Abstract
Pyruvate is the major product of glycolysis in pancreatic β-cells, and its ultimate metabolic fate depends on the relative activities of two enzymes. The first, pyruvate carboxylase (PC) replenishes oxaloacetate withdrawn from the tricarboxylic acid (TCA) cycle via the carboxylation of pyruvate to form oxaloacetate. Flux via PC is also involved in the formation of NADPH, one of several important coupling factors for insulin secretion. In most tissues, PC activity is enhanced by increased acetyl-CoA. The alternative fate of pyruvate is its oxidative decarboxylation to form acetyl-CoA via the pyruvate dehydrogenase complex (PDC). The ultimate fate of acetyl-CoA carbon is oxidation to CO2 via the TCA cycle, and so the PDC reaction results of the irreversible loss of glucose-derived carbon. Thus, PDC activity is stringently regulated. The mechanisms controlling PDC activity include end product inhibition by increased acetyl-CoA, NADH and ATP, and its phosphorylation (inactivation) by a family of pyruvate dehydrogenase kinases (PDHKs 1–4). Here we review new developments in the regulation of the activities and expression of PC, PDC and the PDHKs in the pancreatic islet in relation to islet pyruvate disposition and glucose-stimulated insulin secretion (GSIS).
Keywords: fatty acid, insulin, pyruvate carboxylase, pyruvate dehydrogenase complex, pyruvate dehydrogenase kinase
Introduction
The initial mechanism whereby glucose stimulates insulin secretion requires the metabolism of glucose to pyruvate, and requires its subsequent entry into the tricarboxylic acid (TCA) cycle and oxidation to generate ATP. However, islet metabolism of pyruvate is far more complex and involves a variety of cycles that may have significant roles in generating additional compounds (e.g., NADPH and malonyl-CoA) which facilitate enhanced insulin secretion, for example, in insulin-resistant states. In islets, pyruvate conversion to lactate is limited, and therefore, pyruvate’s metabolic fate depends on the relative activities of pyruvate carboxylase (PC) and the pyruvate dehydrogenase complex (PDC). In this review, we have examined the regulation of these two key steps and how flux, via these enzymes, may influence both the fate of pyruvate and the ability to enhance insulin secretion. Previous studies have tended to focus on the critical role of PC, as GSIS correlates well with rates of pyruvate carboxylation (flux via PC), but not with pyruvate decarboxylation/oxidation (flux via PDC). However, this does not exclude an important role for PDC. Both PC and PDC may have important roles in combating β-cell glucolipotoxicity (β-cell failure and destruction in response to chronic exposure to high glucose and lipid concentrations), and in islet adaptations to enhance insulin secretion to compensate for the development of insulin resistance; these concepts are developed herein.
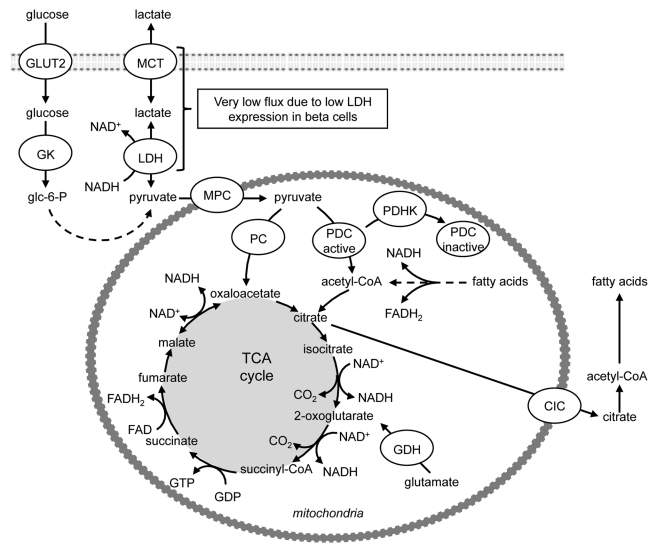
Glucose-stimulated insulin secretion (GSIS) is mediated by metabolic signals whose generation closely parallels β-cell glucose metabolism.1 By virtue of very low expression and activity of lactate dehydrogenase (LDH),2-4 pyruvate is the main end product of glycolysis in pancreatic β-cells (Fig. 1); and glycolysis plus mitochondrial oxidative metabolism and ATP production are both obligatory for GSIS. More than 80% of glucose carbons within the β-cell are oxidized to CO2.2,4 This occurs predominantly via the PDC and the TCA cycle (Fig. 1).
Figure 1. Overview of non-oxidative and oxidative glucose metabolism in islets. By virtue of very low expression and activity of lactate dehydrogenase, pyruvate is the main end product of glycolysis in pancreatic β-cells. Μore than 80% of glucose carbons within the β-cell are oxidized to CO2, which occurs predominantly via the pyruvate dehydrogenase complex and the tricarboxylic acid cycle. Oxaloacetate can be generated from pyruvate via its ATP-dependent carboxylation by pyruvate carboxylase. DIC, dicarboxylate carrier; GDH, glutamate dehydrogenase; GLUT2, glucose transporter 2; GK, glucokinase; LDH, lactate dehydrogenase; MCT, monocarboxylic acid transporter; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; PDHK, pyruvate dehydrogenase kinase.
Acetyl-CoA is conventionally viewed as an initiating metabolite of the TCA cycle. In the fed state this is generated from pyruvate via its decarboxylation by PDC, rather than from fatty acid (FA) β-oxidation (Fig. 1). As in other tissues, the mitochondrial TCA cycle in the β-cell operates in two regulatory spans. The first span generates ATP via NADH production and oxidative phosphorylation, and is initiated by citrate formation. This requires oxaloacetate, which can either be provided via the second span of the TCA cycle and yields energy anaerobically through substrate phosphorylation, or it can be generated from pyruvate via its ATP-dependent carboxylation by PC (Fig. 1). Because oxaloacetate is regenerated in the TCA cycle, small amounts of oxaloacetate will catalyze the oxidation of larger amounts of acetyl-CoA derived from pyruvate. The exit of TCA cycle intermediates from the mitochondria for use for processes such as the biosynthesis of FA from pyruvate, termed cataplerosis, leads to loss of pools of intermediates of the TCA cycle, such as citrate and malate which are replenished by the PC reaction, a process named anaplerosis (Fig. 1).
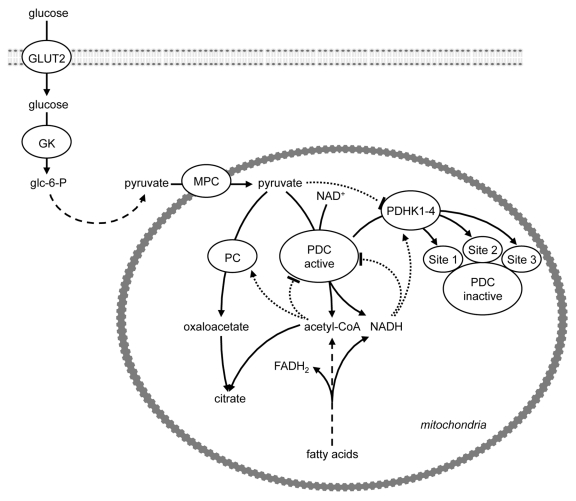
As the ultimate fate of mitochondrial acetyl-CoA is oxidation to CO2, the PDC reaction results in the irreversible loss of glucose-derived carbon. PDC activity is, therefore, stringently regulated when glucose is in short supply, as in prolonged starvation. The mechanisms that control PDC activity include end product inhibition by increased mitochondrial acetyl-CoA, NADH and ATP concentrations (which can also be generated by FA oxidation) and post-translational modification, namely its phosphorylation (inactivation) by a family of pyruvate dehydrogenase kinases (PDHKs 1–4),5,6 (Fig. 2). The pyruvate dehydrogenase component (E1) catalyzes the physiologically irreversible step, and is the PDC component phosphorylated by the PDHKs5,6 (Fig. 2). Phosphorylation of serine-264 (phosphorylation site 1) of E1α of PDC is of primary importance for acute modulation of the percentage of active PDC; whereas additional phosphorylation at serine-271 (phosphorylation site 2) and serine-203 (phosphorylation site 3) of E1α may retard reactivation by dephosphorylation by the pyruvate dehydrogenase phosphatases.7-9 All of the PDHKs phosphorylate sites 1 and 2 of E1α, whereas site 3 of E1α is phosphorylated only by PDHK1.10,11 In the starved state PDC is inhibited, whereas the PDHKs and PC are activated by increased mitochondrial acetyl-CoA derived from FA β-oxidation (Fig. 2). This allows the entry of acetyl-CoA derived from FA oxidation into the first span of the TCA cycle to generate citrate, but the operation of the second span of the TCA cycle becomes dependent on re-oxidation of the NADH and FADH2 that is generated by FA β-oxidation.
Figure 2. Regulation of pyruvate dehydrogenase activity. The mechanisms that control PDC activity include end product inhibition by increased mitochondrial acetyl-CoA, NADH and ATP concentrations (which can also be generated by FA oxidation) and post-translational modification, namely its phosphorylation (inactivation) by a family of pyruvate dehydrogenase kinases (PDHKs 1–4). Phosphorylation of site 1 modulates the percentage of active PDC, whereas phosphorylation of sites 2 and 3 may retard reactivation by dephosphorylation by the pyruvate dehydrogenase phosphatases.7-9 All of the PDHKs phosphorylate sites 1 and 2, whereas site 3 is phosphorylated only by PDHK1. Allosteric activation (arrow) and inhibition (blunt end) is indicated by dotted lines. GK, glucokinase; GLUT, glucose transporter; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; PDHK, pyruvate dehydrogenase kinase.
Pancreatic islets contain PDHKs 1, 2 and 4.12,13 As in other oxidative tissues, the potential therefore exists for multisite phosphorylation of E1α and metabolic inflexibility in switching from FA to glucose as oxidative substrate in islets. Interestingly, exaggerating pyruvate metabolism in the mitochondria, via activation of PDC by knockdown of PDHK1, increases GSIS.14 PC has received much attention because it is highly expressed in pancreatic β-cells, compared with islet non-β-cells;4 it is as highly abundant in β-cells as it is in the gluconeogenic tissues, such as liver and kidney cortex, but serves a completely different role. Nevertheless, a recent study has demonstrated that β-cell-specific PDH deficiency (β-PDHKO) leads to decreased pancreatic insulin content and reduced GSIS demonstrating a key role of PDC in GSIS.15 This review will focus on new developments in our understanding of the roles of flux via PC and PDC in augmenting GSIS in the fed state and suppressing GSIS in starvation, and how relative flux through these two strategic pyruvate- metabolizing enzymes is regulated.
