Abstract
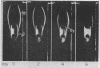
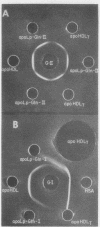
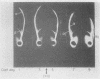
High density lipoproteins (d 1.063-1.210 g/ml) were isolated from the plasma of normal individuals (HDL) and seven homozygous patients with Tangier disease (HDLt). In Tangier patients, the concentration of protein in the high density region (HDLt) was only 0.5-4.5% of normal. Immunochemical studies, including mixing experiments conducted in vivo and in vitro, indicated that HDLt was different from HDL. HDLt was the only high density lipoprotein detectable in the plasma of Tangier homozygotes. In heterozygotes both HDL and HDLt were present. HDLt was not detected in the plasma of over 300 normal persons and 10 patients with secondary high density lipoprotein deficiency and appeared to be a unique marker for Tangier disease.
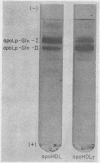
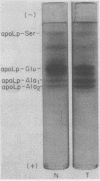
ApoHDL contained two major apoproteins designated apoLp-Gln-I and apoLp-Gln-II; together they comprised 85-90% of the total protein content. Both of the major HDL apoproteins were present in apoHDLt; but apoLp-Gln-I was disproportionately decreased with respect to apoLp-Gln-II, the ratio of their concentrations being 1: 12 in apoHDLt as compared with 3: 1 in apoHDL. Several minor apoprotein components which together comprise 5-15% of apoHDL were present in approximately normal proportions in apoHDLt. In the HDL of Tangier patients it was estimated that, compared with normal individuals, the concentration of apoLp-Gln-I was decreased about 600-fold and the concentration of apoLp-Gln-II about 17-fold. The decrease in these apoproteins was not due to preferential segregation with the lipoprotein fractions of d < 1.063 g/ml or with the plasma proteins of d > 1.21 g/ml. Tangier apoLp-Gln-I and apoLp-Gln-II appeared to be immunochemically identical with their normal counterparts, and no differences between the two sets of apoproteins were detected on polyacrylamide gel electrophoresis at pH 9.4 or 2.9. These results are most compatible with the hypothesis that the hereditary defect in Tangier disease is a mutation in an allele-regulating synthesis of apoLp-Gln-I.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Aladjem F. Precipitation of 125 I-labeled lipoproteins with specific polypeptide antisera. Evidence for two populations with differing polypeptide compositions in human high density lipoproteins. Biochemistry. 1971 Aug 31;10(18):3436–3442. doi: 10.1021/bi00794a019. [DOI] [PubMed] [Google Scholar]
- BAGLIONI C. The fusion of two peptide chains in hemoglobin Lepore and its interpretation as a genetic deletion. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1880–1886. doi: 10.1073/pnas.48.11.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Brewer H. B., Jr, Lux S. E., Ronan R., John K. M. Amino acid sequence of human apoLp-Gln-II (apoA-II), an apolipoprotein isolated from the high-density lipoprotein complex. Proc Natl Acad Sci U S A. 1972 May;69(5):1304–1308. doi: 10.1073/pnas.69.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Further characterization of apolipoproteins from the human plasma very low density lipoproteins. J Biol Chem. 1970 Dec 25;245(24):6588–6594. [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969 Oct 25;244(20):5687–5694. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dern R. J. A new hereditary quantitative variant of glucose-6-phosphate dehydrogenase characterized by a marked increase in enzyme activity. J Lab Clin Med. 1966 Oct;68(4):560–565. [PubMed] [Google Scholar]
