Abstract
Elucidating mechanisms of cell cycle control in normally quiescent human pancreatic β-cells has the potential to impact regeneration strategies for diabetes. Previously we demonstrated that Id3, a repressor of basic Helix-Loop-Helix (bHLH) proteins, was sufficient to induce cell cycle entry in pancreatic duct cells, which are closely related to β-cells developmentally. We hypothesized that Id3 might similarly induce cell cycle entry in primary human β-cells. To test this directly, adult human β-cells were transduced with adenovirus expressing Id3. Consistent with a replicative response, β-cells exhibited BrdU incorporation. Further, Id3 potently repressed expression of the cyclin dependent kinase inhibitor p57Kip2, a gene which is also silenced in a rare β-cell hyperproliferative disorder in infants. Surprisingly, however, BrdU positive β-cells did not express the proliferation markers Ki67 and pHH3. Instead, BrdU uptake reflected a DNA damage response, as manifested by hydroxyurea incorporation, γH2AX expression and 53BP1 subcellular relocalization. The uncoupling of BrdU uptake from replication raises a cautionary note about interpreting studies relying solely upon BrdU incorporation as evidence of β-cell proliferation. The data also establish that loss of p57Kip2 is not sufficient to induce cell cycle entry in adult β-cells. Moreover, the differential responses to Id3 between duct and β-cells reveal that β-cells possess intrinsic resistance to cell cycle entry not common to all quiescent epithelial cells in the adult human pancreas. The data provide a much needed comparative model for investigating the molecular basis for this resistance in order to develop a strategy for improving replication competence in β-cells.
Keywords: DNA damage, regeneration, replication
Introduction
Diabetes is characterized by insulin insufficiency and results from loss of pancreatic β-cell mass (Type I diabetes),1,2 or both loss of β-cell mass and function (Type II diabetes). The molecular and cellular mechanisms that regulate β-cell mass are complex and developing effective diabetes therapies requires a comprehensive understanding of the external cues and cell intrinsic processes that control β-cell regeneration. Regeneration can occur by self-replication or through transdifferentiation of other pancreatic cells3-7 and there is debate about the relative importance of these processes in preserving β-cell mass at different stages of life.8-15
Human β-cell replication has been observed in vivo under certain conditions such as the proximity of islets to gastrinomas.16 To date, however, a robust method for inducing human β-cell proliferation remains elusive. In adult humans, β-cell replication occurs at a rate which is significantly lower than that observed in rodents.15,17-20 Thus, efforts to induce β-cell replication in rodents have not always been predictive of findings in humans. For example, although both rodents and humans exhibit a dramatic age-related decline in β-cell turnover,9,20,21 β-cell replication can be induced in adult rodents in response to the increased metabolic demands of pregnancy and insulin resistance. In contrast, there is debate about the source of the pregnancy-associated increase in β-cell mass in humans.22-24 Species differences are also relevant to in vitro studies, as rodent β-cells more readily proliferate in response to growth factors and mitogenic stimuli in vitro than do human β-cells.25,26
At the molecular level, many members of the cell cycle machinery are differentially expressed between mouse and human β-cells and could account for differences in replication rate. The cyclin dependent kinase inhibitor (cdki) p57Kip2 is more highly expressed in human than rodent islets. Moreover, a role for p57Kip2 in human β-cell replication is suggested by the association between p57Kip2 silencing and β-cell hyperproliferation in focal ‘persistent hyperinsulinemia and hypoglycemia of infancy’ (PHHI).27 Whether or not p57Kip2 plays a role in adult human β-cell replication however, remains unknown.
In the pancreas, basic helix-loop-helix (bHLH) proteins (e.g., E47) are essential mediators of cell fate specification and cell cycle control.28-33 bHLH proteins form obligate homodimers or heterodimers, which bind to regulatory E-box sequences in the DNA of target genes. A layer of regulatory control comes from the Id family of HLH transcriptional repressors. The four mammalian Id (Id1–4) proteins lack the basic amino acid sequence that mediates binding to DNA.34 Thus, Id proteins act as transcriptional repressors by forming inactive dimers sequestering bHLH proteins. Human β-cells express all four Id proteins.35 Studies with mouse and human cell lines have shown that Id2 influences expansion of pancreatic progenitors36 and expression of Ids in human islets is induced by glucose uptake. In turn, Ids may play a role in regulating insulin transcription and secretion37,38 but little is known regarding the relevance of Id proteins to adult human β-cell replication.
Recently, we determined that Id3 mediates efficient cell cycle entry in quiescent human pancreatic duct cells.39 Due to the fact that duct cells act as β-cell progenitors during development and possibly during regeneration,5 we hypothesized that β-cells might respond similarly to Id3. Additional support for a role for Id3 in β-cell replication came from the fact that E47 upregulates p57Kip2 expression and induces growth arrest in a cell line derived from human fetal islets,40 and that E47 and Ids control p57Kip2 expression in other tissues.41 Thus, we hypothesized that Id3 inhibition of E47 activity would downregulate p57Kip2 and potentially induce cell cycle entry in adult human β-cells. Consistent with this possibility, Id3 dramatically suppressed p57Kip2 expression. In addition, Id3 caused a robust increase in nuclear incorporation of (BrdU) in human β-cells. Surprisingly however, BrdU incorporation was not accompanied by upregulation of the cell cycle markers, Ki67, phospho-CyclinE and pHH3. Furthermore, BrdU was localized to nuclear foci which were also found to express DNA repair proteins. The fact that BrdU uptake reflected DNA damage, not proliferation, highlights the limitation of BrdU incorporation as a true measure of β-cell replication. The resistance to cell cycle entry in β-cells was in stark contrast to our recent findings in duct cells. The context specific responses to Id3 in the human pancreas provide a promising comparative model for understanding and potentially resolving β-cell specific resistance to replicative stimuli.
Results
Id3 represses p57Kip2 expression in human β-cells.
