Abstract
Glucose homeostasis depends on adequate control of insulin secretion. We report the association of the cell-adhesion and adiponectin (APN)-binding glycoprotein T-cadherin (Cdh13) with insulin granules in mouse and human β-cells. Immunohistochemistry and electron microscopy of islets in situ and targeting of RFP-tagged T-cadherin to GFP-labeled insulin granules in isolated β-cells demonstrate this unusual location. Analyses of T-cadherin-deficient (Tcad-KO) mice show normal islet architecture and insulin content. However, T-cadherin is required for sufficient insulin release in vitro and in vivo. Primary islets from Tcad-KO mice were defective in glucose-induced but not KCl-mediated insulin secretion. In vivo, second phase insulin release in T-cad-KO mice during a hyperglycemic clamp was impaired while acute first phase release was unaffected. Tcad-KO mice showed progressive glucose intolerance by 5 mo of age without concomitant changes in peripheral insulin sensitivity. Our analyses detected no association of APN with T-cadherin on β-cell granules although colocalization was observed on the pancreatic vasculature. These data identify T-cadherin as a novel component of insulin granules and suggest that T-cadherin contributes to the regulation of insulin secretion independently of direct interactions with APN.
Keywords: adiponectin, confocal microscopy, electron microscopy, exocytosis, glucose homeostasis, hyperglycemic clamp, islets of Langerhans, Secretion, T-cadherin
Introduction
Cadherins constitute a class of calcium-dependent cell adhesion molecules that play central roles in embryonic development and tissue homeostasis.1-4 Classical cadherins are transmembrane proteins that confer homotypic cell-to-cell adhesion and control adhesive strength through dimerization5 and linkage to the actin-based cytoskeleton.6 Regulation of cadherin adhesive functions and linkage to intracellular signaling pathways affects cell behavior and tissue function.7 In the pancreas, β-cells express E-cadherin and N-cadherin on their cell surfaces.8 E-cadherin-mediated adhesion affects the developmental segregation of islets from surrounding non-endocrine tissue9 and contributes to maintaining islet architecture and function.10 N-cadherin is dispensable for pancreatic development and suggested to mildly affect β-cell function by yet unknown mechanisms.11
T-cadherin (T, truncated; cadherin-13, Cdh13) displays the overall ectodomain organization of the classical cadherins and is anchored to the plasma membrane through a glycosylphosphatidyl inositol (GPI) moiety.12,13 This unique cadherin mediates weak calcium-dependent homotypic adhesion13 and binds the adipocyte-secreted, circulating metabolic hormone APN to plasma membranes in responsive tissues including heart, vasculature and muscle.5,13-15 Genome-wide association studies link single nucleotide polymorphisms in the Cdh13 gene to variations in adiponectin concentration that are associated with metabolic control.16-18 This interaction with APN raised our interest in addressing a possible role of T-cadherin in metabolic regulation.
Focusing on islets of Langerhans as a primary organ in controlling glucose homeostasis through regulation of insulin secretion, we report here on a novel and unexpected association of T-cadherin with dense-core insulin-containing granules of β-cells without concomitant localization of APN. Dissecting the specific role for T-cadherin in β-cells in vitro and in vivo, we show that T-cadherin on insulin granules is required to enable sufficient and persistent glucose-stimulated insulin secretion. Systemic loss of T-cadherin in mice impairs glucose homeostasis without concomitant alterations in peripheral insulin sensitivity. These data suggest that T-cadherin, independent of direct interactions with APN, contributes to the regulation of insulin secretion that in turn affects metabolic functions.
Results
T-cadherin is distributed on dense core secretory granules of pancreatic β-cells.
As insulin-secreting β-cells are prime regulators of glucose homeostasis, we investigated the pattern of T-cadherin expression in the adult mouse pancreas. Immunohistochemistry with affinity-purified T-cadherin polyclonal antibodies generated in our laboratory14 revealed T-cadherin in endocrine cells of the islets (Fig. 1A–C) and in the pancreatic vasculature (Fig. 1E). In β-cells, the T-cadherin staining pattern was atypically granular and overlapped to a large extent with vesicles stained for insulin (Fig. 1A). A similar distribution was observed in samples of human islets (Fig. 1B). Some insulin-negative islet cells in the mantle were also T-cadherin positive, suggesting that other endocrine islet cells may also express T-cadherin (Figs. 1A and S1). Surprisingly, β-cells displayed virtually no detectable staining for T-cadherin associating with the cell boundaries, highlighted by the underlying subcortical actin belt (Fig. 1C). This is in sharp contrast to T-cadherin’s cell surface localization elsewhere, including skeletal muscle in vivo (Fig. 1D) and in vitro.13,19
Figure 1. T-cadherin expression in islets delineates intracellular granules and the pancreatic vasculature. (A) Confocal micrographs of WT mouse pancreas and (B) isolated human islet. (C) T-cadherin in mouse islet cells does not associate with cell boundaries. (D) T-cadherin in mouse skeletal muscle associates with myofiber surfaces. T-cadherin in green (A–D). Insulin in red (A and B). Actin (phalloidin) in red (C). DAPI in blue (A–E). (E) T-cadherin (green) is also found on blood vessels in the exocrine pancreas (CD31-red). (F) Adiponectin (green) is localized to blood vessels (CD31-red) in the islet periphery (insulin-blue).
In the pancreatic vasculature, T-cadherin staining overlapped with the endothelial cell surface marker CD31 (Fig. 1E).14 APN, a ligand-binding partner for T-cadherin,20 selectively paralleled the vascular expression of T-cadherin on CD31-positive cells that surrounded the islets (Fig. 1F). APN was undetectable in Æ-cells.
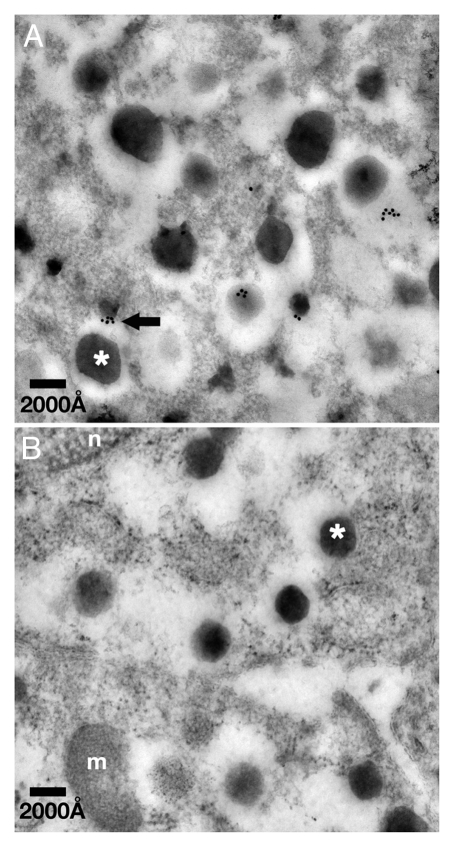
Independent evidence for T-cadherin’s vesicle association was generated by immuno-electron microscopy. T-cadherin was detected with a specific affinity-purified antibody14 followed by 18 nm gold-labeled anti-rabbit immunoglobulin on ultrathin sections of Unicryl-embedded adult mouse islets. Electron microscopic analyses identified the T-cadherin immunogold particles on or in association with electron-dense insulin granules (Fig. 2). Thus, both immunohistochemistry and immunoelectron microscopy distinguish T-cadherin in association with insulin-containing granules in Æ-cells.
