Abstract
Aims/hypothesis: Islet amyloid polypeptide is originally identified as the chief constituent of amyloid in insulinomas and type 2 diabetic islets. This study aimed to identify islet amyloid polypeptide by immunocytochemical staining in pancreatic endocrine tumors including 30 cases of insulinomas and non-β-cell pancreatic endocrine tumors.
Results: In normal islets, 62% of islet cells and 52% of insulin cells were granularly positive for insulin and IAPP, respectively, with more insulin positive cells than IAPP positive cells and some densely positive staining for insulin and IAPP in irregularly shaped a nuclear, degenerating islet β-cells. In pancreatic endocrine tumors, all 10 insulinomas were positive for islet amyloid polypeptide but 2 glucogonomas, 1 somatostatinoma, 6 of 7 pancreatic polypeptidomas, all 7 gastrinomas and all 3 non-functioning pancreatic endocrine tumors were negative for islet amyloid polypeptide whereas one pancreatic polypeptidoma was positive for islet amyloid polypeptide.
Methods: Using commercially available rabbit anti-islet amyloid polypeptide antibody, immunocytochemical staining was performed on 30 cases of pancreatic endocrine tumors, consisting of 10 insulinomas, 2 glucagonomas, 1 somatostatinoma, 7 pancreatic polypeptidomas, 7 gastrinomas and 3 non-functioning pancreatic endocrine tumors. Pancreatic tissues containing pancreatic endocrine tumors were systematically immunostained for insulin, glucagon, somatostatin, pancreatic polypeptide, gastrin and chromogranin A, in addition to islet amyloid polypeptide. When normal pancreatic tissues adjacent to pancreatic endocrine tumors were present, insulin, glucagon, somatostatin and islet amyloid polypeptide positive cells were counted for a total of 20 islets, which were divided into large islets and medium islets for each case.
Conclusions/Interpretations: All 10 insulinomas and 1 pancreatic polypeptidoma were granularly positive for islet amyloid polypeptide, suggesting all 10 insulinomas contained enough insulin granules for IAPP whereas only one non-β-cell pancreatic endocrine tumor was co-localized with islet amyloid polypeptide in their secretary granules.
Keywords: islet amyloid polypeptide, pancreatic endocrine tumors, pancreatic islets
Introduction
Islet amyloid polypeptide (IAPP) is a 37 amino acid polypeptide that is originally identified as the chief constituent of amyloid present in insulinomas1 and in the islets from type 2 diabetics.2-4 IAPP is co-localized in β-granules and co-secreted into the blood stream with insulin in response to glucose- and amino acid- stimulated insulin secretion.3 In fetal islets, IAPP is co-localized in insulin-cells at 50% frequency whereas glucagon and somatostatin (SRIF) cells were co-localized with IAPP at 1.4 and 2.6%, respectively, by immuofluorescence study.5 By double immunocytochemical staining, Iki and Pour reported 50% of insulin cells positive for IAPP and none of the glucagon cells were co-stained for IAPP in non-diabetic islets; whereas there were some islet cells co-expressing both SRIF and IAPP in atrophic type 2 diabetic islets.6 We had previously immunostained pancreatic endocrine tumors (PETs) consisting of insulinomas, glucagonomas, pancreatic polypetidoma (PPomas), gastrinomas and non-functioning tumors, revealing all insulinomas and at least some non-β-cell tumors weakly positive for IAPP, suggesting that some neoplastic non-β-cell tumors were positive for IAPP although non-β-cell tumors were weaker stained than insulinomas.7 We had initially used polyclonal rabbit antihuman IAPP at 1:200 dilution, resulting in overstaining for IAPP in some islet cells and PET cells7 and this study re-examined using more diluted antibody at 1:400 to 1:800 in order to detect granular cytoplasmic immunopositive staining for secretary granules in normal islet cells and PET cells. In this study, we employed immunocytochemical staining using continuous sections for all four pancreatic hormones and IAPP in addition to double immunostaining for pancreatic hormones and IAPP.
Results
Normal islets.