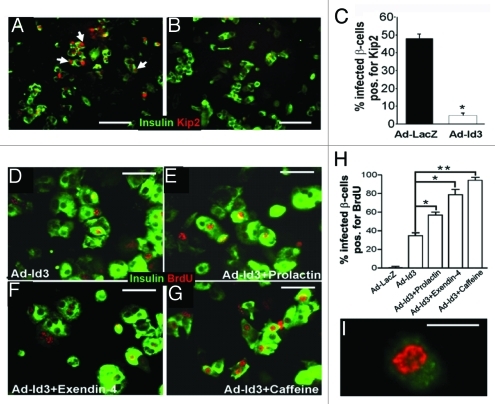
Recently we reported that E47 activated transcription of the cyclin dependent kinase inhibitor (cdki) p57Kip2, inducing cell cycle arrest in a human islet cell line.40 Here, we investigated whether repressing E47 activity would inhibit p57Kip2 in primary adult human β-cells. We chose to downregulate E47 activity by overexpression of the bHLH inhibitor Id3. Islets were cultured as monolayers and infected with either a control adenovirus expressing LacZ (Ad-lacZ) or a virus expressing Id3 (Ad-Id3). Ad-Id3 infection led to efficient expression of Id3 in β-cell cultures (Fig. S1) similar to our recent findings in primary human pancreatic duct cells.39 Forty-eight hours following infection, cells were fixed and p57Kip2 protein expression was determined by immunohistochemistry. Id3 expression resulted in a profound reduction in the number of β-cells expressing p57Kip2 protein, from 48 ± 3% in control infected wells to 4.9 ± 1.4% in Id3 expressing cells (Fig. 1A–C). Moreover, the effect of Id3 on p57Kip2 expression was cell autonomous (Fig. S2).
Figure 1. Id3 mediates cell p57Kip2 downregulation and BrdU incorporation in human β-cells. (A–C) Adult human islets expressing insulin (green) were transduced with Ad-LacZ (A) or Ad-Id3 (B) and analyzed for p57Kip2 (red) expression, quantified in (C) (*p < 0.0005, n = 5). (A) White arrows indicate representative insulin-positive cells expressing p57Kip2. (D–G) Human β-cells (insulin, green) infected with Ad-Id3 and cultured for 48 h in the presence of BrdU alone (D), BrdU+ prolactin (E), BrdU+ exendin-4 (F), or BrdU+ caffeine (G), demonstrate pronounced BrdU incorporation [red, quantified in (H), *p < 0.005, **p < 0.001], in three independent islet cell preparations. Notably, these agents had no effect on BrdU incorporation in the absence of Id3. High power view of a typical nucleus from an Ad-Id3 infected BrdU-positive β-cell demonstrates a punctate, perinuclear pattern of BrdU uptake (I). Blue nuclear counterstain is DAPI. Scale bars, (A–C) = 100 μm, (D–G) = 50 μm, (I) = 10 μm.
Id3 induces BrdU incorporation in human β-cells.
The efficient downregulation of p57Kip2 raised expectations that Id3 also induced β-cell replication. A method commonly used for measuring proliferation in primary β-cells is incorporation of the thymidine analog BrdU into DNA.8,42 Following infection with Ad-Id3 or control virus BrdU was administered to β-cell cultures for 48 h. Consistent with their normally quiescent state, Ad-LacZ-infected cultures exhibited BrdU incorporated in a mere 0.5 ± 0.5% of β-cells. In striking contrast however, BrdU was incorporated into 34.7 ± 2.9% of Id3 expressing β-cells (Figs. 1D, 2B, 3E and F) suggesting that Id3 expressing β-cell were replicating. Surprisingly however, despite BrdU incorporation, no Ki67 or pHH3 expression was observed in β-cells. This BrdU+/Ki67- phenotype was observed in all 17 islet samples tested, independent of donor age (range = 19–62) (Fig. S3). Remarkably, when duct cells and β-cells were isolated from the same donor pancreas, Id3 induced Ki67 expression only in duct cells.39 Thus, the data reveal a fundamental difference in responsiveness to replication stimuli between the two closely related pancreatic epithelial cell populations.
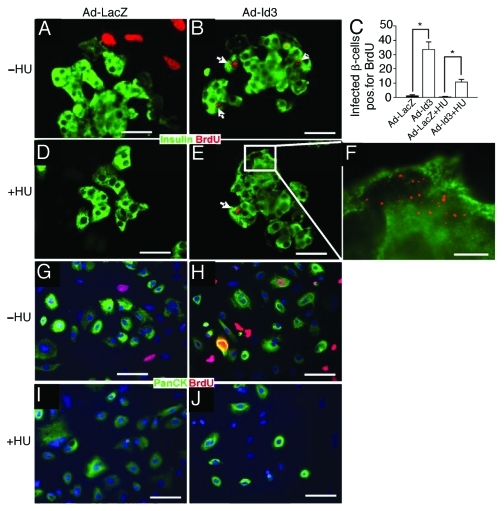
Figure 2. BrdU incorporation proceeds in β-cells, but not exocrine and mesenchymal cells, in the presence of hydroxyurea, a proliferation inhibitor. (A–F) Islet cell cultures (insulin, green) were treated with BrdU (red). BrdU incorporation observed in Id3 infected β-cells (B) was retained in the presence of hydroxyurea (HU) (E and F), suggesting DNA damage, not replication. White arrows depict BrdU positive β-cells and inset (white box) in (E) is magnified in (F), quantified in (C) (*p < 0.005, n = 3) (G–J). In contrast, HU suppressed BrdU incorporation in Ad-Id3 infected exocrine cells (panCK, green) (H) vs. (J) and in mesenchymal cells (insulin and panCK negative, (D) vs. (A), and (G and H vs. I and J), evidence that mesenchymal cells and Id3 infected exocrine cells, are replicating, n = 3. Blue nuclear counterstain is DAPI. Scale bars, (A, B, D and E) = 50 μm, (F) = 10 μm, (G–J) = 100 μm.