Pyruvate Cycles in β-Cells
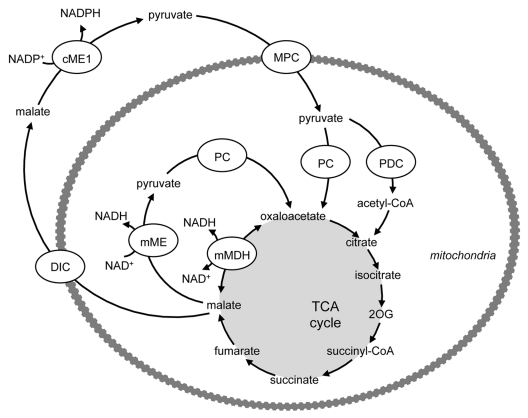
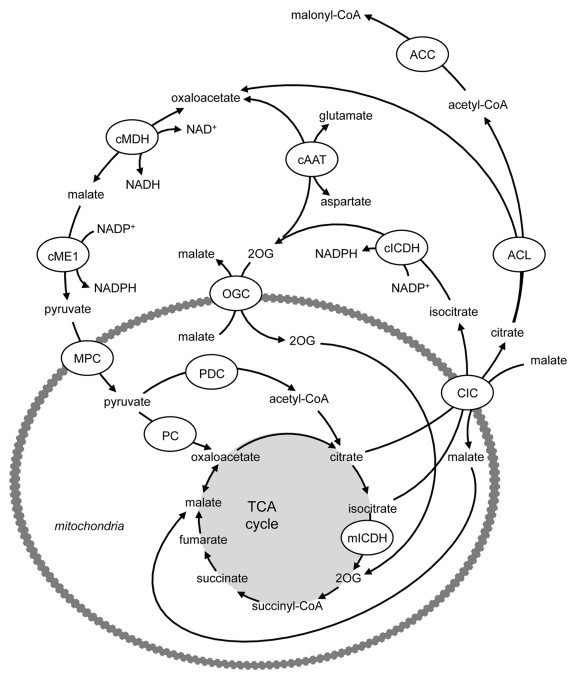
A number of pathways exist in the β-cell that enable the generation of several important coupling factors for insulin secretion (reviewed in ref. 16). The pyruvate cycles involve the generation of oxaloacetate via PC and the subsequent exit of TCA cycle intermediates (notably malate, citrate and isocitrate) from the mitochondria to the cytosol (Figs. 3 and 4). Their further metabolism in the cytosol yields coupling or amplification factors for insulin secretion, with subsequent re-entry of the pathway products into the TCA cycle. Critically, while the cytosolic isoforms of malic enzyme (ME) and isocitrate dehydrogenase (ICDH) are known to generate NAPDH (Figs. 3 and 4), the ME and ICDH isoforms that are expressed in the mitochondria (mME and mICDH) generate NADH rather than NADPH (Figs. 1 and 3). Thus, it is crucial that the TCA cycle intermediates are exported to the cytosol for the formation of the coupling or amplification factors for insulin secretion. The pyruvate/malate pathway (Fig. 3) involves the generation of oxaloacetate from pyruvate by PC, formation of malate by mitochondrial malate dehydrogenase (mMDH), followed by transport of malate into the cytosol by the dicarboxylate carrier (DIC). The coupling factor NADPH is then formed by the conversion of malate to pyruvate by cytosolic ME (cME). Pyruvate can then re-enter the mitochondria for conversion to oxaloacetate. Two additional pathways, the pyruvate/citrate and pyruvate/isocitrate pathways (Fig. 4), are initiated by the conversion of pyruvate to both oxaloacetate (via PC) and acetyl-CoA (via PDC), allowing the formation of citrate and subsequently isocitrate. Both citrate and isocitrate can be transported to the cytosol by the citrate-isocitrate carrier (CIC), where citrate can be converted to isocitrate by cytosolic aconitase. Cytosolic citrate can be cleaved by ATP-citrate lyase (ACL) to acetyl-CoA and oxaloacetate. Oxaloacetate can be converted to malate by cytosolic MDH (cMDH), also generating NADH, and then the coupling factor NADPH can be generated by conversion of malate to pyruvate by cME (as in the pyruvate/malate pathway). Pyruvate can then re-enter the mitochondria completing the cycle. Since the pyruvate/citrate cycle also forms cytosolic acetyl-CoA, it can generate malonyl-CoA. Malonyl-CoA serves both as an intermediate in FA synthesis and also a potent inhibitor of FA oxidation at the level of carnitine palmitoyltransferase (CPT) 1. Cytosolic isocitrate can directly yield NADPH by its conversion to 2OG by cICDH. Potentially, 2OG can be converted to oxaloacetate by cAAT, thereby “joining” the pyruvate/citrate cycle, generating NADPH a second time at the level of cME (see Fig. 4) or alternatively, enter the mitochondria via OGC and join the TCA cycle at the step subsequent to mICDH, and be metabolized to oxaloacetate (Fig. 4).
Figure 3. Generation of cytosolic NADPH by the pyruvate/malate pathway. PC catalyzes the conversion of pyruvate to oxaloacetate, which can yield malate catalyzed by mitochondrial MDH. Malate export into the cytosol via the dicarboxylate carrier can lead to the formation of the coupling factor NADPH through the conversion of malate to pyruvate by cytosolic ME. Pyruvate can then re-enter the mitochondria for conversion to oxaloacetate. Critically, while the cME is known to generate NAPDH, the isoform expressed in the mitochondrion (mME) generates NADH. Thus, it is crucial that the TCA cycle intermediates are exported to the cytosol for the formation of NADPH for insulin secretion. DIC, dicarboxylate carrier; MDH, malate dehydrogenase; ME, malic enzyme; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex.
Figure 4. The pyruvate/citrate and pyruvate/isocitrate pathways are initiated by the conversion of pyruvate to both oxaloacetate (via PC) and acetyl-CoA (via PDC), allowing the formation of citrate and subsequently isocitrate. Both citrate and isocitrate can be transported to the cytosol by the citrate-isocitrate carrier (CIC), where citrate can be converted to isocitrate by cytosolic aconitase. Cytosolic citrate can be cleaved by ATP-citrate lyase (ACL) to acetyl-CoA and oxaloacetate. Oxaloacetate can be converted to malate by cytosolic MDH (cMDH), also generating NADH, and then the coupling factor NADPH can be generated by conversion of malate to pyruvate by cME (as in the pyruvate/malate pathway). Pyruvate can then re-enter the mitochondria completing the cycle. Since the pyruvate/citrate cycle also forms cytosolic acetyl-CoA, it can generate malonyl-CoA. Malonyl-CoA serves both as an intermediate in FA synthesis and also a potent inhibitor of FA oxidation at the level of carnitine palmitoyltransferase (CPT) 1. Cytosolic isocitrate can directly yield NADPH by its conversion to 2OG by cICDH. Potentially, 2OG can be converted to oxaloacetate by cAAT, thereby “joining” the pyruvate/citrate cycle, generating NADPH a second time at the level of cME or alternatively, enter the mitochondria via OGC and join the TCA cycle, at the step subsequent to mICDH, and be metabolized to oxaloacetate. AAT, aspartate aminotransferase; ACC, acetyl-CoA carboxylase; ACL, ATP-citrate lyase; CIC, citrate isocitrate carrier; ICDH, isocitrate dehydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; OGC, 2-oxoglutarate carrier.
Variation of GSIS with PC vs. PDC
13C nuclear magnetic resonance (NMR) and isotopomer analysis using four clonal rat insulinoma cell 1 (INS1)-derived cell lines with varying degrees of glucose responsiveness revealed a connection between exchange of pyruvate with TCA cycle intermediates and GSIS.17 The fraction of acetyl-CoA derived from [U-13C6] glucose was the same in all four cell lines, indicating a poor correlation between the oxidation of pyruvate via PDC (with formation of acetyl-CoA) and glucose responsiveness of insulin secretion. The 13C NMR spectra also demonstrated the possible existence of two separate pyruvate pools, the first exchanging with TCA cycle intermediates and a second feeding acetyl-CoA into the TCA cycle (presumably via PDC). Stimulation of pyruvate cycling with dimethylmalate or its inhibition with phenylacetic acid led to proportional changes in insulin secretion. Further 13C NMR isotopomer analysis studies, quantifying fluxes through PC (with pyruvate derived from glycolytic or from non-glycolytic sources) and glutamate dehydrogenase (GDH) in clonal INS-1 832/13 cells, indicated that GSIS correlates well with rates of pyruvate carboxylation (i.e., flux via PC) but not with pyruvate decarboxylation/oxidation (i.e., flux via PDC).18 This study also indicated that glutamine addition stimulated GDH flux approximately 6-fold without affecting insulin secretion, suggesting that anaplerosis through GDH does not play a major role in insulin secretion in this β-cell line. In another study using four insulinoma cell lines and porcine islets under different culture conditions1913C-glutamate isotopomeric fractions were fitted to metabolic models to estimate relative metabolic fluxes to the TCA cycle in relation to insulin secretion. The cell lines employed included βTC3 cells (that express high levels of proinsulin mRNA20), the growth-regulated β-cell lines βTC-tet and R7T1cells (which maintain normal insulin production and secretion when growth arrested21) and the rat insulinoma cell line INS-1 [832/13] (which exhibits enhanced and stable insulin secretion in response to glucose and several of its known potentiators22). A model was proposed of a pool of pyruvate supplying the TCA cycle via PDC, with anaplerosis via PC and a further undefined route.
