Abstract
Sheep red blood cells can surround, in vitro, some human peripheral blood lymphocytes in a formation called a rosette. The number of rosetteforming cells (RFC) in 50 normal persons had a wide range (4-40%).
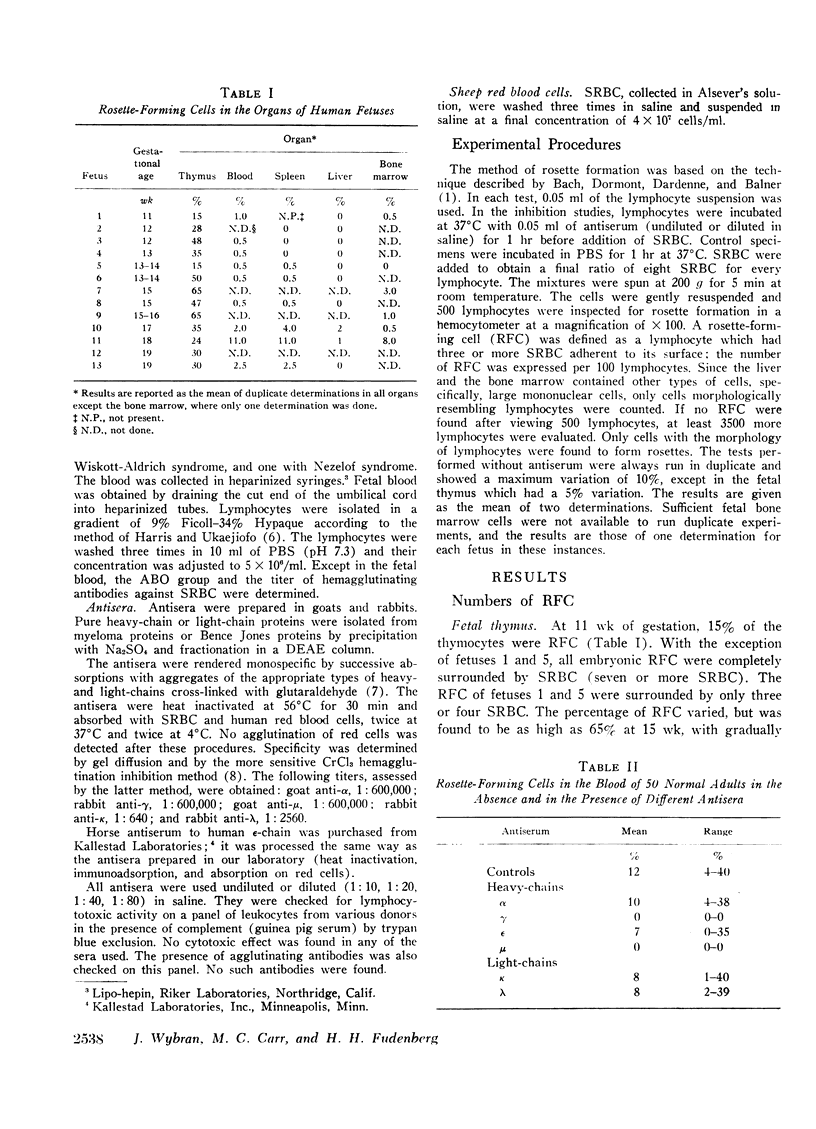
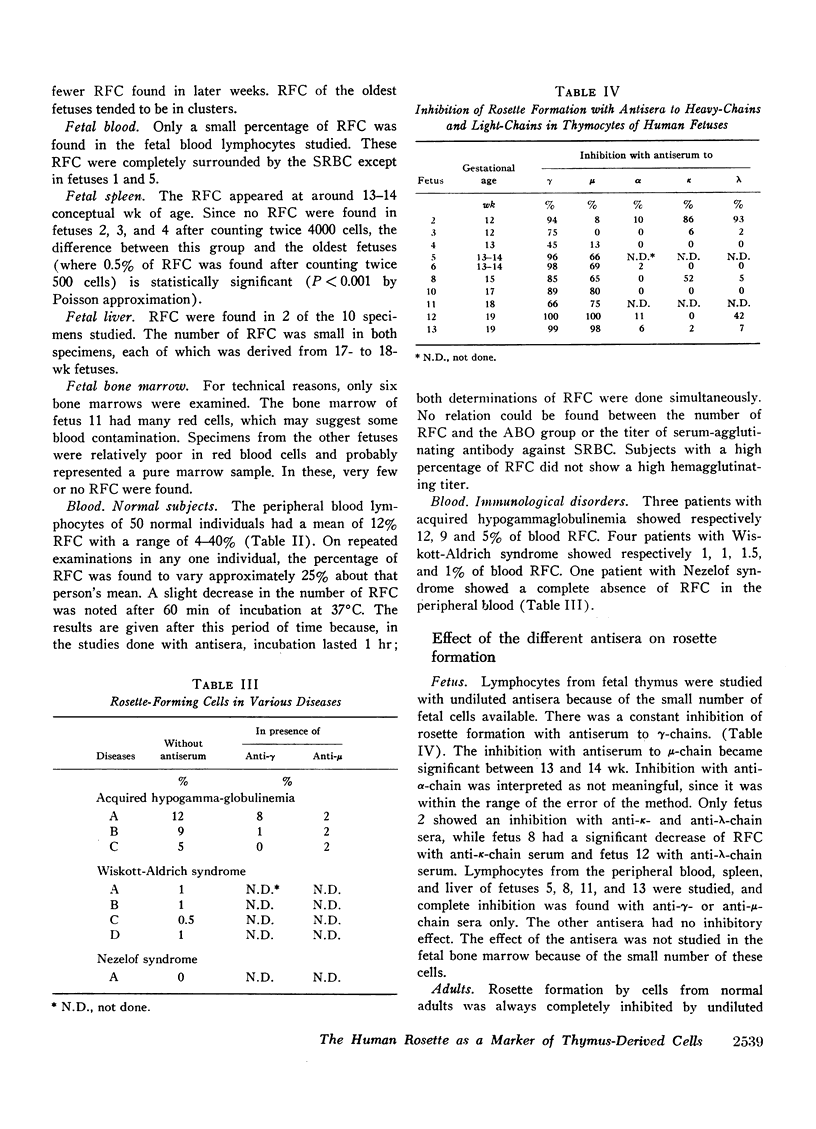
The organs of 13 human fetuses (11-19 wk conceptional age) were examined for the presence of RFC. The thymus possessed the highest percentage of RFC, the maximum being 65% of total thymocytes in two 15-16 wk fetal specimens. Blood RFC were always present and their number slightly increased in the oldest fetuses. The bone-marrow showed 0-8% in the six fetuses studied. RFC were found in the spleen around the 13th wk and in the liver around the 17th wk of gestation. These observations lead to the hypothesis that human blood RFC may be chiefly thymic derived. Studies of patients with immunological disorders support this hypothesis: one patient with Nezelof syndrome had no blood RFC and four patients with Wiskott-Aldrich syndrome had a low number of blood RFC (1 and 1.5%). Patients with acquired hypogammaglobulinemia showed a normal percentage of RFC. With the fetal thymocytes, the percentage of inhibition with anti-μ serum increased with the fetal age to become complete in the oldest fetuses studied. Incubation of the oldest fetal thymocytes or the blood lymphocytes with anti-γ serum of anti-μ serum completely inhibited the rosette formation. These results suggest that μ-chain determinants are present on human fetal thymocytes and blood RFC. The significance of the presence of γ-chain determinants on these cells is unclear.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dardenne M., Davies A. J. Early affect of adult thymectomy. Nat New Biol. 1971 May 26;231(21):110–111. doi: 10.1038/newbio231110a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Muller J. Y. Reconnaissance de l'antigène in vivo par les cellules fomant les rosettes spontanées. C R Acad Sci Hebd Seances Acad Sci D. 1970 Apr 27;270(17):2142–2144. [PubMed] [Google Scholar]