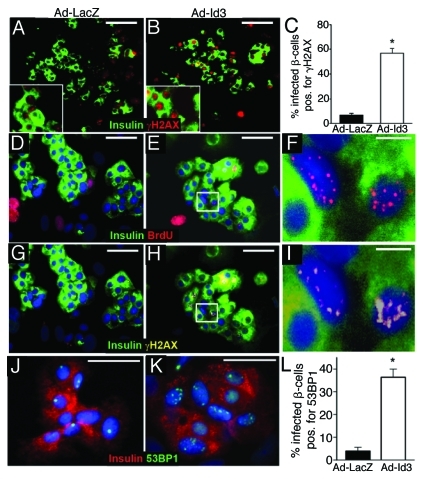
Figure 3. Id3 induces the DNA damage response γH2AX and 53BP1 in human β-cells. Human islets, infected with Ad-LacZ (A, D, G and J) or Ad-Id3 (B, E, H and K) and cultured for 48 h in the presence of BrdU (n = 3), were immunostained for insulin (green) and the DNA damage marker γH2AX (red) in (A and B) and (G–I) (pseudocolored yellow). Greater than five γH2AX foci/nucleus were scored as positive, quantified in (C), *p < 0.0001. Triple immunostaining for insulin (green in D–I), BrdU (red in D–F) and γH2AX (Cy5, pseudocolored yellow in G–I), demonstrated colocalization of BrdU with γH2AX foci in β-cells. (F and I) are magnified from the white boxes in (E and H), respectively. (J–L) Immunostaining for total 53BP1 protein (green) and insulin (red) in control (Ad-LacZ) (J) and Ad-Id3 (K) infected β-cells. More than three foci/nucleus were scored as positive for 53BP1 protein redistribution, quantified in (L), *p < 0.001. Blue nuclear counterstain is DAPI. Scale bars, (A and B) = 100 μm, (D, E, G, H, J and K) = 50 μm, (F and I) = 10 μm.
Id3 induced BrdU incorporation increases in response to prolactin, exendin-4 or caffeine.
It has been reported that human β-cells do not express Ki67 until late in S-phase. We therefore considered the possibility that BrdU positive β-cells entered but did not complete S-phase and that additional replicative stimulus might be necessary to promote cell cycle completion in Id3 expressing β-cells. To test this hypothesis Id3 treated β-cells were exposed to agents which have been reported to induce β-cell replication: prolactin and exendin-4 [an analoe of glucagon-like peptide-1 (GLP-1)].26,43-45 Treatment of Id3-expressing β-cells with either prolactin or exendin-4 had a synergistic effect on BrdU incorporation, with the percentage of BrdU+ β-cells increasing from 34.7 ± 2.9%, to 56.7 ± 3.3% in the presence of prolactin, and to 78.7 ± 5.8% in the presence of exendin-4 (Fig. 1E, F and H). Remarkably, however, expression of Ki67 remained undetectable in prolactin or exendin-4-containing cultures, despite the increase in BrdU content. The data suggest that prolactin and exendin-4 further induced DNA synthesis but did not promote cell cycle progression in Id3 treated β-cells. At the concentrations and stimulation conditions used here, neither prolactin nor exendin-4 induced BrdU uptake or proliferation marker expression in LacZ-expressing β-cells. This is consistent with a report that human islets are significantly less responsive to these agents than that observed in rodent islets.
We considered the possibility that Id3 expressing β-cells were stalled at stress related checkpoints. Caffeine has been shown to circumvent the ATM (ataxia telangiectasia mutated)/ATR (ATM-and Rad3-related) DNA kinase checkpoints in some cells.46 To determine whether caffeine could overcome growth arrest in β-cells, Id3 expressing β-cell cultures were treated with caffeine for 16 h. While caffeine significantly increased the percentage of Id3-expressing β-cells incorporating BrdU, to 94.0 ± 3.1% (Fig. 1G and H), Ki67 was not induced. Thus, caffeine was not sufficient to promote cell cycle completion. No effect of caffeine was observed in Ad-LacZ infected cells.
Id3 activates a DNA repair response in pancreatic β-cells, but not in exocrine or mesenchymal cells.
Upon close inspection it was noted that Ad-Id3 transduced β-cells exhibited a distinctive punctate and perinuclear pattern of BrdU staining, which was qualitatively different from the more homogenous nuclear stain observed in Ad-Id3-expressing ductal cells or in mesenchymal cells (compare Figs. 1D–G, I, 2B, E and F with bright red nuclei in 2A, G and H) and reference 39. This pattern of discrete BrdU foci has been reported to occur in mammalian cells at sites of DNA repair.47 In particular, the perinuclear localization of BrdU is characteristic of repair complexes that localize to DNA double-strand breaks at nuclear pores.48 Thus, we speculated that BrdU incorporation in Id3-expressing β-cells might reflect DNA repair, rather than replication. The two cellular processes can be distinguished by their sensitivity to hydroxyurea (HU), which inhibits ribonucleotide reductase, a critical enzyme for deoxyribonucleotide synthesis. At low doses, HU selectively attenuates DNA replication by depleting the cellular deoxyribonucleotide pool while HU has a less marked effect on DNA repair.49 We examined the effect of 10 mM HU on BrdU incorporation in cultures of Ad-LacZ or Ad-Id3 transduced human pancreatic cells: β-cells, insulin+; exocrine cells, PanCK+, insulin-; mesenchymal cells, PanCK-, insulin-. Consistent with their replicative status, duct and mesenchynal cells exhibited a dramatic reduction in BrdU incorporation in the presence of HU (compare Fig. 2A, G, H vs. D, I, J). In contrast, significant BrdU incorporation was retained in Ad-Id3 infected β-cells following HU treatment, consistent with a DNA damage response (Fig. 2B vs. E and F).