Stable transfection of short hairpin RNA (shRNA) has been used to generate a number of INS-1 832/13-derived β-cell lines with various levels of PC activity. The most PC-deficient cells showed a metabolic crossover at PC, with increased [pyruvate + lactate] (INS-1 cells, unlike β-cells, have relatively high LDH activities) together with decreased [malate + citrate] concentrations, consistent with suppression of PC flux. GSIS was lowered in parallel with decreases in PC activity.23 Of interest, PC was also shown to be important not only for GSIS, but for insulin secretion by INS cells stimulated by non-carbohydrate insulin secretagogues [2-aminobicyclo(2,2,1)heptane-2-carboxylic acid plus glutamine or methyl succinate plus 3-hydroxybutyrate],23 confirming actions linked to TCA cycle flux. Suppression of PC by small interfering RNA (siRNA) in insulinoma-derived β-cells and dispersed rat islet cells was found to impair GSIS, while overexpression of PC in INS-1 cells increased GSIS.24 These studies have recently been the subject of excellent in depth analysis in reference 25.
Inhibition of PC with phenylacetic acid decreases insulin secretion in INS-1 cells and rat islets.17,26,27 Fransson et al. showed, using perifused rat islets, that PC inhibition decreases both the second phase of insulin secretion (during which KATP-channel independent actions of fuel secretagogues for GSIS operate), as well as the first, KATP-channel dependent phase (which would be predicted as the glucose-provoked rise in ATP:ADP ratio) is suppressed by inhibition of PC. Mitochondrial membrane potential, Ca2+-induced exocytosis and KATP-channel conductance were unaffected by PC inhibition. Therefore, it was posited that one role of PC was to allow export of intermediates into the cytosol where they could serve signaling and/or amplification roles in GSIS, in addition to the maintenance of fuel oxidation by the TCA cycle to generate sufficient ATP and the appropriate ATP/ADP ratio to regulate both phases of fuel-induced insulin secretion.
Exposure of β-cells to the sulfonylureas glibencamide and tolbutamide for prolonged periods leads to desensitization to their actions. Although the underlying mechanism is unclear, it is of interest that prolonged exposure of BRIN-BD11 β-cells to tolbutamide results in a change in the ratio of flux through PC and PDC.28
NADH Regeneration and ATP Formation for GSIS vs. Triacylglycerol (TAG) Formation to Combat Lipotoxicity
The utilization of pyruvate for the PDC and PC reactions requires continued generation of pyruvate via glycolysis because of the low expression of LDH and plasma membrane lactate transporters (monocarboxylic acid transporters, MCTs)2-4,29 (Fig. 1). The low expression of LDH also means that the pancreatic β-cell cannot adequately regenerate the NAD+ required for continued glycolysis via the formation of lactate from pyruvate (reviewed in ref. 25) (Fig. 1). As NADH cannot be transported across the inner mitochondrial membrane to be oxidized by the electron transport chain (ETC), redox shuttles are employed to regenerate NAD+ via mitochondrial oxidation.
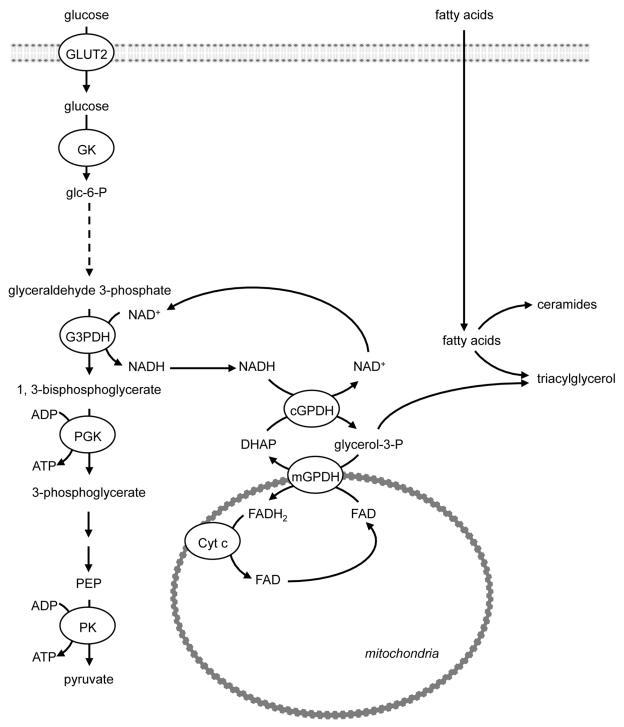
The first of these, the glycerol-3-phosphate shuttle (reviewed in ref. 30) comprises cytosolic and mitochondrial glycerol-3- phosphate dehydrogenases (reviewed in ref. 31) (Fig. 5). Cytoplasmic glycerol-3-phosphate dehydrogenase (cGPDH) catalyzes the conversion of dihydroxyacetone phosphate (DHAP), generated at the glyceraldehyde phosphate dehydrogenase step of glycolysis, to glycerol-3-phosphate. The cGPDH reaction utilizes NADH in this process, and generates NAD+. Glycerol-3-phosphate is converted back to DHAP by mitochondrial GPDH (mGPDH). As the mGPDH isoform is a flavoprotein in which FAD is reduced to FADH2, which enters the ETC via complex II (ubiquinone), the operation of the glycerol-3-phosphate shuttle generates mitochondrial ATP to help fuel exocytosis. Decreased activity of mGPDH or of the glycerol-3-phosphate shuttle is associated with type 2 diabetes.32 Nevertheless, disruption of the gene encoding mGPDH in mice does not impair GSIS,25,33 suggesting that other mechanisms for NAD+ regeneration operate.
Figure 5. The role of the glycerol-3-phosphate shuttle in NADH regeneration and triacylglycerol (TAG) formation to combat lipotoxicity. Cytoplasmic cGPDH catalyzes the conversion of dihydroxyacetone phosphate (DHAP), generated at the glyceraldehyde phosphate dehydrogenase step of glycolysis, to glycerol-3-phosphate and generates NAD+, which facilitates continued ATP and pyruvate production by glycolysis. Mitochondrial GPDH (mGPDH) converts glycerol-3-phosphate back to DHAP and also generates FADH2, which generates mitochondrial ATP to help fuel exocytosis. The esterification of fatty acids to triacylglycerol also utilizes glycerol-3-phosphate, which may act to combat lipotoxicity, thereby limiting the generation of cytotoxic lipids such as ceramide. DHAP, dihydroxyacetone phosphate; GK, glucokinase; GLUT, glucose transporter; GPDH, glycerol-3-phosphate dehydrogenase; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; PK, pyruvate kinase.
The fate of glycerol-3-phosphate is not, however, solely the formation of DHAP. The formation of TAG requires the use of glycerol-3-phosphate for esterification of incoming FA (Fig. 5). The accumulation of TAG within the pancreatic β-cell may act to combat lipotoxicity by sequestering excess FA into an inert form, thereby limiting the generation of cytotoxic lipids such as ceramide (Fig. 5). In Zucker rats, ceramide synthesis is associated with the subsequent induction of nitric oxide (NO) synthase and NO-mediated apoptosis.34,35 Palmitate and oleate induce toxicity in purified rat β-cells; however, this is not due to oxidative stress or NO-mediated cytotoxicity.36 Interestingly, while culture of β-cells with palmitate or oleate for 48 h increases cytosolic TAG content from control levels of 162 pg/ng DNA to 389 and 652 pg/ng DNA respectively, an inverse relationship exists between the cytotoxicity resulting from exposure to palmitate and oleate and cellular TAG accumulation.36 Thus, under conditions when FA are delivered in excess of cellular energy requirements, the β-cell may prioritize TAG formation to oppose the development of lipotoxicity over high rates of GSIS, which requires both ATP generation and the re-oxidation of NADH. An inverse correlation exists between the percentage of dead rat β-cells after 8 d of incubation in the presence of FA and the cellular TAG content of the β-cells after incubation with FA for 2 d.36 An alternative mechanism that may operate to oppose glucolipotoxicity has recently been identified in which β-cell glucolipotoxicity induced in INS-1 β-cells by incubation in the presence of high glucose/palmitate (HG/PA) was opposed by nutritional or pharmacological interventions that enhanced anaplerosis or reduced cataplerosis.37
Links between the NADH-Reoxidizing, ATP-Generating Malate/Aspartate Shuttling and Mitochondrial Anaplerosis via PC
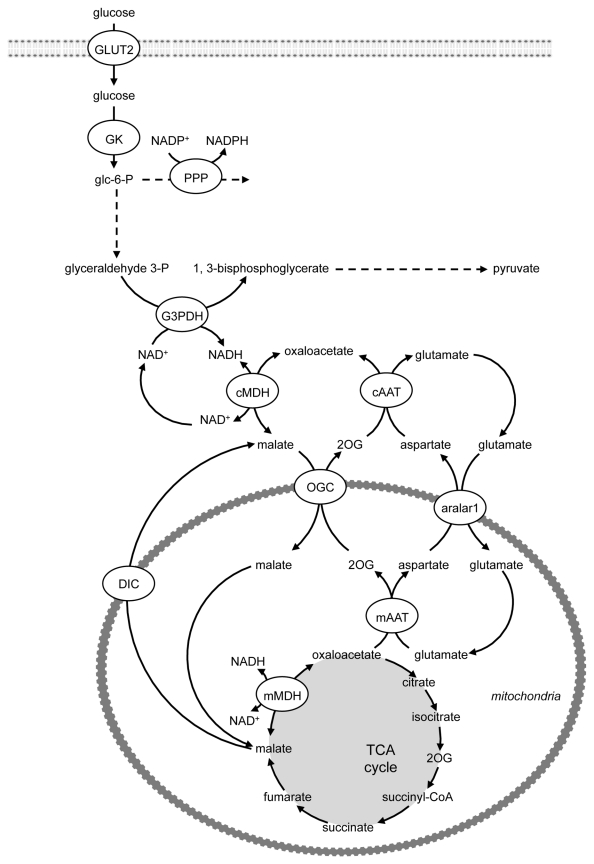
A second shuttle, the malate/aspartate shuttle,38 appears to compensate for the lack of glycerol-3-phosphate shuttling (Fig. 6). In the malate/aspartate shuttle, NADH is transported into the mitochondrion by the interconversion of malate and aspartate via oxaloacetate in the cytoplasm and the mitochondrion.38 Malate, but not oxaloacetate, can cross the inner mitochondrial membrane. The exchange is, therefore, accomplished by interconversion between α-keto and α-amino acids. This involves cytoplasmic and mitochondrial glutamate and 2-oxoglutarate (2OG), also known as α-ketoglutarate, and isoenzymes of glutamate-oxaloacetate transaminase (aspartate aminotransferase, AAT). First, cMDH catalyzes the reaction of oxaloacetate with glycolytically-produced NADH to yield malate and NAD+ in the cytosol. The malate-2OG antiporter imports the malate from the cytosol into the mitochondrial matrix, while simultaneously exporting 2OG from the mitochondrial matrix into the cytosol. After malate reaches the mitochondrial matrix, it is converted by mMDH into oxaloacetate. The NADH, thus generated in the matrix, can potentially be used to pass electrons to the ETC for the synthesis of ATP (with a greater ATP yield than the FADH2 produced by the glycerol-3-phosphate shuttle), whereas the oxaloacetate is converted to aspartate by mitochondrial AAT, using glutamate as amino donor. This reaction results in the formation of 2OG from glutamate. A second mitochondrial antiporter [the mitochondrial aspartate/glutamate carrier aralar1 (aspartate-glutamate carrier 1)] imports glutamate from the cytosol into the matrix and exports aspartate from the matrix to the cytosol. Once in the cytosol, aspartate is converted by cytosolic AAT to replenish cytosolic oxaloacetate. Thus, the net redox effect of the malate-aspartate shuttle is that NADH in the cytosol is oxidized to NAD+, and NAD+ in the matrix is reduced to NADH.