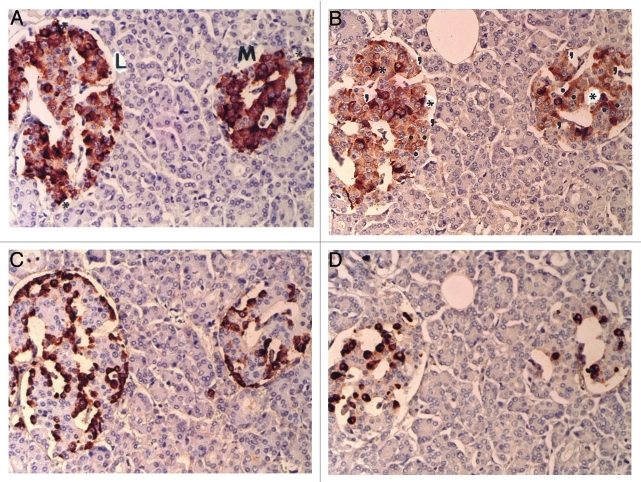
Six normal pancreatic tissues adjacent to the PETs were examined for insulin, glucagon, SRIF and IAPP positive cells. All three pancreatic hormone stainings were granular in the cytoplasm and so was IAPP staining. In continuous sections, cytoplasmic positive staining for both insulin and IAPP was quite variable from moderately to strongly positive whereas non-β-cell cytoplasmic staining was somewhat smaller but uniformly stronger immunostained for the hormones than that of insulin- and IAPP-positive cells (Fig. 1A–D). The periphery of islet was stronger stained for insulin whereas IAPP immunostaining was stronger in mid portion of islet lobule (Fig. 1A and B). Insulin positive staining was 62% in large and medium islets, and was generally stronger stained with granular appearance than that of granular IAPP staining of 35 and 40% in large islets and medium islets, respectively, corresponding to more than half of the insulin secretary granules being positive for IAPP (Fig. 1A and B). Glucagon cells were 26 and 24% and SRIF cells were 10 and 13% in large and medium islets, respectively (Fig. 1C and D and Table 1). Some scattered strongly insulin positive cytoplasm was diffusely and densely positive without granular appearance, representing about 10–15% of the β-cells, as also diffusely stained for IAPP, consistent with positive staining in the degenerated, irregularly twisted β-cell cytoplasm, some of which were without nucleus (Fig. 1A and B). Glucagon cells revealed small, uniformly and strongly stained cytoplasm, located mostly in the periphery of islet and outer islet lobule (Fig. 1C); whereas SRIF cells were located in mid portion of islet lobule adjacent to insulin cells, containing abundant cytoplasm of compatible sizes of insulin cells (Fig. 1D).
Figure 1. Control islets. Insulin cells were the major cells (about 60%) with abundant cytoplasm of variable staining intensity, followed by glucagon cells (about 30%) with small densely immunostained cytoplasm and SRIF cells (about 10%). IAPP-positive cells with abundant cytoplasm accounted for about 35% of total islet cells and about 52% of insulin-positive cells. Both insulin and IAPP immunostaining revealed scattered, dense positive staining in irregular, sickle shaped cytoplasm with lesser staining density for IAPP than that of insulin (*). For counting IAPP-positive cells, granular staining (.) and negative cytoplasmic staining (,) were separately counted. (A) Insulin, (B) IAPP, (C) glucagon and (D) SRIF immunostained. Original magnification x100.
Table1. IAPP immunostaining for pancreatic islets.