In order to extend the hydroxyurea findings we examined expression of additional markers of DNA damage. A DNA damage response can also be visualized by accumulation of the variant histone protein, H2AX, which is phosphorylated at the γ position (γH2AX) at sites of DNA damage and repair.50,51 Consistent with the HU results, approximately 54.8 ± 3.1% of Id3-infected β-cells also expressed nuclear γH2AX, compared with fewer than 2.6 ± 1.3% of LacZ-infected β-cells (Fig. 3A–C). Moreover, foci of γH2AX and BrdU co-localized within cell nuclei (Fig. 3G–I), as expected if BrdU is incorporated at sites of DNA repair.
A third indicator of a DNA damage response is an increase in foci of phosphorylated 53 binding protein 1 (53BP1). In normal nuclei 53BP1 is diffusely distributed, or is restricted to one or two foci, but in response to DNA damage 53BP1 is phosphorylated and relocalizes to multiple sites of repair.52 As expected, we found uniform expression of 53BP1, or fewer than three foci in the nuclei of LacZ-infected β-cells (Fig. 3J). In contrast, Id3 expression induced a 9.3-fold increase in the percentage of β-cells exhibiting multi-focal distribution of 53BP1, from 3.9 ± 1.8% to 36.4 ± 3.6% (Fig. 3K). Together these results demonstrate that Id3 induces a bona fide DNA damage/repair response in β-cells.
BrdU incorporation is observed in β-cells undergoing DNA repair in vivo.
The in vitro results demonstrated that BrdU incorporation in β-cells is not always indicative of replication but can also reflect DNA damage. In order to determine whether the in vitro findings were relevant to in vivo studies, BrdU was administered in drinking water to 4 week old mice for 3 d. Examination of harvested pancreata revealed that BrdU was incorporated into 11.3 ± 1.2% of β-cells. Immunostaining for γH2AX expression revealed that 6.9 ± 2.0% of BrdU positive β-cells also expressed γH2AX (Fig. 4A–C). Thus, under normal conditions approximately 7% of BrdU positive β-cells exhibited DNA damage in situ.
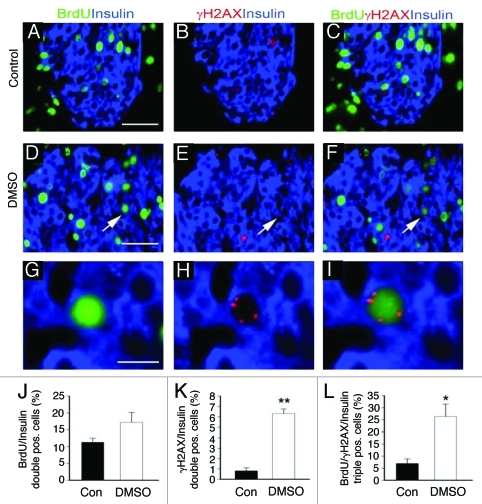
Figure 4. DNA damage in β-cells in vivo. In order to measure DNA synthesis and damage in β-cells in vivo, mice were administered BrdU in drinking water for 3 d. Pancreata were harvested and immunostained for BrdU (green), insulin (blue), and γH2AX (red). Under normal conditions (A–C), approximately 7% of BrdU positive β-cells also exhibited γH2AX (red) expression. In the course of studying small molecules identified in high throughput screens,13 however, we found that intraparenchymal injection of DMSO in the pancreas resulted in expression of γH2AX (red) in over 25% of BrdU positive β-cells (D–F), quantified in (L). Thus, BrdU incorporation can represent DNA damage in a significant percentage of β-cells in vivo as well as in vitro (Fig. 3). Arrows in (D–F) mark region magnified in G–I respectively. *p < 0.01, **p < 0.001. Blue nuclear counterstain is DAPI. Scale bars (A–F) = 50 μm, (G–I) = 10μm.
Recently, we found that a single intraparenchymal injection of DMSO into the murine pancreas significantly increased BrdU incorporation, thus providing a second in vivo model for examining the correlation between BrdU uptake and DNA damage. Following DMSO injection, BrdU was administered for 3 d and pancreata were harvested and immunostained as described above for untreated mice. DMSO increased the proportion of BrdU positive β-cells 1.5-fold, from 11.3 ± 1.2% to 17.2 ± 2.9% (Fig. 4). Remarkably, however, the percentage of BrdU + β-cells expressing H2AX rose 6-fold, to 26.4 ± 5.1% (Fig. 4). The data establish that BrdU incorporation as a measure of β-cell replication is prone to significant error. Moreover, the extent to which BrdU uptake reflects DNA damage differs between in vivo conditions and thus the degree of error cannot easily be anticipated or discounted.
Discussion
In this study, we sought to test the hypothesis that expression of Id3 in adult human β-cells would repress p57Kip2 expression and induce proliferation. The ability of bHLH transcription factors and Id proteins to regulate cell cycle machinery is well documented,53 and is thought to underlie oncogenic transformation of a variety of cell types.54,55 One mechanism by which Ids promote cell cycle entry is through inhibition of bHLH-mediated expression of the cyclin dependent kinase inhibitors (CDKI).56-58 Conversely, Id knockdown is associated in many cell types with increased expression of CDKIs.53,56,59-63 Recently we reported that E47 directly activates p57Kip2 in a human fetal islet cell line.40 In this study Id3 efficiently suppressed p57Kip2. Together the data establish that the E47/Id axis controls p57Kip2 levels in human β-cells, similar to observations in human neuroblastoma cells.41
Our results do not support the hypothesis, however, that loss of p57Kip2 is sufficient to induce proliferation in primary adult human β-cells. In the present study, samples were from donors 19 y and older. Because the association between β-cell hyperplasia (PHHI) and p57Kip2 silencing was reported in patients younger than 12 y of age27 it is possible that β-cell replication in response to p57Kip2 downregulation is age dependent. Age related changes in cell cycle genes have been described in murine β-cells.64 Thus, it is possible that loss of p57Kip2 in neonatal human β-cells would trigger cell cycle entry.