Figure 6. The role of the malate-aspartate shuttle in NADH regeneration. The malate/aspartate shuttle appears to compensate for the lack of glycerol-3-phosphate shuttling. NADH is transported into the mitochondrion by the interconversion of malate and aspartate via oxaloacetate in the cytoplasm and the mitochondrion and involves cytoplasmic and mitochondrial glutamate and 2-oxoglutarate and isoenzymes of aspartate aminotransferase. The net redox effect of the malate-aspartate shuttle is that NADH in the cytosol is oxidized to NAD+, and NAD+ in the matrix is reduced to NADH. AAT, aspartate aminotransferase; GK, glucokinase; GLUT, glucose transporter; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; MDH, malate dehydrogenase; 2OG, 2-oxoglutarate; OGC, 2-oxoglutarate carrier; PPP, pentose phosphate pathway.
If this mitochondrial NADH is not reoxidized to mitochondrial NAD+, then the rise in mitochondrial NADH/NAD+ is predicted to inhibit PDC and activate the PDHKs (Fig. 2). Since the product of the PC reaction is mitochondrial oxaloacetate, which can be converted to mitochondrial malate via mMDH utilizing mitochondrial NADH, there is opportunity for complex regulatory interactions between malate-aspartate shuttling and PC-catalyzed anaplerosis (Fig. 3). In addition, changes in mitochondrial NAD+ are predicted to influence the activity of the mitochondrial sirtuin SIRT3. SIRT3, like other sirtuins, is an NAD+-dependent deacetylase (reviewed in ref. 39). Substrates of SIRT3 include (in mouse liver, at least) mitochondrial metabolic enzymes promoting FA oxidation such as acetyl coenzyme A synthetase 2,40,41 and long-chain acyl-CoA dehydrogenase,42 the latter being one of the major enzymes responsible for the first step of long-chain FA β-oxidation. Deacetylation of long-chain acyl-CoA dehydrogenase causes its activation, and thus, promotion of FA β-oxidation,42 with further production of NADH (and FADH2), resultant activation of the PDHKs, and inhibition of PDC by phosphorylation and end product inhibition.
An isoform of aralar1 is a key component of the malate-aspartate shuttle (Fig. 6). Transduction of INS-1E cells and isolated rat islets with AdCA-aralar1 increases aralar1 protein levels.43 In INS-1E cells, overexpression of aralar1 potentiated metabolism-secretion coupling stimulated by high glucose in association with increased NAD(P)H generation, mitochondrial membrane hyperpolarization, ATP levels and glucose oxidation, and suppressed lactate production.43 Similarly, overexpressing aralar1 in rat islets enhanced insulin secretion at high glucose.43 Overexpression of the aralar1 in BRIN-BD11 cells increases acute and long-term glucose- and amino-acid-stimulated insulin secretion and enhances glucose, L-alanine and L-glutamine metabolism, leading to the suggestion that increased malate-aspartate shuttle activity positively shifts β-cell metabolism to enhance insulin secretion.44,45 Conversely, inhibition of the malate-aspartate shuttle impairs glucose metabolism and i nsulin secretion.45 Knockdown of aralar1 by adenovirus- mediated delivery of shRNA in INS-1E cells impairs glucose oxidation and increases lactate production.46 This highlights the role of aralar1 and the malate/aspartate shuttle in regenerating NAD+ if cells express low levels of LDH. Knockdown of aralar1 was also associated with impaired GSIS at high glucose. AGC1 knockdown in rat islets did not affect their secretory response.
Irrespective of these potential interactions and mechanisms, it is interesting that the low expression of LDH in the β-cell not only funnels pyruvate into the mitochondrion via the PC and PDC reactions but also, if FADH2 and NADH generated by the glycerol-3-phosphate and malate-aspartate shuttles are used to fuel oxidative phosphorylation, produces a large amount of mitochondrial ATP. This can be used to enhance GSIS in concert with augmented glycolytic flux. The role of glutamate in the operation of the malate/aspartate shuttle (Fig. 6) is also of interest. There is evidence that glutamate has a regulatory role in insulin secretion, but how it exerts its effects remains uncertain.47,48 β-cell-specific deletion of glutamate dehydrogenase (GDH) [βGlud1(-/-) mice] results in lowered GSIS in pancreatic perfusions, a response that is reversed by adenovirus-mediated re-expression of GDH in βGlud1(-/-) islets.49 Constitutive or acute adenovirus- mediated knockout of GDH in islets also lowers GSIS.49 In vivo, βGlud1(-/-) mice exhibit lower plasma insulin levels in response to both feeding and a glucose load.49 The mitochondrial glutamate carrier (GC1) is expressed in both INS-1E β-cells and rat islets. Silencing of GC1 in INS-1E cells by adenoviral delivery of short hairpin RNA lowers mitochondrial glutamate transport by approximately 50% and impairs GSIS at high, but not low, glucose.50 The impairment of GSIS was reversed by cell-permeant glutamate (5 mM dimethyl glutamate).50 Silencing of GC1 in rat islets impaired first and second phase GSIS during islet perifusions,50 demonstrating that the recently-identified GC1 is important for maximal glucose response. A recent study51 has shown that ornithine aminotransferase, an enzyme lying on the pathway for conversion of glutamate to ornithine, like LDH, is selectively repressed in pancreatic β-cells. Thus, adequate availability of glutamate is important for maintaining GSIS in part through facilitating the operation of the malate/aspartate shuttle to regenerate cytoplasmic NAD+ required for continued glycolysis.
Metabolic Coupling Factors that Enhance GSIS Require PC, PDC and Pyruvate Cycling
Mitochondrial metabolism has previously been the focus of the search for coupling and amplification signals for GSIS. The lack of cytosolic phosphoenolpyruvate carboxykinase (cPEPCK) and fructose-1,6-bisphosphatase in β-cells indicates that PC does not serve the gluconeogenic role that it has in liver and kidney. Furthermore, while the cytosolic pyruvate pool can be replenished from the TCA cycle intermediates malate or oxaloacetate by either malic enzyme (ME) or PEPCK/pyruvate kinase (PK) in liver, the latter option is unavailable to the β-cell because of the absence of cPEPCK. In the fed state, where there is a requirement for vigorous GSIS, oxaloacetate formed by PC is converted into citrate (by reaction with acetyl-CoA produced by PDC) or to malate (through its reduction by MDH). These compounds exit the mitochondria through the pyruvate/citrate, pyruvate/isocitrate or pyruvate/malate shuttles.
The citrate/isocitrate carrier transfers citrate or isocitrate between mitochondria and cytoplasm (Fig. 4). Citrate exit from the mitochondria can be followed by citrate’s cleavage in the cytoplasm via ACL to yield cytoplasmic acetyl-CoA plus cytoplasmic oxaloacetate. The latter can be recycled back to pyruvate via malate. Alternatively, isocitrate can be converted to 2OG. Metabolic overlap between pyruvate cycling and the malate/aspartate shuttle is clearly apparent, and both malate and 2OG can re-enter the mitochondria and the TCA cycle and contribute to anaplerosis.52,53
The pyruvate/citrate shuttle, the pyruvate/isocitrate shuttle and the pyruvate/malate shuttle all generate NADPH by reactions catalyzed by ME and the cytosolic NADP+-dependent isoform of ICDH54 (Fig. 4). NADPH, like ATP, is critical for exocytosis and has been proposed as a potential coupling/amplification factor for GSIS (reviewed in ref. 16, 53 and 55). Its regulatory roles in GSIS may include its interaction with redox proteins (e.g., thioredoxin and glutaredoxin).56 Alternatively, cellular NADPH may regulate intracellular K+ by binding to the regulatory β-subunit (Kvβ) of the voltage-dependent K+ channel.25 This outward rectifying K+ channel allows K+ efflux, leading to membrane repolarization for the next cycle of GSIS.57 NADPH binding to Kvβ impairs the capacity of the channel to elicit membrane repolarization.25
Recent studies have investigated the roles of the mitochondrial OGC54 and the mitochondrial DIC58 in GSIS using pharmacological and siRNA methodologies. Suppression of OGC in INS 832/13 β-cells and rat islets by adenovirus-mediated delivery of siRNA decreased GSIS, as well as insulin secretion in response to glutamine + a glutamate dehydrogenase activator (2-amino-2-norbornane carboxylic acid). Suppression of OGC significantly decreased the NADPH/NADP+ ratio during stimulation with glucose, but not [glutamine + 2-amino-2- norbornane carboxylic acid]; however, nutrient-stimulated increases in glucose utilization and oxidation, glutamine oxidation and the ATP/ADP ratio were all unaffected. Similarly inhibition of DIC, an important source of cytosolic malate, suppressed glucose-induced increases in the NADPH/NADP+ ratio but did not affect glucose utilization or ATP/ADP.58 It was suggested that the OGC participates in a mechanism of insulin secretion that is common to all fuel secretogogues (glucose, amino acids, organic acids) involving flux through the pyruvate/isocitrate cycling pathway but not necessarily via its effects to generate NADPH.54 Effects of DIC inhibition prompted the conclusion that malate transport by DIC participated in GSIS by providing malate that can act as a counter-substrate for the export of citrate and/or isocitrate, thereby participating in NADPH production mediated by pyruvate cycling.