Figure 2. T-cadherin localizes in association with dense core insulin granules. (A) Immuno-electron micrographs of subcellular T-cadherin localization in a WT mouse β-cell. (B) Negative control WT β-cell where the primary antibody was replaced with normal rabbit serum. Insulin granules were identified by the typical electron dense morphology of the granule core surrounded by an electron sparse halo (asterisk demarcate core of a granule). Tcad was identified by immunostaining with antibodies coupled to 18 nm gold particles [arrow points to 18 nm gold (Tcad) associated with surface of a granule]; n, nucleus; m, mitochondrion. Scale bars: 2,000 ≈.
Ectopically expressed red fluorescent-labeled T-cadherin (Tcad-mRFP) targets to insulin granules in primary islets.
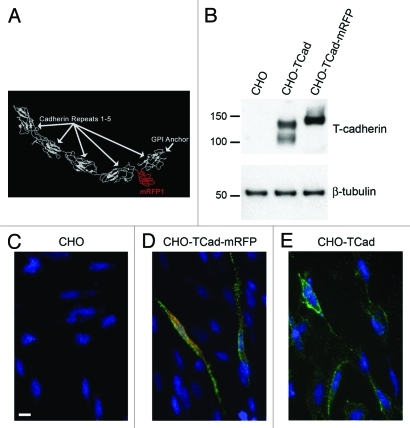
T-cadherin’s unusual localization on secretory vesicles necessitated further evidence to unequivocally demonstrate its association with insulin-containing granules. To accomplish this, we generated and expressed fluorescently tagged T-cadherin (Tcad-mRFP) under the control of the cytomegalovirus (CMV) or the human insulin promoter21 for expression in primary islet cells. The Tcad-mRFP was engineered to express the mRFP insert22 flanked by 3xGly linkers between the fourth and fifth extracellular domain of the T-cadherin protein (Fig. 3A). T-cadherin’s known adhesive functions are mediated by sites within the N-terminal extracellular domains,5 thus the 140 kD Tcad-mRFP protein (Fig. 3B) likely fulfills the structural criteria of a functional protein. Indeed, this positioning seemed to allow proper folding and sorting of the protein and maintenance of the GPI-membrane linkage. Expression of Tcad-mRFP from the general cytomegalovirus promoter (CMV-Tcad-mRFP) validated cell surface targeting in Chinese Hamster Ovary (CHO) cells (Fig. 3D). Reflecting the characteristic localization of GPI-anchored T-cadherin in cell surface domains, Tcad-mRFP protein was expressed in a similar cell surface pattern observed for naive T-cadherin expressed from the unaltered cDNA (compare Fig. 3D and E).
Figure 3. Construction of tagged T-cadherin. (A) Prediction of T-cadherin structure, showing insertion of schematic 3xGly-mRFP-3xGly peptide folding separately in between cadherin motifs four and five. (B) Western blot of CHO cells with or without transfection with wild type T-cadherin or the Tcad-mRFP construct. (C–E) T-cadherin (green) immunostaining performed on living non-permeabilized cells, illustrating Tcad surface localization. Tcad-mRFP fluorescence-red. Scale bar: 10 μm. (C) Wild type CHO cells. (D) CHO cells transfected with Tcad-mRFP. (E) CHO cells transfected with wild type T-cadherin. Note that some Tcad-mRFP is detected in the cytoplasm (D), which probably reflects protein during processing and transport.
Naive overexpressed T-cadherin protein was apparent in CHO-cells as a doublet consisting of the mature 115 kD protein and the ~130 kD proprotein by Western blotting (Fig. 3B; see ref. 13). Tcad-mRFP cDNA encoded a major protein species of ~140 kD reflecting the insertion of the RFP-tag into the mature T-cadherin protein (Fig. 3B). The Tcad-mRFP precursor protein including the propeptide region was undetectable possibly because of the limited stability of this mutated protein isoform.
We used Tcad-mRFP to determine the subcellular localization of the tagged T-cadherin protein in pancreatic endocrine cells. We first expressed Tcad-mRFP from the insulin promoter in granule-containing 832/13 insulinoma cells.24 In contrast to the cell surface expression of Tcad-mRFP into CHO-cells, the tagged protein was localized on intracellular structures (Fig. S2). Using dissociated primary islets from WT adult (2–3 mo old) mice, we next examined ectopic Tcad-mRFP expression in relation to insulin promoter-driven Phogrin-GFP, a marker for the dense core insulin granule in β-cells.25,26 Tcad-mRFP and Phogrin-GFP co-expression was detected after 48 h. As an additional criterion for Tcad-mRFP expression on insulin-containing granules, we examined co-mobilization of Tcad-mRFP and Phogrin-GFP after glucose stimulation to circumvent limitations of optical resolution in a static sample. Confocal images illustrate the granular distribution of Tcad-mRFP in primary β-cells and mobilization of Tcad-labeled Phogrin-positive insulin granules (Fig. 4A and B and Video 1).
Figure 4. Co-migration of mRFP labeled T-cadherin (Tcad-mRFP) and GFP labeled Phogrin in ∆-cell. (A and B) Islet cells were transfected with insulin promoter driving Phogrin-GFP and CMV promoter driving Tcad-mRFP. White arrow points at a yellow mobilized granule. Pictures taken with approximately 2 s interval. (C and D) Cells were instead transfected with CMV promoter driven synaptophysin-GFP and insulin promoter driven Tcad-mRFP. Arrow points to Tcad positive structure mobilized over 5 s that does not overlap with synaptophysin-GFP. See also Video 1. Scale bars: 5 μm. (A and B) are snapshot images from Video 1.
Additional evidence for T-cadherin’s unexpected association with insulin granules came from viewing Tcad-mRFP-labeled granules in relation to the synaptic-like vesicle pool in β-cells. This group of β-cell vesicles is demarcated by synaptophysin27 and distinct from the dense core insulin granules. To determine if T-cadherin is a general component of vesicles in β-cells, we co-expressed synaptophysin-GFP28 and Tcad-mRFP in primary cultured islet cells. Tcad-mRFP expression was controlled from the insulin promoter to restrict transcription to β-cells. Confocal imaging identified Tcad-mRFP expression on a different granule population than that demarcated by synaptophysin-GFP (Fig. 4C and D). No co-migration of Tcad-mRFP and synaptophysin-GFP was observed (Fig. 4C and D and arrow). These data indicate specificity of T-cadherin’s association with Phogrin-positive granule populations that equals insulin granules in β-cells.
In combination, immunolocalization and electron microscopy of endogenous T-cadherin on insulin-containing granules and the expression of RFP-tagged T-cadherin protein on Phogrin-labeled, mobilized granules strongly support the association of T-cadherin with insulin-containing vesicles. T-cadherin’s distribution on insulin granules is specific and complementary to the synaptophysin-labeled vesicle pool.