In our hands, the β-cell replication stimuli prolactin, exendin-4, did not induce proliferation of control (LacZ-expressing) β-cells. Although there is abundant evidence that these agents increase in vivo islet growth and β-cell mass, as well as in vitro replication of rodent β-cells and insulinoma cell lines,26,43-45 we are unaware of similar data that unequivocally demonstrates a stimulatory effect on human β-cells in vitro. In support of our finding, the in vitro proliferative responses of highly purified rat and human β-cells have been compared.25 The GLP-1 analog liraglutide induced BrdU incorporation in insulin-positive rat but not human β-cells. Furthermore, the result was confirmed when β-cells were identified by PDX-1 staining: no PDX-1+Ki67+ or PDX-1+ BrdU+ cells were detected.25 This study is consistent with our finding here that prolactin and exendin-4 are themselves insufficient to induce cell cycle entry in quiescent human β-cells in vitro. Interestingly, we saw a different response under the same conditions with Id3-transduced β-cells. Here, prolactin and exendin-4 synergized with Id3 to increase the percentage of β-cells expressing BrdU. Although the exact explanation remains to be determined, we speculate that prolactin and exendin-4 provide a replication stimulus, but alone it is insufficient to induce cell cycle entry. However, in combination with Id3, these agents are able to increase the proportion of cells attempting DNA synthesis, and subsequently undergoing DNA repair. A similar result was observed following caffeine administration. In caffeine- containing cultures, nearly 100% of insulin + cells were also BrdU-positive, which is consistent with all cells having circumvented the G1/S checkpoint, only to encounter a downstream block that prevents progression through S phase.
Although we observed BrdU incorporation in β-cells in response to Id3, it did not reflect complete cell cycle progression, as the cells lacked expression of the proliferation markers Ki67, pCyclinE or pHH3. Moreover, the BrdU positive cells exhibited evidence of a DNA damage response. Therefore, the data raise a serious concern about relying solely on BrdU uptake as evidence of β-cell replication because BrdU incorporation can signify either DNA repair or replication. Importantly, the same limitation in interpretation applies to other methods which rely on incorporation of an analog into DNA (e.g., tritiated thymidine or fluorogenic deoxyuridine).65
A recent study of replication marker expression in human β-cells demonstrated that co-expression of two or more markers more accurately discriminates between quiescent and replicating β-cells.66 Expression of Ki67 is used routinely as a proliferation marker in immunohistochemical studies. However, in β-cells, Ki67 is expressed at very low levels through G1 and early in S phase and its expression peaks during late S/G2.66 Therefore, β-cells which have begun replication but stalled in early S might not be expected to express Ki67. Interestingly, PCNA is also a commonly used proliferation marker,66-70 but its use as a β-cell replication marker has been suggested to overestimate the true replication frequency.70 PCNA is involved in excision-repair,71 and it is tempting to speculate that at least some PCNA staining may also be due to a stimulus-induced DNA repair response, and not replication.
The punctate BrdU staining pattern was an early indicator that replication was not completed in Id3-expressing β-cells because this pattern was distinct from the more homogenous nuclear staining seen in replicating ductal cells (Figs. 2A, 3D and E) and reference 39. Moreover, upon close inspection BrdU staining colocalized with DNA repair enzymes. BrdU incorporation that occurs during DNA repair is indicative of double strand breaks and collapsed replication forks.47 The DNA damage response is initiated by recruiting repair enzymes, and shunting of damaged DNA to nuclear pores.48 In undamaged nuclei 53BP1 is diffusely distributed, or is restricted to one or two foci, but relocalizes once phosphorylated. Similarly, histone H2AX is rapidly phosphorylated52 in response to S phase DNA double-strand breaks.51,72 Thus, our observation that γH2AX colocalized with foci containing BrdU and that 53BP1 relocalized in Id3-expressing β-cells is entirely consistent with a repair response. Further evidence for a repair response in Id3 expressing β-cells came from hydroxyurea treatment (HU) which preferentially inhibits BrdU incorporation in replicating cells. Mesenchymal and ductal cells exhibited extreme sensitivity to HU, consistent with DNA replication. In contrast, HU treated β-cells retained BrdU uptake in a large proportion of cells.49
Our demonstration that Id3 expressing β-cells failed to enter the cell cycle was unexpected in the light of our finding that Id3 induces robust cell cycle entry of pancreatic ductal cells.39 Ductal and β-cells are closely related developmentally; ducts give rise to neogenic β-cells during pancreas embryogenesis,73 and we have shown that the process can be recapitulated in the adult human pancreas.5 Therefore, the data appear to reinforce the hypothesis that as β-cells age they lose cell cycle machinery or substrates essential for replication. Consistent with this theory, we recently found that the mitosis protein CENP-A declined with age in humans β-cells, while CENP-A levels remained constant in exocrine cells.74 Further, rodent β-cells did not lose CENP-A to the extent seen in human cells. Such findings may shed light on the context dependent effects of Id3 on β-cells compared with other pancreatic epithelial cell types and between species. The ability of Id3 to induce cell cycle entry and progression in duct cells also served as an important control by ensuring that the Id3 expression levels and conditions employed in these studies were not generally toxic to primary human pancreatic epithelial cells.
A recent study has shown that human islets contain a number of key regulatory molecules necessary for G1/S transition, including cyclins and cyclin-dependent kinases (cdks).35 Several of these proteins are also differentially expressed between human and rodent β-cells.35,75 Murine β-cells express abundant cdk-4 and cyclin D2, and genetic mouse models have shown them to be critical for β-cell proliferation and diabetes development.76-79 In contrast, human β-cells contain high levels of the analogous enzyme cdk-6, and cyclins D1 and D3 are thought to play a role. Recently, it was reported that overexpression of cdk-6 and cyclin D1 in isolated human β-cells in vitro induced BrdU incorporation and Ki67 expression.35,80 It will be important to determine whether there is concomitant cell cycle completion and an increase in β-cell number as suggested by transplantation studies.