| |
Large Islets |
Medium Islets |
||||||||||
|
Total Islet |
% Ins |
% Glu |
% SRIF |
% IAPP |
% IAPP/Ins |
Total Islet |
% Ins |
% Glu |
% SRIF |
% IAPP |
% IAPP/Ins |
|
| |
Insulinomas (n = 3) |
|||||||||||
|
Csse 1. 17/F |
65.2 |
64.8 |
21.8 |
12 |
42.6 |
40 |
24.2 |
66.2 |
22.4 |
11.3 |
36.5 |
55.1 |
|
Case 2. 52/M |
53.6 |
61 |
29.7 |
8.7 |
39 |
72.7 |
22 |
53.2 |
33.2 |
10.5 |
43 |
80.8 |
|
Case 3. 66/F |
65.8 |
55.5 |
32.8 |
11.8 |
42.5 |
76.7 |
25.9 |
66.7 |
21.1 |
12.5 |
41.6 |
62.4 |
| |
Somatostatinoma (n = 1) |
|||||||||||
|
Case 1. 42/F |
58.7 |
75 |
16.3 |
7.3 |
24 |
40.9 |
19 |
71.6 |
15.1 |
14 |
40.4 |
56.4 |
| |
PPoma (n = 1) |
|||||||||||
|
Case 1. 33/M |
64 |
58.2 |
27.8 |
14 |
27 |
42.2 |
19.5 |
59.4 |
23.2 |
17.7 |
41.3 |
69.5 |
| |
Non-functioning tumor (n = 1) |
|||||||||||
|
Case 1. 66/M |
94.5 |
58.2 |
29.5 |
9.7 |
36.7 |
38.8 |
26.9 |
58.4 |
37.4 |
13.8 |
37 |
63.3 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Mean (n = 6) |
67 |
62.1 |
26.3 |
10.6 |
35.3 |
51.9 |
22.9 |
62.6 |
23.7 |
13.3 |
40 |
64.6 |
|
SE |
5.8 |
2.9 |
2.5 |
1 |
3.3 |
7.2 |
1.3 |
2.8 |
2.5 |
1 |
1.1 |
3.9 |
Ins, insulin; Glu, glucagon; SRIF, somatostatin; IAPP, islet amyloid polypeptide.
Pancreatic endocrine tumors.
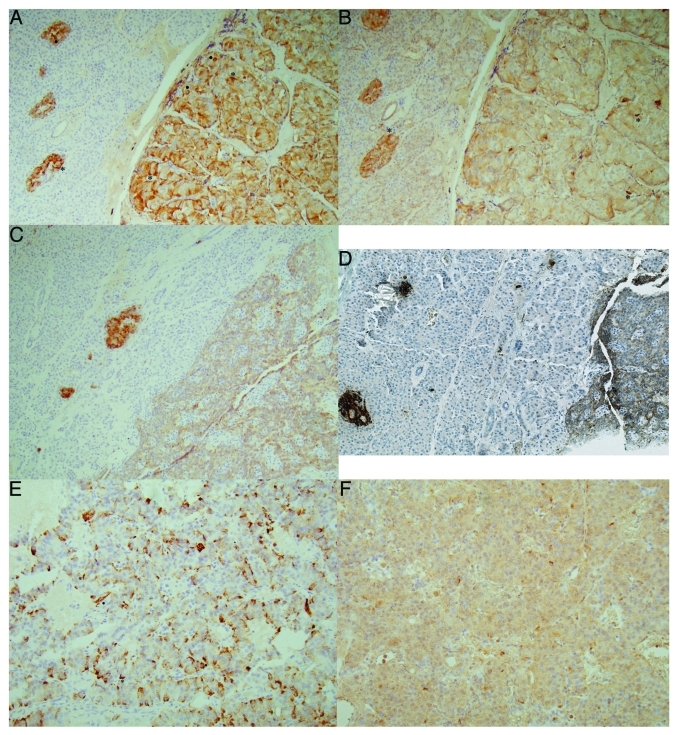
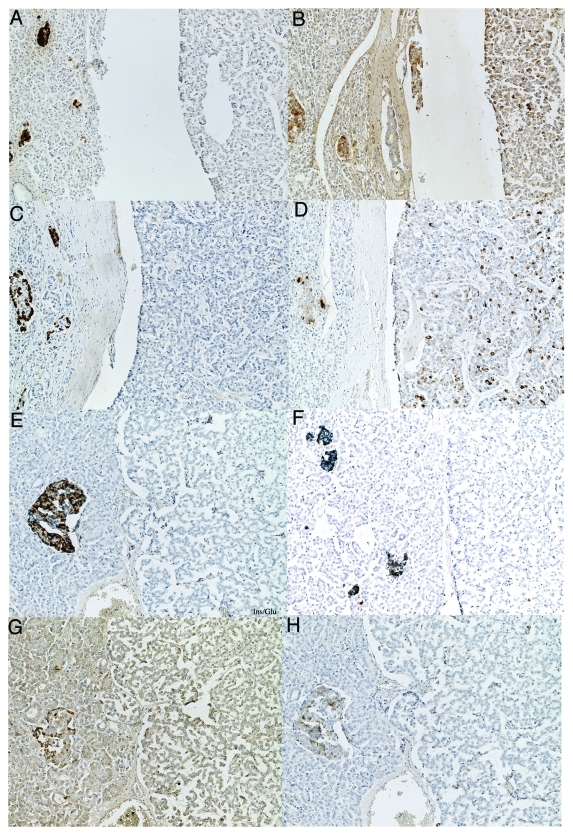
All 10 insulinomas were positive for insulin, which were also positive for IAPP immunostaining with insulin staining stronger than that of IAPP (Fig. 2A–F and Table 2). In the mid portion of insulinoma Case 1, there were scattered nests of irregular, sickle-shaped tumor cells, which were densely positive stained for insulin and IAPP, consistent with degenerating cytoplasmic positive staining in this Case 1 (Fig. 2A and B). In a moderately insulin positive Case 2 (Fig. 2C), IAPP was also moderately positive (Fig. 2D), in which a double immunostaining for insulin and IAPP