- Engel W. K., Dorman J. D., Levy R. I., Fredrickson D. S. Neuropathy in Tangier disease. Alpha-Lipoprotein deficiency manifesting as familial recurrent neuropathy and intestinal lipid storage. Arch Neurol. 1967 Jul;17(1):1–9. doi: 10.1001/archneur.1967.00470250005001. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S. THE INHERITANCE OF HIGH DENSITY LIPOPROTEIN DEFICIENCY (TANGIER DISEASE). J Clin Invest. 1964 Feb;43:228–236. doi: 10.1172/JCI104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- Flatz G., Kinderlerer J. L., Kilmartin J. V., Lehmann H. Haemoglobin Tak: a variant with additional residues at the end of the beta-chains. Lancet. 1971 Apr 10;1(7702):732–733. doi: 10.1016/s0140-6736(71)91994-5. [DOI] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Jan 26;276(4):215–contd. doi: 10.1056/NEJM196701262760406. [DOI] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lindgren F. T. A comparison of heritable abnormal lipoprotein patterns as defined by two different techniques. J Clin Invest. 1969 Nov;47(11):2446–2457. doi: 10.1172/JCI105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan D., Bradford R. H., Alaupovic P., McConathy W. J. Differential activation of lipoprotein lipase from human post-heparin plasma, milk and adipose tissue by polypeptides of human serum Apolipoprotein C. FEBS Lett. 1971 Jun 24;15(3):205–208. doi: 10.1016/0014-5793(71)80312-5. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Kon H. Observations on the conformation of human serum high-density lipoproteins using infrared spectroscopy, circular dichroism, and electron spin resonance. Biochemistry. 1970 Oct 27;9(22):4276–4283. doi: 10.1021/bi00824a006. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Shore V. G., Shore B., Bier D. M. Role of specific glycopeptides of human serum lipoproteins in the activation of lipoprotein lipase. Circ Res. 1970 Oct;27(4):595–600. doi: 10.1161/01.res.27.4.595. [DOI] [PubMed] [Google Scholar]
- Herbert P., Levy R. I., Fredrickson D. S. Correction of COOH-terminal amino acids of human plasma very low density apolipoproteins. J Biol Chem. 1971 Nov 25;246(22):7068–7069. [PubMed] [Google Scholar]
- Hubbard R. W., Kremen D. M. Increased sensitivity of accelerated amino acid ion-exchange chromatography. Anal Biochem. 1965 Sep;12(3):593–602. doi: 10.1016/0003-2697(65)90227-7. [DOI] [PubMed] [Google Scholar]
- Hubbard R. W. Studies in accelerated amino acid analysis. Biochem Biophys Res Commun. 1965 Jun 9;19(6):679–685. doi: 10.1016/0006-291x(65)90310-4. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kocen R. S., Lloyd J. K., Lascelles P. T., Fosbrooke A. S., Willims D. Familial alpha-lipoprotein deficiency (Tangier disease) with neurological abnormalities. Lancet. 1967 Jun 24;1(7504):1341–1345. doi: 10.1016/s0140-6736(67)91759-x. [DOI] [PubMed] [Google Scholar]
- Kostner Gerhard, Alaupovic Petar. Studies of the composition and structure of plasma lipoproteins. C- and N-terminal amino acids of the two nonidentical polypeptides of human plasma apolipoprotein A. FEBS Lett. 1971 Jul 1;15(4):320–324. doi: 10.1016/0014-5793(71)80648-8. [DOI] [PubMed] [Google Scholar]
- LEES R. S., HATCH F. T. Sharper separation of lipoprotein species by paper electrophoresis in albumin-containing buffer. J Lab Clin Med. 1963 Mar;61:518–528. [PubMed] [Google Scholar]
- LEVY R. I., FREDRICKSON D. S. HETEROGENEITY OF PLASMA HIGH DENSITY LIPOPROTEINS. J Clin Invest. 1965 Mar;44:426–441. doi: 10.1172/JCI105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S. A specific apoprotein activator for lipoprotein lipase. Biochem Biophys Res Commun. 1970 Oct 9;41(1):57–62. doi: 10.1016/0006-291x(70)90468-7. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Alaupovic P. Studies of the composition and structure of plasma lipoproteins. Isolation, composition, and immunochemical characterization of low density lipoprotein subfractions of human plasma. Biochemistry. 1970 May 26;9(11):2244–2252. doi: 10.1021/bi00813a004. [DOI] [PubMed] [Google Scholar]