In this study, we found that expression of Id3 in adult human β-cells repressed p57Kip2 expression but did not induce proliferation. Importantly, Id3 mediated efficient cell cycle progression in closely related duct cells thus ruling out adenoviral or Id3 induced general cellular toxicity. The BrdU incorporation and DNA damage markers in β-cells are consistent with a model in which the cells enter S phase in response to Id3, but undergo replication fork stalling due to either an intrinsic limitation in cell cycle machinery or to an abundance of inhibitory factors. Importantly, such questions can be addressed by comparing expression profiles in β-cells vs. ductal epithelial cells. Such studies should lead to a rational strategy for increasing replication competence in adult human β-cells.
Methods
Animals.
Four week old male ICR mice were purchased from Harlan Sprague Dawley, Inc. BrdU water (1 mg/ml, Sigma) administrated for 3 d. In some cohorts of mice 100 ul DMSO (Sigma) was injected a single time intraparenchymally. After 3 d, pancreata were harvested for histology. The study was approved by the animal facility in the Sanford-Burnham Medical Research Institute.
Cell culture.
Primary adult human islets preparations were obtained from the NIH Islet Cell Resources-Administrative and Bioinformatics Coordinating Center (ICR-ABCC) and Dr. James Shapiro in Canada. Cells were cultured in 5.5 mM glucose RPMI 1640/10% FBS/1% Pen/Strep (Gibco) on HTB9 matrix plates in 5% CO2 incubator to form monolayer.
Id3 and LacZ adenovirus infection.
Adeno-Id3 a kind gift from Dr. Colleen McNamara.81 Islet monolayer cells infected with Ad-Id3 or LacZ (MOI 100). After 16 h, viruses were washed and cultured 48 h prior to analysis.
Chemical agents.
Exendin 4 (20 nM, Sigma), prolactin (50 ng/ml, Sigma), hydroxyurea (HU, 10 mM, Sigma) were added to cell cultures upon wash step 16 h after virus addition and incubated 48 h. Caffeine (10 mM, Sigma) was added to cells for 16 h prior to harvest. BrdU (1:1,000, GE) was added to cell media for 48 h after removing virus.
Histology and immunohistochemistry.
Tissues from mice were fixed in 4% paraformaldehyde (USB) for 16–18 h at 4∞C and washed and embedded in OCT freezing media (Sakura Finetek). Samples were sectioned to a mean thickness of 5 ∝m. Cultured cells were fixed in 4% paraformaldehyde for 15 min at 4∞C. For immunostaining, cells were permeabilzed with 0.3% Triton X-100 in PBS for 15 min and blocked for 1 h at room temperature. The samples were incubated with the following primary antibodies overnight at 4∞C: Id3 (Santa Cruz), Insulin (Santa Cruz, US bio), CK19 (DAKO), PanCK (DAKO), Ki67 (Abcam and DAKO), BrdU (GE), phospho-Histon H3 (pHH3, Cell Signaling), γH2AX (Cell Signaling), Kip2 (Diagnostic Biosystems), phospho-CyclinE, 53BP1 (Cell Signaling). Positive and negative controls for each antibody were run in all experiments. For fluorescent imaging, samples were incubated with Alexa 488 (Molecular Probes), Rhodamine or Cy5 (Jackson Immuno Research) fluor-labeled anti-mouse/rabbit/guinea pig and nuclear counterstained with DAPI (Molecular Probes). Digital images of stained sections were captured using a fluorescence microscope with a digital camera (Nikon, Tokyo, Japan). Brightfield and fluorescently labeled sections were analyzed with a conventional inverted microscope (Olympus, PlanFl 40x/0.60) or with a confocal microscope (Bio-Rad Laboratories Inc.) equipped with krypton/argon laser.
Statistical analyses.
Data are presented as means ± SEM. The statistical significance of the differences between groups was analyzed by Student’s t-test.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgements
We thank Dr. Colleen McNamara for Adeno-Id3 and Adeno-lacZ viruses. We thank Tatsuya Kin and Dr. James Shapiro at the University of Alberta, for their invaluable gift of human pancreatic cells. We thank Dr. Rati Fotedar for helpful discussion. We thank Li Huang for technical assistance. This study was funded by an Academic Senate Award from UCSD (P.I.A.), DERC Award from UCSD/UCLA (P.I.A.), CIRM (S.H.L.) and the JDRF Regeneration Program (F.L. and P.I.A.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
S.H.L. performed experiments and analysis. E.H. performed animal surgeries. P.I.A. and F.L. designed experiments. F.L., S.H.L. and P.I.A. wrote the manuscript. P.I.A. provided overall direction, and supervised project planning and execution.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/17923
References
- 1.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 2.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–8. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 3.Levine F, Itkin-Ansari P. beta-cell Regeneration: neogenesis, replication or both? J Mol Med (Berl) 2008;86:247–58. doi: 10.1007/s00109-007-0259-1. [DOI] [PubMed] [Google Scholar]
- 4.Demeterco C, Hao E, Lee SH, Itkin-Ansari P, Levine F. Adult human beta-cell neogenesis? Diabetes Obes Metab. 2009;11(Suppl 4):46–53. doi: 10.1111/j.1463-1326.2009.01105.x. [DOI] [PubMed] [Google Scholar]
- 5.Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, et al. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12:310–6. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 6.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–54. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28:1630–8. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 8.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 9.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–67. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 10.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–26. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–94. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–8. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation. 2009;87:983–91. doi: 10.1097/TP.0b013e31819c86ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC. Increased islet beta cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia. 2006;49:2689–96. doi: 10.1007/s00125-006-0410-5. [DOI] [PubMed] [Google Scholar]