Under glucose-replete conditions, PDC is important for maintaining flux from glucose to citrate. Citrate exiting the mitochondria can be converted by ACL to cytoplasmic acetyl- CoA, which can be used to generate malonyl-CoA (Fig. 4). Malonyl-CoA, like NADPH, has been viewed as a potential metabolic lipid amplification factor for GSIS. It can also act as a precursor for FA synthesis in the cytosol, which is necessary for membrane synthesis to support secretory granule exocytosis. In addition, malonyl-CoA accumulation has been suggested to exert a regulatory function in partitioning incoming FA away from oxidation through inhibition of carnitine palmitoyl transferase I, thereby elevating cytosolic long-chain acyl-CoA.59,60 There is evidence that increased levels of cytosolic long-chain acyl-CoA may stimulate or potentiate GSIS through activation of atypical PKC61 or via interaction at the pore-forming subunit Kir6.2.62 There is also evidence that addition of palmitate, but not palmitoyl-CoA, to mouse islets increases insulin secretion by a KATP channel-independent mechanism.63 This effect of palmitate was exerted at the level of exocytosis and involved augmentation of L-type Ca2+ currents and an increase in the readily releasable pool of secretory granules.63 Stored TAG may also be mobilized to generate lipid-derived signals (e.g., long-chain acyl-CoA and diacylglycerol) required for insulin secretion. Inhibition of islet lipolysis stimulated by glucose and diglyceride lipase activity is abolished by the lipase inhibitor orlistat and is associated with dose-dependent inhibition of GSIS.64 Perifusion of islets with orlistat attenuated second phase insulin secretion in the absence of effects on glucose-induced increases in islet ATP, leading to the suggestion that a lipid coupling factor involved in KATP-independent glucose sensing was affected.64 β-cell specific knockout of hormone-sensitive lipase in mice results in blunting of GSIS in vivo, and an impaired exocytotic response of isolated β-cells to depolarization of the plasma membrane.65 However, the precise role of malonyl-CoA and long-chain acyl-CoA in the regulation of insulin secretion remains controversial. Adenoviral-mediated overexpression in INS-1 cells of malonyl-CoA decarboxylase (AdCMV-MCD), which decarboxylates malonyl-CoA to acetyl-CoA, did not affect GSIS, despite dramatically lowering intracellular malonyl CoA levels at both high and low glucose. It also impaired the effects of high glucose to suppress of palmitate oxidation, and lowered incorporation of palmitate and glucose into cellular lipids at high glucose.66 Similarly, inhibition of long-chain acyl-CoA synthetase by triacsin C fails to alter GSIS despite potent attenuation of palmitate oxidation, lowering of glucose and palmitate incorporation into cellular lipids and decreasing total long-chain acyl-CoA.66 Follow up studies utilized INS-1-derived 832/13 cells, which exhibit robust KATP channel-dependent and KATP channel-independent pathways of GSIS. Treatment of INS-1-derived 832/13 cells with an adenovirus encoding human MCD markedly increased cytosolic MCD activity and prevented the glucose-induced rise in malonyl-CoA, at the same time attenuating the inhibitory effect of glucose on FA oxidation.67 Despite these metabolic changes, MCD overexpression failed to influence KATP channel-dependent or -independent GSIS.67
Signaling Roles for Mitochondrial GTP and PEPCK
Nucleotide-specific isoforms of succinyl-CoA synthetase (SCS) exist that catalyze substrate-level synthesis of mitochondrial GTP and ATP. While the yield of ATP from glucose, coupled with oxidative phosphorylation, varies with each glucose molecule metabolized, the yield of GTP is always approximately 1. Suppression of the GTP-producing pathway (by siRNAs) in INS-1 832/13 cells and cultured rat islets markedly impairs GSIS (by 50%), whereas suppression of the ATP-producing isoform of SCS increases GSIS.68 Insulin secretion correlated with increases in cytosolic calcium rather than changes in NAD(P)H or ATP.68 Consequently, it has been suggested that mitochondrial GTP may regulate GSIS through modulation of mitochondrial metabolism, possibly involving mitochondrial calcium, and may serve as an important molecular signal of TCA-cycle activity.68 It has been proposed that the mitochondrial isoform of phosphoenolpyruvate carboxykinase (mPEPCK) acts as a GTPase that links hydrolysis of mitochondrial GTP to an anaplerotic pathway generating phosphoenolpyruvate (PEP).69 mPEPCK message and protein were detected in INS-1 832/13 cells, rat islets, and mouse islets and PEPCK activity was found to be exclusively mitochondrial.69 mPEPCK was shown to generate between 30–40% of PEP in INS-1 832/13 cells and islets, with glucose stimulation tripling the contribution of mPEPCK.69 Support for a role for mPEPCK was obtained by silencing the PEPCK-M gene, which completely inhibited GSIS.69
Role of a Novel Pathway of Acyl-CoA Synthesis in Human Islets
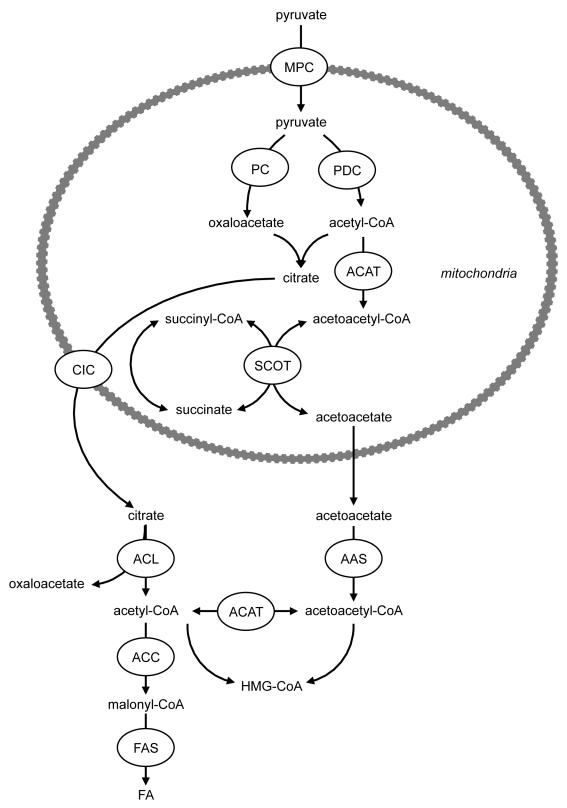
Most studies of the regulation of insulin secretion have relied on the use of rodent islets or cell lines. Studies utilizing human islets have determined that the characteristics of both the triggering and amplifying pathways of insulin secretion are largely similar to those of rodent islets.70 A recent study has shown that the activity and protein expression of the anaplerotic enzyme PC, together with the proportion of pyruvate flux via PC, were much lower in human compared with rodent islets.71 In addition, human islets exhibited lower ACL protein and activity.71 Human islets were characterized by elevated succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and acetoacetyl-CoA synthetase (AAS), which form mitochondrial acetoacetate and permit the formation of cytosolic acyl-CoA respectively (Fig. 7). Knockdown of SCOT or AAS in INS-1 832/13 cells impairs GSIS.23,72 This led to the suggestion that a pathway exists involving acetoacetate synthesis, leading to the production of cytosolic acyl-CoAs71 (Fig. 7), which can act as signaling molecules for insulin exocytosis.73 Importantly, this pathway may be more active in human compared with rodent β-cells due to lower pyruvate carboxylation as a result of lowered PC expression and activity (and thus greater pyruvate decarboxylation via PDC) compared with rodent islets.71
Figure 7. Role of a novel pathway of acyl-CoA synthesis in human islets. Human islets are characterized by elevated succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and acetoacetyl-CoA synthetase (AAS), which form mitochondrial acetoacetate and permit the formation of cytosolic acyl-CoA respectively. This pathway may be more active in human compared with rodent β-cells due to lower pyruvate carboxylation as a result of lowered PC expression and activity (and thus greater pyruvate decarboxylation via PDC) compared with rodent islets. ACAT, acetyl-CoA acetyltransferase; ACC, acetyl-CoA carboxylase; ACL, ATP-citrate lyase; CIC, citrate isocitrate carrier; FAS, fatty acid synthase; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; SCOT, succinyl-CoA:3-ketoacid-CoA transferase.
A Role for Pyruvate in the Acute Augmentation of GSIS through Inhibition of the PDHKs?
Pyruvate generation in situ in the β-cell may have a regulatory role in addition to its function as metabolic substrates for the PC and PDC E1 reactions. Pyruvate inhibits the activity of the PDHKs, particularly that of the “housekeeping” PDHK isoform PDHK2,74 and thereby relieves inhibition of PDC by limiting its phosphorylation by the PDHKs (Fig. 2). ADP both independently suppresses PDHK activity and also acts synergistically with pyruvate to suppress PDHK2.75 An inhibitory effect of increased pyruvate production on PDHK activity in conjunction with increased ATP utilization for exocytosis during glucose stimulation potentially constitutes a feed-forward mechanism of control of PDC flux in the β-cell. As we have previously hypothesized,14 increased flux via PDC could result in increased cataplerosis from the mitochondrion to the cytosol, as citrate/isocitrate exits the mitochondria prior to generation of NADH and FADH2 which drive the respiratory chain and oxidative phosphorylation of ADP to ATP.
Starvation elicits a PDHK isoform shift, with a marked increase in protein expression of PDHK4 in pancreatic islets.12 Increased islet PDHK4 expression is achieved via pharmacological activation of peroxisome proliferator-activated receptor (PPAR)α, implying that islet starvation adaptations could involve generation of an endogenous PPARα ligand. PPARα null mice show fasting hyperinsulinemia,76,77 and analysis of functional characteristics of islets from PPARα null mice indicate that PPARα is involved in the islet adaptation to starvation, namely decreased GSIS.77,78 Contrasting with PDHK2, PDHK4 is relatively insensitive to suppression by the pyruvate analog dichloroacetate.74 Thus, an increased PDHK4/PDHK2 activity ratio could limit the extent to which an increased intracellular pyruvate generation promotes PDC flux. PDHK1 is also relatively insensitive to inactivation by pyruvate. This may in part contribute to the enhanced acute response of insulin secretion to stimulation by glucose in β-cells where PDHK1 is suppressed.14
Roles for PDC, Citrate Cataplerosis and ACL in Mediating Effects of Chronic Exposure to High Glucose on β-Cell Gene Expression?