- Bach J. F., Dardenne M. Signification du phénomène de rosette chez la souris non immunisée. C R Acad Sci Hebd Seances Acad Sci D. 1969 Aug 11;269(6):751–754. [PubMed] [Google Scholar]
- Bach J. F., Dormont J., Dardenne M., Balner H. In vitro rosette inhibition by antihuman antilymphocyte serum. Correlation with skin graft prolongation in subhuman primates. Transplantation. 1969 Sep;8(3):265–280. doi: 10.1097/00007890-196909000-00008. [DOI] [PubMed] [Google Scholar]
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., McConnell I., Munro A. Immunoglobulin determinants on mouse lymphocytes from blood, lymph nodes, bone marrow and thymus. Int Arch Allergy Appl Immunol. 1970;39(2-3):280–291. doi: 10.1159/000230354. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Wilson A. B., Holm G., Lindgren B. Rosette-formation between human lymphocytes and sheep red cells not involving immunoglobulin receptors. Int Arch Allergy Appl Immunol. 1970;39(5-6):658–663. doi: 10.1159/000230390. [DOI] [PubMed] [Google Scholar]
- Fudenberg H., Good R. A., Goodman H. C., Hitzig W., Kunkel H. G., Roitt I. M., Rosen F. S., Rowe D. S., Seligmann M., Soothill J. R. Primary immunodeficiencies. Report of a World Health Organization Committee. Pediatrics. 1971 May;47(5):927–946. [PubMed] [Google Scholar]
- Gitlin D., Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 1969 Aug;48(8):1433–1446. doi: 10.1172/JCI106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Möller E. Studies on antigen-binding cells. I. The origin of reactive cells. Cell Immunol. 1970 Oct;1(4):372–385. doi: 10.1016/0008-8749(70)90015-8. [DOI] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Rapid preparation of lymphocytes for tissue-typing. Lancet. 1969 Aug 9;2(7615):327–327. doi: 10.1016/s0140-6736(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Jones G., Torrigiani G., Roitt I. M. Immunoglobulin determinants on mouse lymphocytes. J Immunol. 1971 Jun;106(6):1425–1430. [PubMed] [Google Scholar]