- 17.Tyrberg B, Anachkov KA, Dib SA, Wang-Rodriguez J, Yoon KH, Levine F. Islet expression of the DNA repair enzyme 8-oxoguanosine DNA glycosylase (Ogg1) in human type 2 diabetes. BMC Endocr Disord. 2002;2:2. doi: 10.1186/1472-6823-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menge BA, Tannapfel A, Belyaev O, Drescher R, Müller C, Uhl W, et al. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes. 2008;57:142–9. doi: 10.2337/db07-1294. [DOI] [PubMed] [Google Scholar]
- 19.Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–30. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- 20.Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95:E234–9. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–72. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genevay M, Pontes H, Meda P. Beta cell adaptation in pregnancy: a major difference between humans and rodents? Diabetologia. 2010;53:2089–92. doi: 10.1007/s00125-010-1848-z. [DOI] [PubMed] [Google Scholar]
- 23.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–76. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21:151–8. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen JH, Galsgaard ED, Møldrup A, Friedrichsen BN, Billestrup N, Hansen JA, et al. Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001;50(Suppl 1):S25–9. doi: 10.2337/diabetes.50.2007.S25. [DOI] [PubMed] [Google Scholar]
- 27.Kassem SA, Ariel I, Thornton PS, Hussain K, Smith V, Lindley KJ, et al. p57(KIP2) expression in normal islet cells and in hyperinsulinism of infancy. Diabetes. 2001;50:2763–9. doi: 10.2337/diabetes.50.12.2763. [DOI] [PubMed] [Google Scholar]
- 28.Itkin-Ansari P, Bain G, Beattie GM, Murre C, Hayek A, Levine F. E2A gene products are not required for insulin gene expression. Endocrinology. 1996;137:3540–3. doi: 10.1210/en.137.8.3540. [DOI] [PubMed] [Google Scholar]
- 29.Itkin-Ansari P, Marcora E, Geron I, Tyrberg B, Demeterco C, Hao E, et al. NeuroD1 in the endocrine pancreas: localization and dual function as an activator and repressor. Dev Dyn. 2005;233:946–53. doi: 10.1002/dvdy.20443. [DOI] [PubMed] [Google Scholar]
- 30.Ball AJ, Abrahamsson AE, Tyrberg B, Itkin-Ansari P, Levine F. HES6 reverses nuclear reprogramming of insulin-producing cells following cell fusion. Biochem Biophys Res Commun. 2007;355:331–7. doi: 10.1016/j.bbrc.2007.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demeterco C, Itkin-Ansari P, Tyrberg B, Ford LP, Jarvis RA, Levine F. c-Myc controls proliferation versus differentiation in human pancreatic endocrine cells. J Clin Endocrinol Metab. 2002;87:3475–85. doi: 10.1210/jc.87.7.3475. [DOI] [PubMed] [Google Scholar]
- 32.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–11. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Yan J, Anderson DA, Xu Y, Kanal MC, Cao Z, et al. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol. 2010;339:26–37. doi: 10.1016/j.ydbio.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-Y. [DOI] [PubMed] [Google Scholar]
- 35.Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58:882–93. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua H, Zhang YQ, Dabernat S, Kritzik M, Dietz D, Sterling L, et al. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem. 2006;281:13574–80. doi: 10.1074/jbc.M600526200. [DOI] [PubMed] [Google Scholar]
- 37.Cordle SR, Whelan J, Henderson E, Masuoka H, Weil PA, Stein R. Insulin gene expression in nonexpressing cells appears to be regulated by multiple distinct negative-acting control elements. Mol Cell Biol. 1991;11:2881–6. doi: 10.1128/mcb.11.5.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wice BM, Bernal-Mizrachi E, Permutt MA. Glucose and other insulin secretagogues induce, rather than inhibit, expression of Id-1 and Id-3 in pancreatic islet beta cells. Diabetologia. 2001;44:453–63. doi: 10.1007/s001250051643. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Hao E, Kiselyuk A, Shapiro J, Shields DJ, Lowy A, et al. The Id3/E47 axis mediates cell-cycle control in human pancreatic ducts and adenocarcinoma. Mol Cancer Res. 2011;9:782–90. doi: 10.1158/1541-7786.MCR-10-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiselyuk A, Farber-Katz S, Cohen T, Lee SH, Geron I, Azimi B, et al. Phenothiazine neuroleptics signal to the human insulin promoter as revealed by a novel high-throughput screen. J Biomol Screen. 2010;15:663–70. doi: 10.1177/1087057110372257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothschild G, Zhao X, Iavarone A, Lasorella A. E Proteins and Id2 converge on p57Kip2 to regulate cell cycle in neural cells. Mol Cell Biol. 2006;26:4351–61. doi: 10.1128/MCB.01743-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung HS, Ahn YR, Oh SH, Kim YS, No H, Lee MK, et al. Enhancement of beta-cell regeneration by islet transplantation after partial pancreatectomy in mice. Transplantation. 2009;88:354–9. doi: 10.1097/TP.0b013e3181b07a02. [DOI] [PubMed] [Google Scholar]
- 43.Brelje TC, Parsons JA, Sorenson RL. Regulation of islet beta-cell proliferation by prolactin in rat islets. Diabetes. 1994;43:263–73. doi: 10.2337/diabetes.43.2.263. [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 45.Movassat J, Beattie GM, Lopez AD, Hayek A. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002;87:4775–81. doi: 10.1210/jc.2002-020137. [DOI] [PubMed] [Google Scholar]
- 46.Cortez D. Caffeine inhibits checkpoint responses without inhibiting the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases. J Biol Chem. 2003;278:37139–45. doi: 10.1074/jbc.M307088200. [DOI] [PubMed] [Google Scholar]
- 47.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Nagai S, Dubrana K, Tsai-Pflugfelder M, Davidson MB, Roberts TM, Brown GW, et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science. 2008;322:597–602. doi: 10.1126/science.1162790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis AA, Blevins RD, Carrier WL, Smith DP, Regan JD. Inhibition of DNA repair in ultraviolet-irradiated human cells by hydroxyurea. Biochim Biophys Acta. 1979;563:385–92. doi: 10.1016/0005-2787(79)90057-1. [DOI] [PubMed] [Google Scholar]