revealed a few non-β-cells were positive for IAPP only by blue staining in adjacent normal islets and most of insulinoma cells were positive for both insulin (brown) and IAPP (blue) (Fig. 2D). One malignant insulinoma Case 3, which metastasized to the liver, tumor cells were diffusely moderately positive for insulin (Fig. 2E) and were diffusely weakly positive for IAPP (Fig. 2F) with a scattered strongly positive tumor cell cytoplasm for both insulin and IAPP (Fig. 2E and F). Two glucagonomas and one SRIFoma were negative for IAPP (Table 2). One PPoma showed granular moderately positive staining for PP and IAPP (Fig. 3B and D) whereas it was negative for insulin, glucagon (Fig. 3A and C) and SRIF. Six of seven PPomas, all seven gastrinomas and all three non-functioning tumors were negative for IAPP (Table 2), in the latter tumor cells were negative for insulin, IAPP, glucagon and PP by double immunostianing (Fig. 3E, F and H) and were negative for SRIF immunostaining as well (Fig. 3G).
Figure 2. Insulinoma cases. Case 1 (A and B), Case 2 (C and D) and Case 3 (E and F). Case 1, benign insulinoma, revealed diffusely strongly positive staining for insulin (A) and diffusely moderately positive staining for IAPP (B) with scattered dense staining for insulin (.), some of which were dense sickle-shaped cytoplasm, often showing no nucleus (*). Case 2, another benign insulinoma, was moderately positive for insulin (C) and weakly positive for IAPP. A double immunostianing for insulin (brown) and IAPP (blue) revealed the same tumor cells positive for both insulin and IAPP (D). Case 3, malignant insulinoma, revealed diffusely moderately positive staining for insulin (E) and diffusely weak staining for IAPP (F) with some scattered strongly positive cells for insulin (.) only. (A, C and E) Insulin, (B and F) IAPP, (D) a double immunostained for insulin (brown) and IAPP (blue).