Loss of T-cadherin in ®-cells from knockout mice.
T-cadherin’s distribution on insulin granules raises interest in functions in β-cells and regulation of glucose homeostasis in vivo. We used a loss-of-function approach and studied mice deficient for T-cadherin gene expression (Tcad-KO) to address this issue.14 Immunohistochemistry of paraffin-embedded pancreas sections showed intracellular T-cadherin staining in β-cells of wild-type (WT) mice. No staining signal was detected in the Tcad-KO condition (Fig. 5A and B).
Figure 5. Characterization of mutant mice. (A and B) T-cadherin immunostaining (brown DAB, hematoxylin counterstain) demonstrating expression in WT islets (A) and no expression in KO islets (B). (C) Western blot of tissues confirming expression in WT tissue and deletion in KO tissue (note that irrelevant lanes were omitted between pancreas and heart samples).
We further confirmed deletion of T-cadherin in the null mutant mice by analyzing tissue lysates by Western blotting. In whole pancreas, heart and muscle, T-cadherin was expressed as both mature and proprotein species of ~100 kD and ~130 kD, respectively (Figs. 5C and S3: islets and other tissues). Minor variations in the molecular masses of T-cadherin in different tissues result largely from tissue-specific glycosylation (reviewed in ref. 29 and unpublished data). Importantly, T-cadherin was absent from any tissue examined from the knockout mice (Fig. 5A–C). Thus, the null mutation had reliably ablated protein expression in the pancreas.
Tcad-KO pancreata show normal morphology and insulin content.
No differences were noted between genotypes in the overall size and appearance of pancreata. We examined the pancreata from 1 mo old and older Tcad-KO mice for morphological and functional differences in comparison with the WT. First, examination of pancreatic morphology identified no overt differences between genotypes in morphology and overall islet architecture. Islets in both Tcad-KO and WT mice contained large numbers of β-cells and comparable populations of glucagon-expressing α- and somatostatin-containing δ-cells (Fig. 6A and B). These immunohistochemical observations suggest that islet architecture development occurs independent of T-cadherin. Second, as an estimate for insulin biosynthesis and storage, we measured insulin content in equivalent numbers of islets isolated from 3 mo old mice of both genotypes. Comparable insulin levels were found in WT and Tcad-KO islet tissue (Fig. 6C; n = 12–15 mice) suggesting that insulin synthesis/ storage is unaffected by T-cadherin. Third, we investigated pancreatic insulin content of the intact organ reflecting an estimate of the number of β-cells per pancreas. Again, we found no differences between genotypes at 1–8 mo of age (Fig. 6D; n = 7–14). These results suggest that the T-cadherin mutation neither affected the development of pancreatic islets, nor limited the availability of insulin by affecting Æ-cell number, insulin biosynthesis or storage.
Figure 6. T-cadherin affects second phase insulin secretion, but not islet morphology and insulin content. (A and B) Immunofluorescent analysis of islet architecture in 1 mo old Tcad WT (A) and KO (B) pancreas (insulin-red, somatostatin-blue, glucagon-green). (C) Insulin content of isolated islets from 3 mo old mice. (D) Whole pancreas insulin content as a measure of β-cell mass (no differences within age groups). (E) Insulin secretion in vitro of isolated pancreatic islets from 3 mo old mice, demonstrating a reduced response to glucose but not to KCl in Tcad KO islets. Data expressed as ratio of stimulated secretion over baseline secretion for each batch of islets. (F) In vivo insulin secretion during hyperglycemic clamp, demonstrating severely impaired or delayed second phase (beyond hatched line) insulin secretion in KO mice. Inset shows area under the curve analyses. **p < 0.01, ***p < 0.001.
T-cadherin affects insulin secretion from pancreatic β-cells.
To test if T-cadherin affects insulin-release properties of Æ-cells, we assessed insulin secretion from cultured primary islets isolated from Tcad-KO and WT mice at 3 mo of age. An equal number of islets from each WT and Tcad-KO mice were stimulated by the addition of 22.4 mM glucose for 45 min. The amount of insulin released into the culture medium was quantified by ELISA. WT islets secreted 0.346 ± 0.047 pmol/10 islets/h insulin. In contrast, T-cadherin mutant islets released 54% less insulin (0.154 ± 0.023 pmol/10 islets/h) (Fig. 6E; n = 10–12). Glucose stimulation elicits a rapid first phase of insulin secretion characterized by the release of granules in proximity to the plasma membrane and a prolonged second phase that requires recruitment and priming of the reserved vesicle pool. To distinguish which part of the secretion process is affected by T-cadherin, we assessed insulin secretion by the readily releasable granule pool close to the plasma membrane. To accomplish this, we depolarized isolated pancreatic islets with 35 mM KCl in the absence of glucose, and measured insulin secreted from Tcad-KO or WT islets by ELISA after 15 min KCl exposure. Our data revealed no differences in KCl-stimulated insulin release between genotypes (Fig. 6E; n = 6) suggesting that T-cadherin-deficient β-cells rapidly release insulin upon stimulation. Stimulation of islets with 35 mM KCl in combination with 22.4 mM glucose for 45 min resulted in an increased response in both genotypes, but a 58% reduction of insulin secretion in Tcad-KO islets (n = 8). In fact, the addition of glucose had no further effect on Tcad-KO islet insulin secretion than KCl alone. These data support the conclusion that T-cadherin deficiency, while not contributing to the depolarization phase of insulin release from β-cells (induced here by KCl), affects the prolonged phase of insulin secretion, characterized by processes that require ATP from glucose or other fuels.
To directly assess the role of T-cadherin in the classical two phases of insulin secretion and thereby better understand its role, we examined insulin secretion in WT and Tcad-KO mice during a hyperglycemic clamp. Insulin concentrations in blood sampled for the first 5 min of the clamp, corresponding to the first phase of insulin release, were comparable between genotypes. However, blood collected from Tcad-KO mice over the subsequent 55 min showed significantly reduced insulin content compared with the WT values (Fig. 6F; n = 6–8). These data suggest that loss of T-cadherin from β-cells leads to a significant impairment in the second phase of insulin release characterized by ATP-dependent processes, such as priming, recruitment and release of the reserved vesicle pool.30-32
Impaired glucose tolerance in T-cadherin null mutant mice.
Impaired insulin secretion in Tcad-KO mice together with the expression of T-cadherin in tissues targeted by insulin (such as muscle, Fig. 1D) in WT mice, poses the question of T-cadherin’s role in overall glucose metabolic control. To examine this, we analyzed WT and Tcad-KO male C57Bl/6 mice fed a normal diet for peripheral insulin sensitivity, baseline blood glucose levels and their ability to acutely clear intraperitoneally administered glucose from the bloodstream (IPGTT).
To evaluate peripheral insulin sensitivity, we applied a hyperinsulinemic-euglycemic clamp to male mice from both genotypes at 4 mo of age. The steady-state glucose infusion rate, Ginf, for each animal recorded at the end of the euglycemic clamp, was used as a measure of insulin sensitivity. Accordingly, insulin sensitivity is proportional to the exogenous Ginf necessary to maintain euglycemia. No differences in Ginf were observed between Tcad-KO and WT mice (Fig. 7A) suggesting that T-cadherin-deficiency has no major effect on insulin sensitivity and glucose utilization in peripheral tissues, such as muscle.