As described above, the operation of one pyruvate cycling pathway requires malate export from the mitochondria and NADP+-dependent decarboxylation of malate to pyruvate by cME1. It has been reported that mouse β-cells lack ME1 activity (c.f. rat β-cells) and, although adenoviral-mediated overexpression of ME1 greatly augments GSIS in rat insulinoma INS-1 832/13 cells, it does not affect GSIS in mouse islets.79 However, other studies have identified ME activity and mRNA in C57BL/6 mouse islets and MIN-6 cells, as well as impaired ME activity in streptozotocin-treated mouse islets and increased ME activity in obese agouti-L mouse islets.80 Furthermore, ME siRNA inhibited ME activity and reduced GSIS and lowered NADPH.80 A further study,81 using both INS-1 832/13 cells and glucose-responsive INS-1 832/3 cells, demonstrated that siRNA- mediated suppression of either mME or cME lowered GSIS, the latter in association with decreased NADPH. However, while adenovirus-mediated delivery of siRNAs specific to cME and mME to isolated rat islets suppressed the targets transcripts, it failed to alter GSIS.81 Furthermore, islets isolated from MEc-null MOD1(-/-) mouse islets are also characterized by normal glucose- and KCl-stimulated insulin secretion.81 These findings suggest that citrate/isocitrate may have another, as yet unidentified role in GSIS.
Recently, Wellen et al. examined the effect of suppressing ACL expression in several different mammalian cell types, including cultured adipocytes. It was observed that siRNA suppression of ACL reduced global histone acetylation. Moreover, histone acetylation depended on glucose, with FA unable to substitute. This observation is consistent with a requirement for a cytosolic or nuclear (but not mitochondrial) pool of acetyl-CoA. Suppressing ACL in differentiating adipocytes has metabolic consequences: it prevented the expression of the major adipocyte glucose transporter, GLUT4, with reduced H3 and H4 histone acetylation found at the promoter of the Glut4-encoding gene.82
Suppressing ACL in differentiating adipocytes also prevented the expression of several glucose-metabolizing enzymes [hexokinase 2, phosphofructokinase-1 and LDH-A]. Therefore, it is possible that, as in cultured adipocytes, chromatin modification by histone acetylation in pancreatic β-cells is dependent upon acetyl-CoA derived from glucose via the concerted actions of PDC, citrate/isocitrate transport and ACL. If so, ACL could provide a mechanism for the longer term changes in gene expression that affect β-cell glucose handling seen when there is chronic exposure to elevated glucose, which is known to alter expression levels of over 180 genes in pancreatic β-cells.83
Islet Compensation for Insulin Resistance: Roles for PC and PDHK1?
While many insulin-resistant individuals do not develop diabetes because the pancreatic β-cells adapt to meet the body’s markedly increased demand for insulin secretion, some individuals are more susceptible to diabetes; the factors and mechanism(s) underlying this are far from clear. PC activity is preserved in the islets of obese animals.84 In contrast, PC activity is suppressed in the islets of animal models of type 2 diabetes and in islets of patients with type 2 diabetes.85,86 Thus, PC may be important in β-cell adaptation to obesity-related insulin resistance, and a reduction in PC activity could lead to β-cell failure when the islet is excessively challenged to secrete more insulin in response to the development of insulin resistance.
Culture of C57BL/6 mouse islets in the presence of palmitate suppresses PDC activity and upregulates mRNA and protein expression of PDHK1, PDHK2 and PDHK4 (as in earlier studies12 PDHK3 was not detected), together with increased PDHK activity.13 In this study,13 high glucose also increased PDHK1 and PDHK2 mRNA expression, but lowered PDK4 mRNA expression in cultured islets. In addition, PDC activity is significantly lowered in pancreatic islets of obese and diabetic animals (reviewed in ref. 87). Unusually, PDHK1 is highly expressed in pancreatic islets.12 Silencing PDHK1, a negative regulator of PDC, in INS-1-derived 832/13 clonal β-cells increases PDC activity in conjunction with specific augmentation of GSIS.14 Therefore, we hypothesized that modulation of the islet PDHK isoform profile could be important in situations requiring increased GSIS to counter the developments of insulin resistance, such as high saturated-fat feeding.88-90 Therefore, in a pilot study, we analyzed the effects of high saturated fat feeding on the islet PDHK isoform profile. Significant suppression of islet PDHK1 expression was observed as a consequence of high saturated fat feeding (Holness and Sugden unpublished data). Although PDHK4 expression increases in skeletal muscle in response to the provision of a diet high in saturated fat (which causes insulin resistance with respect to peripheral glucose disposal91), high saturated fat feeding failed to modify islet PDHK4 protein expression (Holness and Sugden unpublished data). Maintenance of low PDHK1 expression, keeping PDC in a relatively dephosphorylated and active state, may be important for β-cells to achieve glucose metabolic flux rates sufficient to fuel the augmented GSIS required for islet compensation for lipid-induced insulin resistance.
The Transition to Type 2 Diabetes: Roles for PDHK1 and PDHK2 in Combating Oxidative Stress?
Mitochondrial performance is suppressed by oxidative damage as reactive oxygen species (ROS) are generated in excess. Oxidative stress markers such as 8-hydroxy-2'-deoxyguanosine, 4-hydroxy-2-nonenal-modified proteins and heme oxygenase-1 are increased in β-cells of islets of diabetic rodents.92,93 Mechanisms to defend against excessive and/or chronic ROS production, ROS toxicity and oxidative damage in pancreatic β-cells include induction of antioxidant/detoxification enzymes including the superoxide dismutases (SODs), catalase, glutathione peroxidase-1, glutaredoxin and the thioredoxins (thioredoxin1 and thioredoxin2).94 Pancreatic β-cells are particularly vulnerable to ROS cytotoxicity, attributable to relatively low levels of antioxidant enzymes in islets.95-97 For example, (cytoplasmic) Cu/Zn SOD and (mitochondrial) Mn SOD expression levels in islets are in the range of 30–40% of those in the liver.96 In other studies, these investigators found that glutathione peroxidase-1 gene expression was 15% of those in liver and that catalase gene expression was not detectable in pancreatic islets.97 As we have noted previously, changes in mitochondrial NAD+ are predicted to influence the activity of the mitochondrial sirtuin SIRT3. Therefore, it is interesting that SIRT3 levels in cardiac myocytes increase during stress, protecting cells from apoptosis,98 SIRT3-mediated deacetylation of evolutionarily-conserved lysine 122 activates Mn SOD activity in response to stress, and infection of Sirt3-/- MEFs with lenti-Mn SOD (K122-R) inhibited decreased mitochondrial superoxide.99 We have found that SIRT3 is highly expressed in mouse islets (unpublished observations), and further studies will establish whether SIRT3 subserves a protective role against oxidative stress in pancreatic β-cells.
A shift to anaerobic ATP production from ATP production via the TCA cycle reduces ROS production; in tumor cells, hypoxia via HIF-1 induces overexpression of PDHK1, which then acts to lower PDC activity.100,101 Selectively blocking HIF-induced expression of PDHK1 induced cellular apoptosis, and one possible mechanism suggested was that PDC activation due to PDHK suppression led to enhanced production of ROS, which induced apoptosis. Similarly, we observed recently in INS-1 832/13 cells cultured for 48 h at 2.8 or 16.7 mM glucose that, of the PDHK isoforms, gene expression of PDHK1 was selectively increased (a >2-fold increase in PDHK1 compared with a 0.5-fold increase in PDHK2) in conjunction with impaired GSIS.14 Therefore, we hypothesize that enhanced expression of PDHK1 in β-cells may be part of a mechanism to optimise β-cell survival under conditions of oxidative stress at the expense of GSIS, a strategy analogous to the protection against lipotoxicity afforded from the use of glycerol-3- phosphate for TAG synthesis rather than to support the glycerol-3-phosphate shuttle.
Ectopic tissue lipid accumulation, as can occur when dietary lipid delivery is excessive, has been correlated with persistent activation of members of the protein kinase C (PKC) family, and the conventional (classical, e.g., α, β, γ) and novel (e.g., δ, ε, η, θ) subgroups of the PKC family can be activated by lipid second messengers such as diacylglycerol. PKC™, a member of the novel PKC isoform subclass, also responds to redox state. Exposure of purified PKC™ to the thiol-specific oxidant diamide and reduced glutathione results in PKC™ activation.102 PDHK2 contains an autophosphorylation site (reviewed in ref. 103) and there may be an involvement of PKCδ in acute activation of PDHK2 by its phosphorylation.104 In heart, translocation of PKCδ to the mitochondria can prevent reactivation and/or promote continued PDC inactivation by phosphorylation and activation of PDHK2.104 In a recent study, Hennige et al. generated transgenic mice overexpressing a catalytically inactive form of PKC™ specifically in β-cells. The rationale was that the inactive PKCδ would compete with endogenous PKC™ and inhibit its activity. The transgenic mice exhibited improved glucose intolerance when mice were maintained on a high saturated fat diet (but not on normal diet) and GSIS was enhanced. Islet size was also increased and lipoapoptosis lowered. Various mechanisms were proposed to explain these effects, including the possibility that PKCδ might link endoplasmic reticulum stress with β-cell lipoapoptosis106,107 or with mitochondrial apoptosis.105 Suppression of PKC™ in β-cells through changes in redox state or through dissipation of its activator diacylglycerol could also be perceived as a mechanism to enhance PDC activity, augmenting ATP synthesis and allowing increased production of amplification factors for GSIS in conjunction with suppression of apoptosis, whereas activation of PKC™ in β-cells through altered mitochondrial redox state or through accumulation of mitochondrial lipid could prevent these effects and exacerbate lipotoxicity.