Table 2. IAPP immunostaining for pancreatic endocrine tumors.
| Types of PETs |
Total Numbers |
Locations |
IAPP Immunostaining |
|
| Insulinomas |
10 |
Pancreas—9 Liver meta—1 |
Positive—9 Positive—1 |
|
| Glucagonomas |
2 |
Pancreas—1 Liver meta—1 |
|
Negative—1 Negative—1 |
| Somatostatinoma |
1 |
Pancreas—1 |
|
Negative—1 |
| PPomas |
7 |
Pancreas—7 |
Positive—1 |
Negative—6 |
| Gastrinomas |
7 |
Pancreas—5 Duodenum—1 Liver meta—1 |
|
Negative—5 Negative—1 Negative—1 |
| Non-functioning | 3 | Pancreas—3 | Negative—3 | |
Meta, Metastasis
Figure 3. IAPP-positive PPoma (A–D) and IAPP-negative non-functioning tumor (E–H). This PPoma revealed many strongly PP positive cells (D) and scattered moderately IAPP positive cells (B) whereas tumor cells were negative for insulin (A), glucagon (C) and SRIF. This non-functioning tumor was negative for IAPP (F and H) and was also negative for insulin (E and F), glucagon (E), SRIF (G) and PP (H). (A) Insulin, (B) IAPP, (C) glucagon, (D) PP, (E) insulin/glucagon double stained, (F) IAPP/Insulin double stained, (G) SRIF, (H) IAPP/PP double immunostained.
Discussion
IAPP was originally isolated from amyloid stroma of insulinoma1 tissues and interstitial amyloid deposits from type 2 diabetic islets.2-4 There is a general agreement on that β-cell secretary granules contain IAPP and that insulin and IAPP are co-secreted into the blood in glucose- and amino acid-stimulated secretion.3 With fetal pancreas, Portela-Gomez et al. initially reported that about 50% of insulin cells were positive for IAPP,5 but fetal pancreas is different from adult pancreas in that fetal pancreatic islets contain less insulin and IAPP and that fetal islets contain immature islet cells with multi-potential differentiation.8 Rindi et al. reported co-localization of insulin and IAPP in β-cell granules, but no presence of IAPP in non-β-cell granules by immunoelectron microscopy,9 which is less sensitive than light microscopic immunocytoche-mical staining.
Our previous work using IAPP antibody at 1:200 dilution revealed that practically all islet cells were positive for IAPP with more IAPP positive cells than insulin cells.7,10 The current study using more diluted anti-IAPP antibody at 1:400 and 1:800 dilutions, revealed less IAPP positive cells than insulin cells, however, there were definitely more IAPP-negative cells than insulin-negative cells using the higher diluted antibody (Fig. 1A and B). Some IAPP-negative cells corresponded to the location of non-β-cells including glucagon and SRIF cells (Fig. 1B–D).
There were scattered densely positive cells for insulin and IAPP in irregular, sickle-shaped cytoplasm as compared with granular positive staining in the majority of plump and round cytoplasm of morphologically viable β-cells, the former were consistent with degenerating islet β-cells without nucleus in some cells, which appeared to be on the process of apoptosis, and more frequently observed in the islets from both type 1 and type 2 diabetic subjects (non-published data). Similar twisted and densely positive cytoplasm for insulin and IAPP was observed in scattered insulinoma cells (Fig. 2A, B, E and F) but not in one IAPP-positive PPoma (Fig. 3A and D).
Both Portela-Gomez et al. with fetal islets and Iki and Pour with adult islets reported about 50% of insulin cells positive for IAPP,5,6 and our results showed about 15–20% of anuclear insulin cells were strongly and densely positive for IAPP and about 50–60% of islet cells including the majority of insulin cells and a few non-β-cells were at least moderately and granularly positive for IAPP (Table 1 and Fig. 1). This lesser IAPP immunostaining than insulin staining in β islet cells corresponds to a ratio of insulin to IAPP of 20:1 in the serum levels.11
There is a disagreement on immunopositive staining for IAPP in non-β-cells. In fetal islets, Portela-Gomez et al. reported that glucagon and SRIF cells were positive for IAPP at 1.4 and 2.6%, respectively,5 whereas with normal adult islets Iki and Pour reported none of the glucagon cells were positive for IAPP, but in type 2 diabetics, some islets in the atrophic islets were positive for both SRIF and IAPP.6 Our current results also support that at least a few non-β-cells are positive for IAPP at weaker than insulin cells (Fig. 1).