Figure 7. T-cadherin contributes to glucose tolerance, but not peripheral insulin sensitivity. (A) Submaximal hyperinsulinemic-euglycemic clamp demonstrating that 4 mo old male WT and KO mice are equally sensitive to insulin. (B and C) Intra-peritoneal glucose tolerance tests demonstrating an impaired glucose tolerance in KO compared with WT 5 mo old male C57Bl/6 mice (B) and 5 mo old male Black-Swiss mice (C). Solid line, WT. Hatched line KO. *p < 0.05, **p < 0.01, ***p < 0.001.
Nonetheless, intraperitoneal glucose tolerance tests (IPGTT) on male mice from both genotypes at 1, 2, 3, 5 and 15 mo of age, demonstrated progressive impairment of glucose tolerance in the Tcad-KO group. WT and Tcad-KO littermates cleared glucose equally well at ages ranging between 1–3 mo (not shown). However, by 5 mo of age, the KO mice were glucose intolerant (Fig. 7B; n = 8), without any notable differences in fasting blood glucose levels (Fig. 7B). To ensure that the observed impairment of glucose tolerance was independent of the mouse genetic background, we repeated the IPGTT with WT and Tcad-KO on the mixed Black-Swiss background at 5 mo of age (Fig. 7C). The same temporal appearance and severity of glucose intolerance were observed in both the Tcad-KO Black-Swiss (clone 64) and the C57Bl6 (clone 92) mouse lines, thus firmly establishing the phenotype. We note that both WT and Tcad-KO Black-Swiss mice were obese at 5 mo of age [Black-Swiss: 41.1 ± 1.3 g, n = 6; vs. C57Bl/6: 26.6 ± 0.8 g, n = 16; no differences between genotypes (not shown)], and that the WT Black-Swiss control animals displayed decreased glucose tolerance compared with C57Bl/6 mice (compare Fig. 7B and C). Still, loss of T-cadherin resulted in significantly further reduced glucose clearance from the blood stream (Fig. 7C).
In summary, the results from our study suggest that T-cadherin on insulin granules contributes to second phase insulin secretion in mice, which may affect consequent glucose metabolic control without notable influence on peripheral insulin resistance.
Discussion
In the current work, we identify T-cadherin in association with insulin granules in pancreatic ∆-cells, and link T-cadherin functions to the insulin secretory machinery responsible for the second phase of insulin release. We correlate this finding with the development of glucose intolerance in T-cadherin deficient mice fed a normal diet without an apparent impairment of peripheral insulin sensitivity. Assessing insulin resistance and β-cell function prior to the onset of metabolic dysfunction assured that we evaluated the effect of T-cadherin loss-of-function on these parameters rather than recording secondary effects from prolonged hyperglycemia.33-35 Our combined data suggest that T-cadherin primarily affects insulin secretion, which in turn contributes to dysregulation of glucose homeostasis in the Tcad KO mice. Clearly, our work does not exclude the possibility that T-cadherin in peripheral tissues contributes to regulation of insulin sensitivity in other circumstances, such as during high-fat diet induced stress.
Cadherin association with secretory vesicles in β-cells is unprecedented and was asserted through several independent lines of experimentation. First, affinity-purified, highly specific antibodies detected T-cadherin in association with insulin- containing granules in WT, but not in the Tcad-KO mice. Second, T-cadherin immuno-electron microscopy showed T-cadherin in association with the dense-core insulin granules. Third, mRFP-tagged T-cadherin expressed in CHO cells resided on the plasma membrane, while T-cadherin protein transcribed from the same cDNA was targeted to insulin granules in primary Æ-cells. The mRFP-labeled T-cadherin protein expressed in β-cells co-migrated with GFP-labeled Phogrin, an insulin granule marker, upon glucose stimulation. This suggests that T-cadherin, in contrast to its plasma membrane localization elsewhere, is differentially processed and targeted for association with insulin granules in β-cells. Due to limitations of optical microscopy resolution and electron microscopy sample preparation, we are unable to determine if T-cadherin is expressed on all or only a subset of insulin granules (e.g., the reserve pool), which may be assessed in the future by novel super-resolution microscopy techniques (nanoscopy).36 This could further shed light on the function of T-cadherin in the insulin secretory process.
The association of T-cadherin with insulin granules is dispensable during pancreatic development: we detect no overt defects in pancreatic architecture and insulin availability in T-cadherin-deficient adult mice compared with the WT. However, we demonstrate that T-cadherin on insulin granules contributes to the insulin-release properties of β-cells. Insulin release following glucose stimulation of primary Tcad-KO pancreatic islets is more than 50% less effective than from islets isolated from corresponding WT mice.
Insulin granules in β-cells comprise at least two pools based on availability, the readily releasable pool close to the plasma membrane and the reserved pool localized deeper inside the cells.30,37,38 Upon physiological fuel stimulation (glucose, etc.) of Æ-cells, membrane depolarization and concomitant Ca2+ influx lead to the immediate first phase of insulin secretion in which the readily releasable granules fuse with the plasma membrane to release insulin content. Fuel driven secretion is achieved by producing ATP, leading to ATP-dependent closure of KATP-channels, resulting in membrane depolarization. This is followed by the second, prolonged phase of insulin release, requiring energy-dependent priming and recruitment of the reserved vesicle pool to the cell surface.32,39-44 Dissecting the specific role of T-cadherin in the insulin release process, we show that acute insulin release remains intact after stimulation of primary Tcad-KO β-cells by direct depolarizing action of KCl in vitro and by glucose-driven ATP production in vivo. However, we provide in vitro and in vivo evidence that prolonged glucose-dependent insulin secretion is impaired by the Tcad-KO mutation.
How could T-cadherin associated with insulin granules affect insulin release properties? First, prolonged insulin release is dependent on ATP generation, and T-cadherin may directly or indirectly affect energy availability. We do not favor this interpretation as glucose stimulated first phase insulin secretion, which depends on ATP closure of KATP-channels to cause β-cell depolarization, as described above, is normal in Tcad-KO mice. Moreover, comparative microarray gene expression data from isolated WT and Tcad-KO islets did not identify reductions in genes regulating metabolic pathways (not shown).