- 50.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–62. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 51.Liu JS, Kuo SR, Melendy T. Comparison of checkpoint responses triggered by DNA polymerase inhibition versus DNA damaging agents. Mutat Res. 2003;532:215–26. doi: 10.1016/j.mrfmmm.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Anderson L, Henderson C, Adachi Y. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol Cell Biol. 2001;21:1719–29. doi: 10.1128/MCB.21.5.1719-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zebedee Z, Hara E. Id proteins in cell cycle control and cellular senescence. Oncogene. 2001;20:8317–25. doi: 10.1038/sj.onc.1205092. [DOI] [PubMed] [Google Scholar]
- 54.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–30. doi: 10.1016/S1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 55.Fong S, Debs RJ, Desprez PY. Id genes and proteins as promising targets in cancer therapy. Trends Mol Med. 2004;10:387–92. doi: 10.1016/j.molmed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Yokota Y, Mori S. Role of Id family proteins in growth control. J Cell Physiol. 2002;190:21–8. doi: 10.1002/jcp.10042. [DOI] [PubMed] [Google Scholar]
- 57.Prabhu S, Ignatova A, Park ST, Sun XH. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol Cell Biol. 1997;17:5888–96. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagliuca A, Gallo P, De Luca P, Lania L. Class A helix-loop-helix proteins are positive regulators of several cyclin-dependent kinase inhibitors’ promoter activity and negatively affect cell growth. Cancer Res. 2000;60:1376–82. [PubMed] [Google Scholar]
- 59.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–7. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 60.Mori S, Nishikawa SI, Yokota Y. Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO J. 2000;19:5772–81. doi: 10.1093/emboj/19.21.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–70. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 62.Ouyang XS, Wang X, Ling MT, Wong HL, Tsao SW, Wong YC. Id-1 stimulates serum independent prostate cancer cell proliferation through inactivation of p16(INK4a)/pRB pathway. Carcinogenesis. 2002;23:721–5. doi: 10.1093/carcin/23.5.721. [DOI] [PubMed] [Google Scholar]
- 63.Chassot AA, Turchi L, Virolle T, Fitsialos G, Batoz M, Deckert M, et al. Id3 is a novel regulator of p27kip1 mRNA in early G1 phase and is required for cell-cycle progression. Oncogene. 2007;26:5772–83. doi: 10.1038/sj.onc.1210386. [DOI] [PubMed] [Google Scholar]
- 64.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–7. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 65.Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A. Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. Biotechniques. 2008;44:927–9. doi: 10.2144/000112812. [DOI] [PubMed] [Google Scholar]
- 66.Köhler CU, Kreuter A, Rozynkowski MC, Rahmel T, Uhl W, Tannapfel A, et al. Validation of different replication markers for the detection of beta-cell proliferation in human pancreatic tissue. Regul Pept. 2010;162:115–21. doi: 10.1016/j.regpep.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986;166:209–19. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- 68.Bruno S, Crissman HA, Bauer KD, Darzynkiewicz Z. Changes in cell nuclei during S phase: progressive chromatin condensation and altered expression of the proliferation-associated nuclear proteins Ki-67, cyclin (PCNA), p105, and p34. Exp Cell Res. 1991;196:99–106. doi: 10.1016/0014-4827(91)90460-C. [DOI] [PubMed] [Google Scholar]
- 69.Tsurusawa M, Ito M, Zha Z, Kawai S, Takasaki Y, Fujimoto T. Cell-cycle-associated expressions of proliferating cell nuclear antigen and Ki-67 reactive antigen of bone marrow blast cells in childhood acute leukemia. Leukemia. 1992;6:669–74. [PubMed] [Google Scholar]
- 70.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–5. doi: 10.1210/en.141.12.4600. [DOI] [PubMed] [Google Scholar]
- 71.Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, et al. Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol. 2005;25:9350–9. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ward IM, Wu X, Chen J. Threonine 68 of Chk2 is phosphorylated at sites of DNA strand breaks. J Biol Chem. 2001;276:47755–8. doi: 10.1074/jbc.C100587200. [DOI] [PubMed] [Google Scholar]
- 73.Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–67. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- 74.Lee SH, Itkin-Ansari P, Levine F. CENP-A, a protein required for chromosome segregation in mitosis, declines with age in islet but not exocrine cells. Aging (Albany NY) 2010;2:785–90. doi: 10.18632/aging.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cozar-Castellano I, Haught M, Stewart AF. The cell cycle inhibitory protein p21cip is not essential for maintaining beta-cell cycle arrest or beta-cell function in vivo. Diabetes. 2006;55:3271–8. doi: 10.2337/db06-0627. [DOI] [PubMed] [Google Scholar]
- 76.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 77.Martín J, Hunt SL, Dubus P, Sotillo R, Néhmé-Pélluard F, Magnuson MA, et al. Genetic rescue of Cdk4 null mice restores pancreatic beta-cell proliferation but not homeostatic cell number. Oncogene. 2003;22:5261–9. doi: 10.1038/sj.onc.1206506. [DOI] [PubMed] [Google Scholar]
- 78.Marzo N, Mora C, Fabregat ME, Martín J, Usac EF, Franco C, et al. Pancreatic islets from cyclin-dependent kinase 4/R24C (Cdk4) knockin mice have significantly increased beta cell mass and are physiologically functional, indicating that Cdk4 is a potential target for pancreatic beta cell mass regeneration in Type 1 diabetes. Diabetologia. 2004;47:686–94. doi: 10.1007/s00125-004-1372-0. [DOI] [PubMed] [Google Scholar]
- 79.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, et al. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol. 2005;25:3752–62. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, et al. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes. 2010;59:1926–36. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumura ME, Li F, Berthoux L, Wei B, Lobe DR, Jeon C, et al. Vascular injury induces posttranscriptional regulation of the Id3 gene: cloning of a novel Id3 isoform expressed during vascular lesion formation in rat and human atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:752–8. doi: 10.1161/01.ATV.21.5.752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.