In PETs, two reports in favor for IAPP immunoreactive PETs were reported. Rindi et al. reported IAPP immunostaining on β-cell granules by immunoelectron miscroscopy, whereas 20 of 21 PETs (95%) consisting of 10 insulinomas, 1 glucagonoma, 2 gastrinomas, 1 vipoma and 7 non-functioning tumors were at least partially positive for IAPP by immunocytochemical staining.9 Using the same IAPP antibody as ours, Eissele et al. studied 65 cases of PETs in which 13 of 15 insulinomas (85%), 3 of 21 gastrinomas (14%) and 2 of 29 non-functioning tumors (7%) with a total of 18 of 65 cases (28%) positive for IAPP.12 These studies suggest that neoplastic PETs may present IAPP immunoreactivity as much as non-neoplastic gastropancreatic endocrine cells.9,12
Our current study revealed less IAPP immunostaining in PETs than the reported two studies in reference 9 and 11, and our previous study in reference 7, since only granular positive staining in the cytoplasm was considered as positive excluding weak and diffuse cytoplasmic staining. Thus, the weak non-granular diffuse staining was considered as non-specific cytoplasmic staining also shown by the other several neuroendocrine cell markers.13
Regarding the current IAPP immunostaining in PETs, all 10 insulinomas were positive for IAPP. Two glucagonomoma, 1 SRIFoma, 6 of 7 PPomas, all 7 gastrinomas and all 3 non-functioning PETs were negative for IAPP (Table 2), however, 1 PPoma showed granular positive staining, supporting secretary granules being positive for IAPP in this PPoma (Fig. 3B and D). Our previous report on more IAPP positive PETs appeared to be diffuse staining in the cytoplasm instead of granular staining in the secretary granules and we conclude now that the previous positive PETs were over-stained and/or over-interpreted for less specific diffuse staining, which is less specific than granular staining in the secretary granules.13 Granular staining for pancreatic hormones and IAPP tended to be more heterogenous in distribution with darker staining in one area than the others and even focal granular staining with negative staining in the other cytoplasmic parts of the same cells. In PPomas, not all tumor cells were strongly granularly positive for PP by immunocytochemical staining (Fig. 3D), however, electron microscopy revealed all PPoma cells containing secretary granules from a few dozens to densely packed small granules.14 In the diffuse, non-granular cytoplasmic staining for enzymes including matrix metalloproteinases and tissue inhibitors of metalloproteinases15 and metallothionine16 revealed diffuse cytoplasmic staining without granular appearance.
The disagreement on how many islet cells and PET cells are positive for IAPP depends on the specificity of each IAPP utilized by each investigators. Portela-Gomez et al. used a different antibody from ours,5 and Iki and Pour also used different IAPP antibody from ours,6 who reported about the same immunopositive cells as ours despite using the similar polyclonal antibody. We incubated the sections with primary IAPP antibody overnight at 4°C, which may have achieved stronger IAPP positive staining than the others and Iki and Pour did not disclose the specific incubation condition.6
Our results support a majority of β-cells, about 52–63% of β-cells were positive for IAPP and there were scattered strongly, cytoplasmic positive staining for insulin and IAPP in anuclear, sickle-shaped degenerating β-cells in normal islets and in insulinoma cells, which may well be in the degenerating process leading to apoptosis. These strongly immunostained cytoplasm may be a post-apoptosis marker for degenerating cells as also revealed by a nuclear apoptosis marker by nuclear staining in islet cells and PET cells by cleaved caspase-3 immunostaining previously reported in reference 17. In PETs, all 10 insulinomas were positive for IAPP of variable staining intensity depending on the abundance of insulin secretary granules, and at least one PPoma was positive for IAPP, and perhaps more non-β-cell PETs may well be at least partially or focally positive for IAPP regardless of the pancreatic hormone production and secretion status.
