuity composition in Central Amazonia, Brazil. Animal Conservation. 2010;13:204–216. [Google Scholar]
- 122.Maass J, Balvanera P, Castillo A, Daily GC, Mooney HA, et al. Ecosystem services of tropical dry forests: insights from long-term ecological and social research on the Pacific Coast of Mexico. Ecology and Society. 2005;10:17. [Google Scholar]
- 123.Rendón-Carmona H, Martínez-Yrízar A, Balvanera P, Pérez-Salicrup D. Selective cutting of woody species in a Mexican tropical dry forest: Incompatibility between use and conservation. Forest Ecology and Management. 2009;257:567–579. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ordination of sampling sites based on the structural attributes of vegetation.
(DOC)
Percentage of variation in population, ensemble and assemblage-level parameters, associated with the variation of the habitat attributes.
(DOC)
Most plausible models (95%) explaining the variation in population, ensemble and assemblage-level parameters.
(DOC)
Chiropterophylic and chiropterochoric species occurring in the Chamela-Cuixmala region.
(DOC)
Description of the image classification process.
(DOC)