- Levy R. I., Lees R. S., Fredrickson D. S. The nature of pre beta (very low density) lipoproteins. J Clin Invest. 1966 Jan;45(1):63–77. doi: 10.1172/JCI105324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Hirz R., Shrager R. I., Gotto A. M. The influence of lipid on the conformation of human plasma high density apolipoproteins. J Biol Chem. 1972 Apr 25;247(8):2598–2606. [PubMed] [Google Scholar]
- MARIER J. R., ROSE D. DETERMINATION OF CYANATE, AND A STUDY OF ITS ACCUMULATION IN AQUEOUS SOLUTIONS OF UREA. Anal Biochem. 1964 Mar;7:304–314. doi: 10.1016/0003-2697(64)90135-6. [DOI] [PubMed] [Google Scholar]
- McConathy W. J., Quiroga C., Alaupovic P. Studies of the composition and structure of plasma lipoproteins. C- and N-terminal amino acids of C-I polypeptide ("R-Val") of human plasma apolipoprotein C. FEBS Lett. 1972 Jan 1;19(4):323–326. doi: 10.1016/0014-5793(72)80071-1. [DOI] [PubMed] [Google Scholar]
- Milner P. F., Clegg J. B., Weatherall D. J. Haemoglobin-H disease due to a unique haemoglobin variant with an elongated alpha-chain. Lancet. 1971 Apr 10;1(7702):729–732. doi: 10.1016/s0140-6736(71)91992-1. [DOI] [PubMed] [Google Scholar]
- Nichols A. V., Lux S., Forte T., Gong E., Levy R. I. Degradation products from human serum high density lipoproteins following dehydration by rotary evaporation and solubilization. Biochim Biophys Acta. 1972 May 23;270(1):132–148. doi: 10.1016/0005-2760(72)90186-5. [DOI] [PubMed] [Google Scholar]
- Piazzi S. E. A simple method for preliminary immunodiffusion test of antigen-antibody systems having unknown ratios of reaction. Anal Biochem. 1969 Feb;27(2):281–284. doi: 10.1016/0003-2697(69)90033-5. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Rudman D., Garcia L. A., Howard C. H. A new method for isolating the nonidentical protein subunits of human plasma alpha-lipoprotein. J Clin Invest. 1970 Feb;49(2):365–372. doi: 10.1172/JCI106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C., Lim C. T. Effect of disulfide cleavage on the molecular weight of one of the major polypeptides of human serum high density lipoprotein. FEBS Lett. 1971 Nov 1;18(2):305–307. doi: 10.1016/0014-5793(71)80472-6. [DOI] [PubMed] [Google Scholar]
- Scanu A., Cump E., Toth J., Koga S., Stiller E., Albers L. Degradation and reassembly of a human serum high-density lipoprotein. Evidence for differences in lipid affinity among three classes of polypeptide chains. Biochemistry. 1970 Mar 17;9(6):1327–1335. doi: 10.1021/bi00808a005. [DOI] [PubMed] [Google Scholar]
- Scanu A., Toth J., Edelstein C., Koga S., Stiller E. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 1969 Aug;8(8):3309–3316. doi: 10.1021/bi00836a027. [DOI] [PubMed] [Google Scholar]
- Shore B., Shore V. Isolation and characterization of polypeptides of human serum lipoproteins. Biochemistry. 1969 Nov;8(11):4510–4516. doi: 10.1021/bi00839a043. [DOI] [PubMed] [Google Scholar]
- Shore V., Shore B. Some physical and chemical studies on two polypeptide components of high-density lipoproteins of human serum. Biochemistry. 1968 Oct;7(10):3396–3403. doi: 10.1021/bi00850a013. [DOI] [PubMed] [Google Scholar]
- Simons K., Ehnholm C., Renkonen O., Bloth B. Characterization of the Lp(a) lipoprotein in human plasma. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):459–466. doi: 10.1111/j.1699-0463.1970.tb04328.x. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Amino acid substitution (histidine to tyrosine) in a glucose-6-phosphate dehydrogenase variant (G6PD Hektoen) associated with over-production. J Mol Biol. 1970 Sep 28;52(3):483–490. doi: 10.1016/0022-2836(70)90414-6. [DOI] [PubMed] [Google Scholar]
- ungenberg de Jong J. J., Marsh J. B. Biosynthesis of plasma lipoproteins by rat liver ribosomes. J Biol Chem. 1968 Jan 10;243(1):192–199. [PubMed] [Google Scholar]