IAPP plays an important role in glucose-homeostasis by suppressing postprandial glucose excursion through suppressing glucagon surge, thus compliments the effects of insulin by regulating the rate of glucose flow to the blood stream.18 IAPP signals the stomach to regulate the rate of gastric emptying and also reduces food intake by centrally stimulating satiety.19 A synthetic IAPP, Pramlintide25 (Pro-h-IAPP with three amino acid replacements for IAPP) was approved by the Federal Drug Administration for treating diabetes and has been used for glycemic control in type 1 and type 2 diabetics, who are treated with insulin for IAPP deficient secretion.19,20
Finally, IAPP is depleted in β-cells from type 1 and type 2 diabetics, whereas amyloid accumulates in perivascular interstitium of type 2 diabetic islets. Freshly prepared intermediate IAPP polymers (25–6,000 IAPP molecules) have a toxic effects on islet β-cells and relocation and misfolding of granular IAPP in islet β-cell cytoplasm to perivascular, amorphous interstitial amyloid deposits in islet without granular appearance consisting of IAPP polymers (>106 IAPP molecules per particle) is a much debated subject as a cause or result for type 2 diabetes.4,20-24 By our immunocytochemical staining, β-cell granules were more densely stained for IAPP than non-granular interstitial amyloid deposits in type 2 diabetic islets, for the latter, pretreatment of the deparaffinized sections with formic acid enhances immunocytochemical staining for amyloid and IAPP.25
Materials and Methods
A total of 30 cases of PETs from the University of Kansas Medical Center, obtained between 1974 and 2001, were included for this study, and were selected among the previously reported cases,7 which were immunostained for IAPP using 1:400 to 1:800 dilution of the antibody, so that both strongly to weakly granular immunostained cells could be identified in both normal islets and PETs. The PETs consisted of 10 insulinomas, 2 glucagonomas, 1 SRIFoma, 7 PPomas, 7 gastrinomas and 3 non-functioning tumors. All tissue specimens were routinely fixed in 10% neutral formalin and embedded in paraffin. For immunocytochemical staining, all deparaffinized sections were treated with 0.1 N citrate buffer (pH 6.2) at 100°C for 10 min using a high pressure cooker (Biocare Medical). For IAPP immunostaining, the sections were initially incubated overnight at 4°C with rabbit antihuman IAPP 1–13 at 1:400 to 1:800 dilution (Peninsula Laboratory). All the continuous tissue sections were systematically immunostained for insulin, glucagon, SRIF, PP, gastrin and CgA. The normal islets adjacent to PETs were calculated for hormone positive cells including, insulin, glucagon and SRIF cells, except PP cells as PP cells represented the smallest percentage of islet cells and their relative percentages were so variable from one case to the other and even from one slide to the others in the same cases. In each normal pancreas, six normal pancreatic tissues containing more than 20 islets were systematically counted for insulin, glucagon and SRIF cells to count the total islet cell numbers by adding all three pancreatic hormone containing cells. Normal islets were divided into large islets containing 67.0 ± 5.8 (40–130) cytoplasms and medium islets containing 22.9 ± 1.3 (15–39) cytoplasms and islet cytoplasms were similarly calculated for pancreatic hormone and IAPP positive cells, respectively, whereas small islets of less than 14 islet cytoplasms were excluded as these small islets tended to show a wide variation of each pancreatic hormone cells and IAPP positive cells as also recognized for a variable cleaved caspase-3 positive islet cells reported previously by us in reference 16. For a double immunostaining, the sections were initially immunostained using diaminobenzidine tetrahydrochloride (Dojindo Molecular Technology) for brown color, then sections were incubated with the second antibody from the different animal source from the first immunostaining, and streptavidin-alkaline phosphatase and alkaline phosphatase substrate kit IV (both from Vector Laboratories) were used for the second immunostaining for blue color as previously reported by us in reference 17.