Concluding Remarks
We have focused on the involvement of pyruvate cycling, PDC and PC in the metabolic redox shuttles that are employed to regenerate NAD+ via mitochondrial oxidation to maintain glycolytic flux. We have extended this to illustrate how the glycerol-3-phosphate shuttle may generate glycerol-3- phosphate for esterification of incoming FA and thereby combat lipotoxicity by sequestering excess FA into an inert form, TAG, limiting the generation of cytotoxic lipids such as ceramide. We have also highlighted the role that these shuttles, for example that involving the mitochondrial 2-OG carrier, may undertake in generating key signaling molecules (e.g., NADPH and malonyl-CoA) that may facilitate the enhancement of insulin secretion which is required in response to an increased insulin demand elicited by the development of insulin resistance. We have raised the potential for PDC, citrate cataplerosis and ATP citrate lyase in mediating effects of chronic exposure to high glucose on β-cell gene expression by affecting chromatin modification by histone acetylation in pancreatic β-cells. Finally, we have addressed the potential involvement of specific PDHK isoforms, which inactivate PDC by phosphorylation, in combating the development of oxidative stress.
Acknowledgments
The authors’ work cited in this review was supported by funding from Diabetes UK (BDA:RD08/0003665, BDA:RD07/0003568, BDA:RD06/0003424, BDA:RD 04/0002863 and BDA:RD03/0002725).
Glossary
Abbreviations:
- AAS
acetoacetyl-CoA synthetase
- AAT
aspartate aminotransferase
- ACC
acetyl-CoA carboxylase
- ACL
ATP-citrate lyase
- CIC
citrate isocitrate carrier
- CPT
carnitine palmitoyltransferase
- DHAP
dihydroxyacetone phosphate
- DIC
dicarboxylate carrier
- ETC
electron transport chain
- FA
fatty acid
- GDH
glutamate dehydrogenase
- GK
glucokinase
- GLUT
glucose transporter
- GPDH
glycerol-3-phosphate dehydrogenase
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- GSIS
glucose-stimulated insulin secretion
- ICDH
isocitrate dehydrogenase
- INS1
insulinoma cell 1
- LDH
lactate dehydrogenase
- MCT
monocarboxylic acid transporter
- MDH
malate dehydrogenase
- ME
malic enzyme
- MPC
mitochondrial pyruvate carrier
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- 2OG
2-oxoglutarate
- OGC
2-oxoglutarate carrier
- PC
pyruvate carboxylase
- PDC
pyruvate dehydrogenase complex
- PDHK
pyruvate dehydrogenase kinase
- PEP
phosphoenolpyruvate
- PEPCK
phosphoenolpyruvate carboxykinase
- PGK
phosphoglycerate kinase
- PK
pyruvate kinase
- PKC
protein kinase C
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- SCOT
succinyl-CoA:3-ketoacid-CoA transferase
- SCS
succinyl-CoA synthetase
- SOD
superoxide dismutases
- TAG
triacylglycerol
- TCA
tricarboxylic acid
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/17806
References
- 1.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–60. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 2.Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–902. [PubMed] [Google Scholar]
- 3.Berman HK, Newgard CB. Fundamental metabolic differences between hepatocytes and islet beta-cells revealed by glucokinase overexpression. Biochemistry. 1998;37:4543–52. doi: 10.1021/bi9726133. [DOI] [PubMed] [Google Scholar]
- 4.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–9. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 5.Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112:139–49. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 6.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284:E855–62. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 7.Holness MJ, Sugden MC. Pyruvate dehydrogenase activities during the fed-to-starved transition and on re-feeding after acute or prolonged starvation. Biochem J. 1989;258:529–33. doi: 10.1042/bj2580529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugden MC, Holness MJ. Effects of re-feeding after prolonged starvation on pyruvate dehydrogenase activities in heart, diaphragm and selected skeletal muscles of the rat. Biochem J. 1989;262:669–72. doi: 10.1042/bj2620669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugden PH, Simister NE. Role of multisite phosphorylation in the regulation of ox kidney pyruvate dehydrogenase complex. FEBS Lett. 1980;111:299–302. doi: 10.1016/0014-5793(80)80814-3. [DOI] [PubMed] [Google Scholar]
- 10.Kolobova E, Tuganova A, Boulatnikov I, Popov KM. Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem J. 2001;358:69–77. doi: 10.1042/0264-6021:3580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korotchkina LG, Patel MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem. 2001;276:37223–9. doi: 10.1074/jbc.M103069200. [DOI] [PubMed] [Google Scholar]
- 12.Sugden MC, Bulmer K, Augustine D, Holness MJ. Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-alpha: implications for glucose-stimulated insulin secretion. Diabetes. 2001;50:2729–36. doi: 10.2337/diabetes.50.12.2729. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Han J, Epstein PN, Liu YQ. Regulation of PDK mRNA by high fatty acid and glucose in pancreatic islets. Biochem Biophys Res Commun. 2006;344:827–33. doi: 10.1016/j.bbrc.2006.03.211. [DOI] [PubMed] [Google Scholar]
- 14.Krus U, Kotova O, Spégel P, Hallgard E, Sharoyko VV, Vedin A, et al. Pyruvate dehydrogenase kinase 1 controls mitochondrial metabolism and insulin secretion in INS-1 832/13 clonal beta-cells. Biochem J. 2010;429:205–13. doi: 10.1042/BJ20100142. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan M, Choi CS, Ghoshal P, Pliss L, Pandya JD, Hill D, et al. Beta-cell-specific pyruvate dehydrogenase deficiency impairs glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2010;299:E910–7. doi: 10.1152/ajpendo.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:E1287–97. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci U S A. 2002;99:2708–13. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004;279:44370–5. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- 19.Simpson NE, Khokhlova N, Oca-Cossio JA, Constantinidis I. Insights into the role of anaplerosis in insulin secretion: A 13C NMR study. Diabetologia. 2006;49:1338–48. doi: 10.1007/s00125-006-0216-5. [DOI] [PubMed] [Google Scholar]
- 20.Vieau D, Seidah NG, Day R. Mouse insulinoma beta TC3 cells express prodynorphin messenger ribonucleic acid and derived peptides: a unique cellular model for the study of prodynorphin biosynthesis and processing. Endocrinology. 1995;136:1187–96. doi: 10.1210/en.136.3.1187. [DOI] [PubMed] [Google Scholar]
- 21.Fleischer N, Chen C, Surana M, Leiser M, Rossetti L, Pralong W, et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998;47:1419–25. doi: 10.2337/diabetes.47.9.1419. [DOI] [PubMed] [Google Scholar]
- 22.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–30. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 23.Hasan NM, Longacre MJ, Stoker SW, Boonsaen T, Jitrapakdee S, Kendrick MA, et al. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J Biol Chem. 2008;283:28048–59. doi: 10.1074/jbc.M804170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Han J, Long YS, Epstein PN, Liu YQ. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia. 2008;51:2022–30. doi: 10.1007/s00125-008-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53:1019–32. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–26. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 27.Fransson U, Rosengren AH, Schuit FC, Renström E, Mulder H. Anaplerosis via pyruvate carboxylase is required for the fuel-induced rise in the ATP:ADP ratio in rat pancreatic islets. Diabetologia. 2006;49:1578–86. doi: 10.1007/s00125-006-0263-y. [DOI] [PubMed] [Google Scholar]
- 28.Brennan L, Hewage C, Malthouse JP, McClenaghan NH, Flatt PR, Newsholme P. Investigation of the effects of sulfonylurea exposure on pancreatic beta cell metabolism. FEBS J. 2006;273:5160–8. doi: 10.1111/j.1742-4658.2006.05513.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Wilson MC, Schuit F, Halestrap AP, Rutter GA. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes. 2001;50:361–6. doi: 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald MJ. High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J Biol Chem. 1981;256:8287–90. [PubMed] [Google Scholar]
- 31.MacDonald MJ. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990;39:1461–6. doi: 10.2337/diabetes.39.12.1461. [DOI] [PubMed] [Google Scholar]
- 32.Rasschaert J, Malaisse-Lagae F, Sener A, Leclercq-Meyer V, Herberg L, Malaisse WJ. Impaired FAD-glycerophosphate dehydrogenase activity in islet and liver homogenates of fa/fa rats. Mol Cell Biochem. 1994;135:137–41. doi: 10.1007/BF00926516. [DOI] [PubMed] [Google Scholar]
- 33.Ravier MA, Eto K, Jonkers FC, Nenquin M, Kadowaki T, Henquin JC. The oscillatory behavior of pancreatic islets from mice with mitochondrial glycerol-3-phosphate dehydrogenase knockout. J Biol Chem. 2000;275:1587–93. doi: 10.1074/jbc.275.3.1587. [DOI] [PubMed] [Google Scholar]
- 34.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimabukuro M, Ohneda M, Lee Y, Unger RH. Role of nitric oxide in obesity-induced beta cell disease. J Clin Invest. 1997;100:290–5. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771–7. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 37.Choi SE, Lee YJ, Hwang GS, Chung JH, Lee SJ, Lee JH, et al. Supplement of TCA cycle intermediates protects against high glucose/palmitate-induced INS-1 beta cell death. Arch Biochem Biophys. 2011;505:231–41. doi: 10.1016/j.abb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–5. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 39.Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–75. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A. 2006;103:10224–9. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–5. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubi B, del Arco A, Bartley C, Satrustegui J, Maechler P. The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J Biol Chem. 2004;279:55659–66. doi: 10.1074/jbc.M409303200. [DOI] [PubMed] [Google Scholar]
- 44.Bender K, Maechler P, McClenaghan NH, Flatt PR, Newsholme P. Overexpression of the malate-aspartate NADH shuttle member Aralar1 in the clonal beta-cell line BRIN-BD11 enhances amino-acid-stimulated insulin secretion and cell metabolism. Clin Sci (Lond) 2009;117:321–30. doi: 10.1042/CS20090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender K, Newsholme P, Brennan L, Maechler P. The importance of redox shuttles to pancreatic beta-cell energy metabolism and function. Biochem Soc Trans. 2006;34:811–4. doi: 10.1042/BST0340811. [DOI] [PubMed] [Google Scholar]
- 46.Casimir M, Rubi B, Frigerio F, Chaffard G, Maechler P. Silencing of the mitochondrial NADH shuttle component aspartate-glutamate carrier AGC1/Aralar1 in INS-1E cells and rat islets. Biochem J. 2009;424:459–66. doi: 10.1042/BJ20090729. [DOI] [PubMed] [Google Scholar]