Second, T-cadherin associated with insulin granules is a candidate to confer trafficking of the reserved vesicles to the plasma membrane through interactions with protein complexes bound to microtubules. Insulin granule exocytosis depends largely on kinesin motor complexes that traffic along microtubules to the plasma membrane.32,39 Recently, calsyntenins, members of the cadherin superfamily, were localized to secretory granules in the anterior pituitary and the pancreatic α-cell.45 In parallel to calsyntenin functions in excitatory neurons, T-cadherin could promote interactions with kinesin to regulate anteriograde vesicle transport.46
Finally, T-cadherin may serve a role in priming the reserved insulin granules for membrane fusion, a process necessary for glucose-augmented prolongation of insulin release. Different proteins have recently been associated with second phase insulin secretion, including H+/Cl- transporters,47 Munc13-1,41,48 and the Rho family GTPase Cdc42.43 T-cadherin, in the context of cell migration, is engaged in RhoA and Rac signaling,49 thus a plausible function in β-cells is the association with the granular Cdc42-caveolin1-VAMP2 complex that regulates prolonged insulin release.50-52 Alternatively, T-cadherin overexpression alters Akt/Gsk3Æ signaling leading to Æ-catenin redistribution in endothelial cells.53 This may mean that T-cadherin is involved in actin cytoskeleton reorganization in β-cells, which is critical for insulin granule exocytosis through cdc42-dependent mechanisms.54 Future experiments will need to provide mechanistic insights into T-cadherin’s functions in the insulin release process.
Continuously limited availability of insulin may affect the development of progressive glucose intolerance of the Tcad-KO mice. However, a diverse range of other factors also influences the regulation of glucose metabolic control and could contribute to metabolic abnormalities in the Tcad-KO mice. T-cadherin expression in heart, muscle and blood vessels14,15 (Figs. 1D–F and 5C) could affect overall metabolic regulation by sequestering the adipocyte-secreted metabolic hormone APN.20 Indeed, in Tcad-KO mice, APN fails to associate with the vasculature and insulin-sensitive heart and skeletal muscle, and APN serum levels are more than five-fold increased over WT,14,55 which in theory would make APN abundantly available for interactions with other receptors. Even with a possible overstimulation of other APN-receptors, Tcad-KO mice are, however, glucose intolerant on a normal diet, while APN-KO mice show no deficits under baseline feeding conditions.56 Studies addressing the role of APN in β-cell functions have led to inconsistent results.61-64 In the pancreas in vivo, we detect APN in association with T-cadherin only on the pancreatic vasculature where this interaction may indirectly affect signals regulating β-cell function. Islet vascularization is a critical factor for β-cell function.65 Thus, T-cadherin-APN interactions in the pancreatic vasculature may contribute to metabolic control in addition to direct actions of T-cadherin in insulin release. We note that our in vitro model identified defective insulin secretion from isolated Tcad-KO islets in the absence of vascular or other cellular components or APN. Still, defective endothelium-islet cell interactions could lead to changes in extracellular matrix composition that may have the potential to affect insulin secretion even in vitro.66 Nevertheless, the data presented here suggests that defective insulin secretion reflects a separate function of intracellular T-cadherin that does not seem to require direct interactions with APN. The possible misbalance of T-cadherin/APN functions in islets and other organs will need to be considered in separate studies of the metabolic phenotype of the Tcad-KO mice.
In summary, our work identifies a novel and unexpected localization of T-cadherin on insulin granules that affects second phase insulin release from β-cells, and thus, may contribute to the glucose intolerance observed in Tcad-KO mice. Although the baseline metabolic phenotype of the T-cadherin KO mice somewhat parallels that of mice deficient for APN,56,67 our data do not support a model in which T-cadherin binds APN to regulate pancreatic functions. However, our studies do not rule out the possibility that T-cadherin—APN interactions contribute to metabolic control by regulating insulin release indirectly or confound functions of other organs, such as liver. Defining the molecular interactions of T-cadherin in the pancreas and in other insulin-sensitive tissues will be an important contribution to understanding the role of this unique molecule in metabolic regulation.
Methods
Animals.
Unless noted, the T-cadherin KO mice were used after back breeding onto the pure C57Bl/6 background. Generation and genotyping of these mice were previously described in reference 14. For critical experiments, the findings were confirmed with Tcad KO mice on a mixed C57Bl6/SVJ129/BlackSwiss background. Males were used for all in vivo experiments. All animals were housed under controlled light (12/12 h) and temperature conditions, and had free access to food and water unless specifically indicated. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Animal Subjects Committee of the University of California, San Diego and Sanford-Burnham Medical Research Institute.
Immunochemistry.
For immunohistochemistry, pancreata were extirpated after intra-cardiac perfusion of the animal with 4% paraformaldehyde (PFA), cryopreserved in 30% sucrose in PBS at 4°C, embedded in O.C.T. compound (4583, Sakkura Finetek), and cryosectioned at 5μm slices. Isolated islets were drop-fixed, then cryopreserved and cryosectioned as above. Rabbit anti- mouse T-cadherin anti-sera were generated in rabbits against recombinant T-cadherin protein representing EC1–EC4 as described in reference 14. The resulting antisera were used after affinity-purification on soluble T-cadherin recombinant protein and specifically detected T-cadherin protein species in various tissues. Other antibodies included mouse-α-insulin (2D11-H5, Santa Cruz), guinea pig α-insulin (I7660, US Biologicals), goat α-somatostatin (D-20, Santa Cruz), mouse α-glucagon (K79bB10, Sigma), rat α-CD31 (clone 390, PharMingen), rabbit α-adiponectin (ACRP, PA1-054, Affinity Bioreagents), Alexa 488- and Alexa 594-conjugated donkey antibodies (A-11055, A-21202, A-21206, A-21208, A-11058, A-21203, A-21207, A-21209; Invitrogen), and Rhodamine RedX- and DyLight 649-conjugated donkey antibodies (706-295-148, 706-495-148, 705-495-147, 712-495-153; Jackson Immuno). Immunofluorescent staining was performed by incubation with primary antibodies at 4°C overnight in PBS with 0.1% TritonX-100, 1% normal donkey serum (017-000-121, Jackson Immuno), 0.2% BSA and 0.2% cold-water fish gelatin (G7041, Sigma). Secondary antibodies were incubated for 1 h at RT in the same dilution buffer. Counterstaining was with DAPI nuclear dye (D21490, Invitrogen) and in some cases TexasRed-Phallodin to label actin filaments (T7471, Invitrogen). Specimen were mounted in Fluorescent Mounting Media (S3023, Dako) and visualized in a Confocal Laser Scanning Microscope (MRC 1024MP, Bio-Rad Laboratories) equipped with krypton/argon laser and two-photon Ti-Sapphire femtosecond laser system Millenia-Tsunami (Spectra-Physics) to visualize UV-excited fluorophores. T-cadherin histochemical staining was performed with HRP labeled secondary antibody (K0679, DAKO LSAB+, Dako) and DAB visualization in combination with Mayer’s Hematoxylin nuclear counterstain.
Electron microscopy.