Acknowledgments
I sincerely thank Dr. Ov Slayden for allowing me to use his research laboratory to perform immunocytochemical staining at the Reproductive Science Division, Oregon National Primate Center, Beaverton, OR. This study was in part supported by ONRRC Core Grant: NIH RR 000163.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/17866
References
- 1.Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–31. doi: 10.1016/0006-291X(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84:8628–32. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–53. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 4.Höppener JW, Ahrén B, Lips CJM. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343:411–9. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 5.Portela-Gomes GM, Johansson H, Olding L, Grimelius L. Co-localization of neuroendocrine hormones in the human fetal pancreas. Eur J Endocrinol. 1999;141:526–33. doi: 10.1530/eje.0.1410526. [DOI] [PubMed] [Google Scholar]
- 6.Iki K, Pour PM. Distribution of pancreatic endocrine cells including IAPP-expressing cells in non-diabetic and type 2 diabetic cases. J Histochem Cytochem. 2007;55:111–8. doi: 10.1369/jhc.6A7024.2006. [DOI] [PubMed] [Google Scholar]
- 7.Tomita T. Amylin in pancreatic islets and pancreatic endocrine neoplasms. Pathol Int. 2003;53:591–5. doi: 10.1046/j.1440-1827.2003.01532.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayek A, Beattie GM. Experimental transplantation of human fetal and adult pancreatic islets. J Clin Endocrinol Metab. 1997;82:2471–5. doi: 10.1210/jc.82.8.2471. [DOI] [PubMed] [Google Scholar]
- 9.Rindi G, Terenghi G, Westermark G, Westermark P, Moscoso G, Polak J. Islet amyloid polypeptide in proliferating pancreatic ®-cells during development. Am J Pathol. 1991;138:1321–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Tomita T. Amylin in human adult pancreatic islets. Pathol Int. 2003;53:591–5. doi: 10.1046/j.1440-1827.2003.01532.x. [DOI] [PubMed] [Google Scholar]
- 11.Fineman M, Weyer C, Maggs DG, Strobel S, Kolterman OG. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–8. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- 12.Eissele R, Neuhaus C, Trautmann ME, Funk A, Arnold R, Höfler H. Immunoreactivity and expression of amylin in gastroenteropancreatic endocrine tumors. Am J Pathol. 1993;143:283–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Bordi C, Pilato FP, D’Adda T. Comparative study of seven neuroendocrine markers in pancreatic endocrine tumours. Virchows Arch A Pathol Anat Histopathol. 1988;413:387–98. doi: 10.1007/BF00716987. [DOI] [PubMed] [Google Scholar]
- 14.Tomita T, Friesen SR, Kimmel JR, Doull V, Pollock HG. Pancreatic polypeptide-secreting islet-cell tumors. A study of three cases. Am J Pathol. 1983;113:134–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita T, Iwata K. Gelatinases and inhibitors of gelatinases in pancreatic islets and islet cell tumors. Mod Pathol. 1997;10:47–54. [PubMed] [Google Scholar]
- 16.Tomita T. Metallothionein in pancreatic endocrine neoplasms. Mod Pathol. 2000;13:389–95. doi: 10.1038/modpathol.3880064. [DOI] [PubMed] [Google Scholar]
- 17.Tomita T. Immunocytochemical localisation of caspase-3 in pancreatic islets from type 2 diabetic subjects. Pathology. 2010;42:432–7. doi: 10.3109/00313025.2010.493863. [DOI] [PubMed] [Google Scholar]
- 18.Kolterman OG, Gottlieb A, Moyses C, Colburn W. Reduction of postprandial hyperglycemia in subjects with IDDM by intravenous infusion of AC137, a human amylin analogue. Diabetes Care. 1995;18:1179–82. doi: 10.2337/diacare.18.8.1179. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MR, Tyagi SC. “A” is for amylin and amyloid in type 2 diabetes mellitus. JOP. 2001;2:124–39. [PubMed] [Google Scholar]
- 20.Buse JB, Weyer C, Maggs DG. Amylin replacement with Pramlintide in type 1 and type 2 diabetes: A physiological approach to overcome barriers with insulin therapy. Clin Diabetes. 2002;20:137–44. doi: 10.2337/diaclin.20.3.137. [DOI] [Google Scholar]
- 21.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–60. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 22.Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta. 2001;1537:179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 23.Marzban L, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exp Gerontol. 2003;38:347–51. doi: 10.1016/S0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 24.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–43. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 25.Kitamoto T, Ogomori K, Tateishi J, Prusiner SB. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987;57:230–6. [PubMed] [Google Scholar]