- Klein E., Eskeland T., Inoue M., Strom R., Johansson B. Surface immunoglobulin-moieties on lymphoid cells. Exp Cell Res. 1970 Sep;62(1):133–148. doi: 10.1016/0014-4827(79)90515-9. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Atwell J. L., Cone R. E. Isolation of surface immunoglobulin from lymphocytes from human and murine thymus. Nat New Biol. 1972 Feb 23;235(60):240–242. doi: 10.1038/newbio235240a0. [DOI] [PubMed] [Google Scholar]
- NEZELOF C., JAMMET M. L., LORTHOLARY P., LABRUNE B., LAMY M. L'HYPOPLASIE HEREDITAIRE DU THYMUS: SA PLACE ET SA RESPONSABILITE DANS UNE OBSERVATION D'APLASIE LYMPHOCYTAIRE, NORMOPLASMOCYTAIRE ET NORMOGLOBULINEMIQUE DU NOURRISSON. Arch Fr Pediatr. 1964 Oct;21:897–920. [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiernik M. Correlation of lymphocyte transformation and morphology in the human fetal thymus. Blood. 1970 Oct;36(4):470–479. [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Grey H. M. Immunoglobulins on the surface of lymphocytes. 3. Bursal origin of surface immunoglobulins on chicken lymphocytes. J Immunol. 1971 May;106(5):1418–1420. [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J., MARTIN E., RIOTTON G. L'apparition des diverses composantes antigéniques du sérum au cours du développement foetal. Schweiz Med Wochenschr. 1956 Mar 3;86(9):224–226. [PubMed] [Google Scholar]
- Schlesinger M. Anti-theta antibodies for detecting thymus-dependent lymphocytes in the immune response of mice to SRBC. Nature. 1970 Jun 27;226(5252):1254–1256. doi: 10.1038/2261254a0. [DOI] [PubMed] [Google Scholar]
- Siegel I. Natural and antibody-induced adherence of guinea pig phagocytic cells to autologous and heterologous thymocytes. J Immunol. 1970 Oct;105(4):879–885. [PubMed] [Google Scholar]
- TYAN M. L., COLE L. J. MOUSE FETAL LIVER AND THYMUS: POTENTIAL SOURCES OF IMMUNOLOGICALLY ACTIVE CELLS. Transplantation. 1963 Jul;1:347–350. doi: 10.1097/00007890-196301030-00011. [DOI] [PubMed] [Google Scholar]
- Taylor R. B. Pluripotential stem cells in mouse embryo liver. Br J Exp Pathol. 1965 Aug;46(4):376–383. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Rosette formation, a test for cellular immunity. Trans Assoc Am Physicians. 1971;84:239–247. [PubMed] [Google Scholar]