Isolated islets were fixed in 4% depolymerized paraformaldehyde and 0.2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.3, for 45 min, at 4°C, and processed essentially as previously described in reference 68: Samples were dehydrated in successive alcohol series from 30% to 100% ethanol, at -20°C, infiltrated with a mixture of ethanol: PELCOÆ Acrylic Embedding Resin (18190, Ted Pella International) (2:1) and (1:2), for 30 min each, at -20°C, followed by two changes of pure resin, for 1 h each, and left over night at -20°C. Infiltrated material was transferred to freshly made resin and polymerized at -20°C under a UV source (360 nm, 2 x 6 W black light lamps, Sylvania) placed 10 cm above the specimen. Polymerization was performed in two steps: for 4 d at -20∞C, followed by 7 d at 4∞C. Ultrathin sections, 500–700 ≈ thick, were obtained with an ultramicrotome (Ultracut-E, Reichert-Jung). Sections were mounted on formvar-carbon coated grids and blocked with 2.5% normal goat serum, 1% BSA and 0.002% Glycine in 0.1 M Cacodylate buffer, pH 7.3, for 1 h, at RT. Immunostaining was performed polyclonal rabbit α-T-cadherin antibody (1:20),14 for 2 h, at RT. To control for unspecific binding some samples were instead incubated with normal rabbit serum at the same protein concentration as for primary antibody. Sections were washed and incubated with secondary goat-α-rabbit 18 nm gold conjugates (1:25, 111-215-144, Jackson Immuno). Immunostained sections were contrasted with 2% uranyl acetate in water followed by lead citrate69 and visualized in an electron microscope (H-600A, Hitachi), at 75 kV and different instrumental magnifications.
Generation of fluorescent T-cadherin.
cDNA encoding monomeric red fluorescent protein (mRFP, kind gift from Dr. Tsien22) with the addition of 3xGly linkers on each side and minus the stop codon was inserted into a unique AseI site between cadherin motif 4 and 5 in mouse T-cadherin cDNA (Tcad-mRFP, Fig. 3A). The construct was subcloned into an expression vector under the control of the CMV promoter, or into the pFOX1.4 hIns1 expression vector21 (kind gift from Dr. German, UCSF), that drives expression from the human insulin promoter. Phogrin-EGFP under the control of either the rat insulin promoter or the CMV promoter were kind gifts of Dr. Hutton,70 and Synaptophysin-GFP under the control of the CMV promoter of Dr. Reichardt.28 Proper processing and surface localization of the Tcad-mRFP protein was confirmed by transfecting T-cadherin deficient CHO cells with naÔve T-cadherin or Tcad-mRFP, followed by immunostaining of live cells at 4°C (which prevents labeling of internal structures) with the T-cadherin antibody. Staining was visualized by Alexa488-labeled anti-rabbit antibody, and after fixation, DAPI counterstaining (see above).
Islet isolation.
Islets were isolated by hand-picking after 2.5 mg/ml collagenase P (11249002001, Roche) digestion of the pancreas in Hank’s Balanced Salt Solution (HBSS) essentially as previously described in reference 71 and 72, except that pancreata were perfused with 3 ml ice-cold collagenase solution through the main pancreatic duct73 before digestion at 37°C for 12–15 min at 400 rpm in an orbital shaker. Islets were cultured at 37°C and 5% CO2, free-floating in non-treated plastic bacterial dishes in RPMI 1640 media (11 mM glucose) supplemented with 10% fetal bovine serum, 0.17 mM benzylpenicillin and 0.17 mM streptomycin for 2–5 d before any experimentation. Human islets were isolated at a JDRF islet isolation center and shipped by over-night air. Human islets were cultured as mouse islets, with the exception that the glucose concentration was 5.6 mM.
Live microscopy.
Isolated islets were trypsinized in 0.125% trypsin in Versene (15040-066, Invitrogen) for 4 min at 37∞C with occasional agitation. Cells were immediately rinsed in a large amount of RPMI with serum and then seeded on glass-bottom Petri dishes (P35GC-1.5-10-C, Mat-Tek) coated with HTB9 cellular matrix.74 832/13 insulinoma cells (kind gift of Dr. Newgard24) were seeded on the same type of dishes without HTB9 matrix. Cells were transfected with Tcad-mRFP in combination with Phogrin-EGFP or Synaptophysin-GFP with Lipofectamine 2000 (11668-027, Invitrogen) after seeding. Six hours after transfection the media was changed and the cells left in culture for 48 h before experimentation. Approximately 5% of the islet cells and 25% of the 832/13 cells were expressing both transgenes at this point, as revealed by GFP and mRFP expression. Cells were then observed in RPMI with 1% BSA and the addition of 5.5 or 30 mM glucose at 37°C in a spinning-disc confocal microscope equipped with an Ar/Kr laser and a heat-controlled chamber (Perkin-Elmer). Pictures were taken with an approximate frame rate of 10 Hz and a lag time between red and green fluorescence of 10 msec. Cells were also observed in a conventional point-scanning confocal system with heat- controlled chamber (Bio-Rad Radiance) with simultaneous capture of red and green fluorescence (no lag time) with an approximate frame rate of 1 Hz. Possible non-specific interactions between phogrin-GFP and Tcad-mRFP were ruled out by expressing CMV-promoter-driven Tcad-mRFP and CMV-Phogrin-GFP in L929 fibroblasts. These cells do not contain the insulin secretory machinery, and thus, segregated the two proteins into different subcellular compartments. Tcad-mRFP was observed in the established cell surface distribution in L929 fibroblast cells, while Phogrin-GFP was localized in a diffuse pattern in the cytoplasm (Fig. S4).
Western blotting.
Cells were lysed in either low salt RIPA buffer (50 mM NaCl) or in Brij97 buffer (1% Brij97, 50 mM Tris, 50 mM NaCl) with protease inhibitors (1/100 Protease inhibitor cock-tail, 539131, EMD) in a glass homogenizer with 15 strokes. Cellular debris and nuclei were spun out at 10,000 rpm for 10 min at 4°C in a tabletop centrifuge. All samples were normalized in respect to total protein as assessed by Bradford Reagent (500-0001, BioRad). Proteins were separated on large 8% polyacrylamide gels and blotted. After incubation over night with rabbit-α-T-cadherin and mouse-α-Æ-actin (A3853, Sigma) antibodies, blots were developed with a chemiluminescent reagent (DuraECL, 34075, Thermo Scientific) and exposed to photographic film (Amersham Hyperfilm, 28-9068-35, GE Healthcare).
Islet insulin secretion and content.
Islets were preincubated for 2 h in normal culture media with 1.67 mM glucose. Triplicates of 10 islets were then incubated for 45 min at 37°C and 5% CO2 in 100 μl of RPMI 1640 without glucose supplemented with 1% BSA, the appropriate glucose concentration and 35 mM KCl when applicable. All experiments were paired with the same islets subjected to a base-line incubation in 1.67 mM glucose followed by the stimulatory media. Insulin was analyzed by insulin ELISA (Rat Ultrasensitive, 10-1251-01, Mercodia). As the amount of secreted insulin at baseline varied between batches of isolated islets we compared insulin secretion by dividing secreted insulin at stimulation over secreted insulin at baseline for each batch of 10 islets (Stimulation Index). Insulin content was determined after homogenization of a 10–15 mg sample from the pancreatic tail or 10 islets in water and over-night extraction of insulin in acid ethanol (0.18 M HCl in 95% ethanol) at 4°C. All acid ethanol samples were diluted >100x, as acid ethanol interferes with the ELISA at higher concentrations.
Hyperglycemic and submaximal hyperinsulinemic- euglycemic clamps.