- 47.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–9. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald MJ, Fahien LA. Glutamate is not a messenger in insulin secretion. J Biol Chem. 2000;275:34025–7. doi: 10.1074/jbc.C000411200. [DOI] [PubMed] [Google Scholar]
- 49.Carobbio S, Frigerio F, Rubi B, Vetterli L, Bloksgaard M, Gjinovci A, et al. Deletion of glutamate dehydrogenase in beta-cells abolishes part of the insulin secretory response not required for glucose homeostasis. J Biol Chem. 2009;284:921–9. doi: 10.1074/jbc.M806295200. [DOI] [PubMed] [Google Scholar]
- 50.Casimir M, Lasorsa FM, Rubi B, Caille D, Palmieri F, Meda P, et al. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J Biol Chem. 2009;284:25004–14. doi: 10.1074/jbc.M109.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pullen TJ, Khan AM, Barton G, Butcher SA, Sun G, Rutter GA. Identification of genes selectively disallowed in the pancreatic islet. Islets. 2010;2:89–95. doi: 10.4161/isl.2.2.11025. [DOI] [PubMed] [Google Scholar]
- 52.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 53.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 54.Odegaard ML, Joseph JW, Jensen MV, Lu D, Ilkayeva O, Ronnebaum SM, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285:16530–7. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 56.Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in ’t Veld P, Renström E, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–42. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 57.MacDonald PE, Wheeler MB. Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia. 2003;46:1046–62. doi: 10.1007/s00125-003-1159-8. [DOI] [PubMed] [Google Scholar]
- 58.Huypens P, Pillai R, Sheinin T, Schaefer S, Huang M, Odegaard ML, et al. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia. 2011;54:135–45. doi: 10.1007/s00125-010-1923-5. [DOI] [PubMed] [Google Scholar]
- 59.Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45:273–83. doi: 10.2337/diabetes.45.3.273. [DOI] [PubMed] [Google Scholar]
- 60.Deeney JT, Gromada J, Høy M, Olsen HL, Rhodes CJ, Prentki M, et al. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells) J Biol Chem. 2000;275:9363–8. doi: 10.1074/jbc.275.13.9363. [DOI] [PubMed] [Google Scholar]
- 61.Yaney GC, Korchak HM, Corkey BE. Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal beta-cells. Endocrinology. 2000;141:1989–98. doi: 10.1210/en.141.6.1989. [DOI] [PubMed] [Google Scholar]
- 62.Bränström R, Leibiger IB, Leibiger B, Klement G, Nilsson J, Arhem P, et al. Single residue (K332A) substitution in Kir6.2 abolishes the stimulatory effect of long-chain acyl-CoA esters: indications for a long-chain acyl-CoA ester binding motif. Diabetologia. 2007;50:1670–7. doi: 10.1007/s00125-007-0697-x. [DOI] [PubMed] [Google Scholar]
- 63.Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557:935–48. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulder H, Yang S, Winzell MS, Holm C, Ahrén B. Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes. 2004;53:122–8. doi: 10.2337/diabetes.53.1.122. [DOI] [PubMed] [Google Scholar]
- 65.Fex M, Haemmerle G, Wierup N, Dekker-Nitert M, Rehn M, Ristow M, et al. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia. 2009;52:271–80. doi: 10.1007/s00125-008-1191-9. [DOI] [PubMed] [Google Scholar]
- 66.Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB. Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem. 1998;273:16146–54. doi: 10.1074/jbc.273.26.16146. [DOI] [PubMed] [Google Scholar]
- 67.Mulder H, Lu D, Finley J, 4th, An J, Cohen J, Antinozzi PA, et al. Overexpression of a modified human malonyl-CoA decarboxylase blocks the glucose-induced increase in malonyl-CoA level but has no impact on insulin secretion in INS-1-derived (832/13) beta-cells. J Biol Chem. 2001;276:6479–84. doi: 10.1074/jbc.M010364200. [DOI] [PubMed] [Google Scholar]
- 68.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5:253–64. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, et al. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009;284:26578–90. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–7. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 71.MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, et al. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. 2011;286:18383–96. doi: 10.1074/jbc.M111.241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald MJ, Smith AD, 3rd, Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem. 2007;282:30596–606. doi: 10.1074/jbc.M702732200. [DOI] [PubMed] [Google Scholar]
- 73.Corkey BE, Deeney JT, Yaney GC, Tornheim K, Prentki M. The role of long-chain fatty acyl-CoA esters in beta-cell signal transduction. J Nutr. 2000;130(Suppl):299S–304S. doi: 10.1093/jn/130.2.299S. [DOI] [PubMed] [Google Scholar]
- 74.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–6. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pratt ML, Roche TE. Mechanism of pyruvate inhibition of kidney pyruvate dehydrogenasea kinase and synergistic inhibition by pyruvate and ADP. J Biol Chem. 1979;254:7191–6. [PubMed] [Google Scholar]
- 76.Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ. Peroxisome-proliferator-activated receptor-alpha (PPARalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J. 2002;364:361–8. doi: 10.1042/BJ20011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gremlich S, Nolan C, Roduit R, Burcelin R, Peyot ML, Delghingaro-Augusto V, et al. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146:375–82. doi: 10.1210/en.2004-0667. [DOI] [PubMed] [Google Scholar]
- 78.Holness MJ, Greenwood GK, Smith ND, Sugden MC. Peroxisome proliferator-activated receptor-alpha and glucocorticoids interactively regulate insulin secretion during pregnancy. Diabetes. 2006;55:3501–8. doi: 10.2337/db06-0666. [DOI] [PubMed] [Google Scholar]
- 79.Heart E, Cline GW, Collis LP, Pongratz RL, Gray JP, Smith PJ. Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2009;296:E1354–62. doi: 10.1152/ajpendo.90836.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu J, Han J, Long YS, Lock J, Weir GC, Epstein PN, et al. Malic enzyme is present in mouse islets and modulates insulin secretion. Diabetologia. 2008;51:2281–9. doi: 10.1007/s00125-008-1155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ronnebaum SM, Jensen MV, Hohmeier HE, Burgess SC, Zhou YP, Qian S, et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem. 2008;283:28909–17. doi: 10.1074/jbc.M804665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–80. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuit F, Flamez D, De Vos A, Pipeleers D. Glucose-regulated gene expression maintaining the glucose-responsive state of beta-cells. Diabetes. 2002;51(Suppl 3):S326–32. doi: 10.2337/diabetes.51.2007.S326. [DOI] [PubMed] [Google Scholar]
- 84.Liu YQ, Jetton TL, Leahy JL. beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002;277:39163–8. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- 85.MacDonald MJ, Longacre MJ, Langberg EC, Tibell A, Kendrick MA, Fukao T, et al. Decreased levels of metabolic enzymes in pancreatic islets of patients with type 2 diabetes. Diabetologia. 2009;52:1087–91. doi: 10.1007/s00125-009-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacDonald MJ, Tang J, Polonsky KS. Low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of Zucker diabetic fatty rats. Diabetes. 1996;45:1626–30. doi: 10.2337/diabetes.45.11.1626. [DOI] [PubMed] [Google Scholar]
- 87.Zhou YP, Ostenson CG, Ling ZC, Grill V. Deficiency of pyruvate dehydrogenase activity in pancreatic islets of diabetic GK rats. Endocrinology. 1995;136:3546–51. doi: 10.1210/en.136.8.3546. [DOI] [PubMed] [Google Scholar]
- 88.Holness MJ, Smith ND, Greenwood GK, Sugden MC. Acute omega-3 fatty acid enrichment selectively reverses high-saturated fat feeding-induced insulin hypersecretion but does not improve peripheral insulin resistance. Diabetes. 2004;53:166–71. doi: 10.2337/diabetes.53.2007.S166. [DOI] [PubMed] [Google Scholar]
- 89.Holness MJ, Greenwood GK, Smith ND, Sugden MC. Diabetogenic impact of long-chain omega-3 fatty acids on pancreatic beta-cell function and the regulation of endogenous glucose production. Endocrinology. 2003;144:3958–68. doi: 10.1210/en.2003-0479. [DOI] [PubMed] [Google Scholar]
- 90.Holness MJ, Smith ND, Greenwood GK, Sugden MC. Acute (24 h) activation of peroxisome proliferator-activated receptor-alpha (PPARalpha) reverses high-fat feeding-induced insulin hypersecretion in vivo and in perifused pancreatic islets. J Endocrinol. 2003;177:197–205. doi: 10.1677/joe.0.1770197. [DOI] [PubMed] [Google Scholar]
- 91.Holness MJ, Kraus A, Harris RA, Sugden MC. Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes. 2000;49:775–81. doi: 10.2337/diabetes.49.5.775. [DOI] [PubMed] [Google Scholar]
- 92.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–32. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 93.Gorogawa S, Kajimoto Y, Umayahara Y, Kaneto H, Watada H, Kuroda A, et al. Probucol preserves pancreatic beta-cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2002;57:1–10. doi: 10.1016/S0168-8227(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 94.Lacraz G, Figeac F, Movassat J, Kassis N, Coulaud J, Galinier A, et al. Diabetic beta-cells can achieve self-protection against oxidative stress through an adaptive upregulation of their antioxidant defenses. PLoS ONE. 2009;4:6500. doi: 10.1371/journal.pone.0006500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–7. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- 96.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–42. doi: 10.2337/diabetes.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 97.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–6. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 98.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28:6384–401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Chu F, Ward NE, O’Brian CA. Potent inactivation of representative members of each PKC isozyme subfamily and PKD via S-thiolation by the tumor-promotion/progression antagonist glutathione but not by its precursor cysteine. Carcinogenesis. 2001;22:1221–9. doi: 10.1093/carcin/22.8.1221. [DOI] [PubMed] [Google Scholar]
- 103.Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res. 2004;38:1083–92. doi: 10.1080/10715760400004168. [DOI] [PubMed] [Google Scholar]
- 104.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 105.Hennige AM, Ranta F, Heinzelmann I, Düfer M, Michael D, Braumüller H, et al. Overexpression of kinase-negative protein kinase Cdelta in pancreatic beta-cells protects mice from diet-induced glucose intolerance and beta-cell dysfunction. Diabetes. 2010;59:119–27. doi: 10.2337/db09-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 107.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–63. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]