T-cadherin KO and WT mice underwent glucose clamps experiments at 3–4 mo of age to directly assess insulin sensitivity and the pattern of insulin secretion. Surgery was performed under isoflurane anesthesia to implant two catheters into the right jugular vein (Micro-renathane, MRE-025, 0.025” outer diameter, 0.012” inner diameter, Braintree Scientific). Catheters were tunneled subcutaneously, exteriorized at the back of the neck, and filled with heparinized saline. Four days after surgery, the animal was fasted for 5 h and then placed in a restrainer to which it was accustomed. A basal blood sample was collected from the tail tip for immediate glucose determination. For the hyperglycemic clamp the mouse was promptly injected with a low dose of regular insulin (Novolin R; Novo Nordisk 0.2 mU/kg bw, IP) to minimize stress-induced hyperglycemia and maintain Æ-cell insulin content, anesthetized with sevoflurane, and placed on a heating pad to maintain body temperature at 37∞C for the remainder of the experiment. Thirty minutes later at t = 0, a primed infusion of 50% dextrose was begun. Blood samples (5 μl) were taken at 0, 1, 5, 10, 20, 30, 40, 50 and 60 min for the immediate determination of blood glucose (B-Glucose Analyzer; HemoCue). Based on these values, dextrose was variably infused into the jugular catheter to maintain the blood glucose concentration between 16–18 mM for 60 min (Fig. S5). Additional blood samples (60 μl) were collected in heparinized capillary tubes at 0, 1, 5, 20, 40 and 60 min for later determination of plasma insulin levels with ELISA, as described above. The hyperinsulinemic-euglycemic clamps were instead started by infusion of 4 mU/kg/min regular human insulin into one jugular catheter. The insulin infusate was diluted with saline containing 0.1% BSA. Blood samples (5 μl) were drawn at 10 min intervals for the immediate determination of blood glucose. Based on these values, 50% dextrose was variably infused into the other jugular catheter to maintain the plasma glucose concentration at ~8 mM. Steady-state (stable plasma glucose concentration and exogenous glucose infusion rate, Ginf) was generally achieved within 90–120 min, at which time a blood sample (225 μl) was collected. The animals were promptly euthanized after the procedures.
Intra peritoneal glucose tolerance test.
Blood glucose was analyzed from tail vein with an Accucheck (Roche) blood glucose monitor. Conscious mice were fasted over night and then injected i.p. with 3 g/kg BW glucose in 0.9% NaCl solution. Blood glucose was measured before the glucose injection and at 15, 30, 60, 90, 120, 150 and 180 min thereafter.
Statistics.
Samples were compared with Student’s t-test or whenever appropriate, ANOVA followed by Bonferroni post hoc tests for multiple comparisons.
Supplementary Material
Supplementary PDF file supplied by authors.
Supplementary video file supplied by authors.
Acknowledgments
We thank Ms. Xuan-Dao Duong-Polk for assistance with the breeding of the T-cadherin-deficient mice and genotyping, Ulrika Bergstràm for her expert contributions in perfusing the mice for immunohistochemistry, Ergeng Hao and Roxane Pasquier for assistance with collagenase perfusion and islet isolation, Annelie Abrahamsson for help with generating the Tcad-mRFP vector and Anna Monosova for assistance with electron microscopy. This work was supported by grants from the National Institute of Health (HL102680) to B.R.; NIDDK to F.L.; and the Larry L. Hillblom Foundation and NIDDK (DK0889059) to B.T. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
These authors contributed equally to this work.
Previously published online: www.landesbioscience.com/journals/islets/article/17705
References
- 1.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 2.Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol. 2005;17:499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Pentecost M, Otto G, Theriot JA, Amieva MR. Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog. 2006;2:e3. doi: 10.1371/journal.ppat.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- 5.Ciatto C, Bahna F, Zampieri N, VanSteenhouse HC, Katsamba PS, Ahlsen G, et al. T-cadherin structures reveal a novel adhesive binding mechanism. Nat Struct Mol Biol. 2010;17:339–47. doi: 10.1038/nsmb.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–74. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 8.Dahl U, Sjødin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895–902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- 9.Rouiller DG, Cirulli V, Halban PA. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol. 1991;148:233–42. doi: 10.1016/0012-1606(91)90332-W. [DOI] [PubMed] [Google Scholar]
- 10.Rogers GJ, Hodgkin MN, Squires PE. E-cadherin and cell adhesion: a role in architecture and function in the pancreatic islet. Cell Physiol Biochem. 2007;20:987–94. doi: 10.1159/000110459. [DOI] [PubMed] [Google Scholar]
- 11.Johansson JK, Voss U, Kesavan G, Kostetskii I, Wierup N, Radice GL, et al. N-cadherin is dispensable for pancreas development but required for beta-cell granule turnover. Genesis. 2010;48:374–81. doi: 10.1002/dvg.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991;7:391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- 13.Vestal DJ, Ranscht B. Glycosyl phosphatidylinositol--anchored T-cadherin mediates calcium-dependent, homophilic cell adhesion. J Cell Biol. 1992;119:451–61. doi: 10.1083/jcb.119.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res. 2008;68:1407–16. doi: 10.1158/0008-5472.CAN-07-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15:3256–64. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jee SH, Sull JW, Lee JE, Shin C, Park J, Kimm H, et al. Adiponectin concentrations: a genome-wide association study. Am J Hum Genet. 2010;87:545–52. doi: 10.1016/j.ajhg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling H, Waterworth DM, Stirnadel HA, Pollin TI, Barter PJ, Kesäniemi YA, et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity (Silver Spring) 2009;17:737–44. doi: 10.1038/oby.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Li Y, Lange EM, Croteau-Chonka DC, Kuzawa CW, McDade TW, et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet. 2010;19:4955–64. doi: 10.1093/hmg/ddq423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc) 2001;66:1174–86. doi: 10.1023/A:1012445316415. [DOI] [PubMed] [Google Scholar]
- 20.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.German MS, Wang J. The insulin gene contains multiple transcriptional elements that respond to glucose. Mol Cell Biol. 1994;14:4067–75. doi: 10.1128/mcb.14.6.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:7877–82. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 24.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–30. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 25.Wasmeier C, Hutton JC. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem. 1996;271:18161–70. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- 26.Pouli AE, Emmanouilidou E, Zhao C, Wasmeier C, Hutton JC, Rutter GA. Secretory-granule dynamics visualized in vivo with a phogrin-green fluorescent protein chimaera. Biochem J. 1998;333:193–9. doi: 10.1042/bj3330193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffa R, Rindi G, Sessa F, Gini A, Capella C, Jahn R, et al. Synaptophysin immunoreactivity and small clear vesicles in neuroendocrine cells and related tumours. Mol Cell Probes. 1987;1:367–81. doi: 10.1016/0890-8508(87)90018-1. [DOI] [PubMed] [Google Scholar]
- 28.Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–31. doi: 10.1016/S0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippova M, Joshi MB, Kyriakakis E, Pfaff D, Erne P, Resink TJ. A guide and guard: the many faces of T-cadherin. Cell Signal. 2009;21:1035–44. doi: 10.1016/j.cellsig.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 30.Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48:1686–90. doi: 10.2337/diabetes.48.9.1686. [DOI] [PubMed] [Google Scholar]
- 31.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18:451–63. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 32.Varadi A, Tsuboi T, Johnson-Cadwell LI, Allan VJ, Rutter GA. Kinesin I and cytoplasmic dynein orchestrate glucose-stimulated insulin-containing vesicle movements in clonal MIN6 beta-cells. Biochem Biophys Res Commun. 2003;311:272–82. doi: 10.1016/j.bbrc.2003.09.208. [DOI] [PubMed] [Google Scholar]
- 33.Reaven GM, Sageman WS, Swenson RS. Development of insulin resistance in normal dogs following alloxan-induced insulin deficiency. Diabetologia. 1977;13:459–62. doi: 10.1007/BF01234496. [DOI] [PubMed] [Google Scholar]
- 34.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–5. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser N, Leibowitz G, Nesher R. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16:5–22. doi: 10.1515/JPEM.2003.16.1.5. [DOI] [PubMed] [Google Scholar]
- 36.Toomre D, Bewersdorf J. A new wave of cellular imaging. Annu Rev Cell Dev Biol. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]
- 37.Olofsson CS, Göpel SO, Barg S, Galvanovskis J, Ma X, Salehi A, et al. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- 38.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–45. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 39.Varadi A, Ainscow EK, Allan VJ, Rutter GA. Involvement of conventional kinesin in glucose-stimulated secretory granule movements and exocytosis in clonal pancreatic beta-cells. J Cell Sci. 2002;115:4177–89. doi: 10.1242/jcs.00083. [DOI] [PubMed] [Google Scholar]
- 40.Olsen HL, Hoy M, Zhang W, Bertorello AM, Bokvist K, Capito K, et al. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells. Proc Natl Acad Sci U S A. 2003;100:5187–92. doi: 10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang L, He Z, Xu P, Fan J, Betz A, Brose N, et al. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 2006;3:463–8. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki M, Betz A, et al. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55:1421–9. doi: 10.2337/db05-1263. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–46. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mourad NI, Nenquin M, Henquin JC. Metabolic amplification of insulin secretion by glucose is independent of β-cell microtubules. Am J Physiol Cell Physiol. 2011;300:C697–706. doi: 10.1152/ajpcell.00329.2010. [DOI] [PubMed] [Google Scholar]
- 45.Rindler MJ, Xu CF, Gumper I, Cen C, Sonderegger P, Neubert TA. Calsyntenins are secretory granule proteins in anterior pituitary gland and pancreatic islet alpha cells. J Histochem Cytochem. 2008;56:381–8. doi: 10.1369/jhc.7A7351.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V, et al. Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell. 2006;17:3651–63. doi: 10.1091/mbc.E06-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiernet P, Guiot Y, Gilon P, Henquin JC. Glucose acutely decreases pH of secretory granules in mouse pancreatic islets. Mechanisms and influence on insulin secretion. J Biol Chem. 2006;281:22142–51. doi: 10.1074/jbc.M513224200. [DOI] [PubMed] [Google Scholar]
- 48.Sheu L, Pasyk EA, Ji J, Huang X, Gao X, Varoqueaux F, et al. Regulation of insulin exocytosis by Munc13-1. J Biol Chem. 2003;278:27556–63. doi: 10.1074/jbc.M303203200. [DOI] [PubMed] [Google Scholar]
- 49.Philippova M, Ivanov D, Allenspach R, Takuwa Y, Erne P, Resink T. RhoA and Rac mediate endothelial cell polarization and detachment induced by T-cadherin. FASEB J. 2005;19:588–90. doi: 10.1096/fj.04-2430fje. [DOI] [PubMed] [Google Scholar]
- 50.Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, et al. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98:540–55. doi: 10.1172/JCI118822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nevins AK, Thurmond DC. A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J Biol Chem. 2005;280:1944–52. doi: 10.1074/jbc.M409528200. [DOI] [PubMed] [Google Scholar]
- 52.Nevins AK, Thurmond DC. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J Biol Chem. 2006;281:18961–72. doi: 10.1074/jbc.M603604200. [DOI] [PubMed] [Google Scholar]
- 53.Joshi MB, Ivanov D, Philippova M, Erne P, Resink TJ. Integrin-linked kinase is an essential mediator for T-cadherin-dependent signaling via Akt and GSK3beta in endothelial cells. FASEB J. 2007;21:3083–95. doi: 10.1096/fj.06-7723com. [DOI] [PubMed] [Google Scholar]
- 54.Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol. 2003;285:C698–710. doi: 10.1152/ajpcell.00093.2003. [DOI] [PubMed] [Google Scholar]
- 55.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–52. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 57.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–81. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002 doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 59.Yano W, Kubota N, Itoh S, Kubota T, Awazawa M, Moroi M, et al. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr J. 2008;55:515–22. doi: 10.1507/endocrj.K08E-093. [DOI] [PubMed] [Google Scholar]
- 60.Khetani SR, Chen AA, Ranscht B, Bhatia SN. T-cadherin modulates hepatocyte functions in vitro. FASEB J. 2008;22:3768–75. doi: 10.1096/fj.07-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staiger K, Stefan N, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, et al. Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J Clin Endocrinol Metab. 2005;90:6707–13. doi: 10.1210/jc.2005-0467. [DOI] [PubMed] [Google Scholar]
- 62.Winzell MS, Nogueiras R, Dieguez C, Ahrén B. Dual action of adiponectin on insulin secretion in insulin-resistant mice. Biochem Biophys Res Commun. 2004;321:154–60. doi: 10.1016/j.bbrc.2004.06.130. [DOI] [PubMed] [Google Scholar]
- 63.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003;312:1118–22. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 64.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–4. doi: 10.1016/S0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 66.Huang G, Ge G, Wang D, Gopalakrishnan B, Butz DH, Colman RJ, et al. α3(V) collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues. J Clin Invest. 2011;121:769–83. doi: 10.1172/JCI45096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 68.Gounon P. Low-temperature embedding in acrylic resins. In: Hajibagheri MA, Ed. Electron microscopy methods and protocols. Totowa NJ, USA: Humana Press 1999; 117-23. [DOI] [PubMed] [Google Scholar]
- 69.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–12. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bright NA, Walters J, Wasmeier C, Hutton JC. Targeting of a phogrin-green fluorescent protein chimaera to insulin secretory granules of pancreatic beta-cells in transgenic mice. Diabetes Metab. 2002;28:329–36. [PubMed] [Google Scholar]
- 71.Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978;14:397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- 72.Moskalewski S. Isolation and culture of the islets of Langerhans of guinea-pig. Gen Comp Endocrinol. 1965;44:342–53. doi: 10.1016/0016-6480(65)90059-6. [DOI] [PubMed] [Google Scholar]
- 73.Shapiro AM, Hao E, Rajotte RV, Kneteman NM. High yield of rodent islets with intraductal collagenase and stationary digestion--a comparison with standard technique. Cell Transplant. 1996;5:631–8. doi: 10.1016/S0963-6897(96)00084-X. [DOI] [PubMed] [Google Scholar]
- 74.Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS. Growth factor/matrix-induced proliferation of human adult beta-cells. Diabetes. 1995;44:1458–60. doi: 10.2337/diabetes.44.12.1458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.
Supplementary video file supplied by authors.