Abstract
The circadian clock has been shown to regulate metabolic homeostasis. Mice with a deletion of Bmal1, a key component of the core molecular clock, develop hyperglycemia and hypoinsulinemia suggesting β-cell dysfunction. However, the underlying mechanisms are not fully known. In this study, we investigated the mechanisms underlying the regulation of β-cell function by Bmal1. We studied β-cell function in global Bmal1-/- mice, in vivo and in isolated islets ex vivo, as well as in rat insulinoma cell lines with shRNA-mediated Bmal1 knockdown. Global Bmal1-/- mice develop diabetes secondary to a significant impairment in glucose-stimulated insulin secretion (GSIS). There is a blunting of GSIS in both isolated Bmal1-/- islets and in Bmal1 knockdown cells, as compared with controls, suggesting that this is secondary to a loss of cell-autonomous effect of Bmal1. In contrast to previous studies, in these Bmal1-/- mice on a C57Bl/6 background, the loss of stimulated insulin secretion, interestingly, is with glucose but not to other depolarizing secretagogues, suggesting that events downstream of membrane depolarization are largely normal in Bmal1-/- islets. This defect in GSIS occurs as a result of increased mitochondrial uncoupling with consequent impairment of glucose-induced mitochondrial potential generation and ATP synthesis, due to an upregulation of Ucp2. Inhibition of Ucp2 in isolated islets leads to a rescue of the glucose-induced ATP production and insulin secretion in Bmal1-/- islets. Thus, Bmal1 regulates mitochondrial energy metabolism to maintain normal GSIS and its disruption leads to diabetes due to a loss of GSIS.
Keywords: Bmal1, circadian clock, diabetes, insulin secretion, mitochondria, β-cells
Introduction
The evolutionarily conserved circadian clock, in mammals, is composed of a web of cell-autonomous and self-sustained oscillators that reside in the hypothalamic suprachiasmatic nucleus (central clock) and also in every cell of peripheral tissues (peripheral clocks), including the pancreatic islets.1-6 These coordinately serve to allow the organism to anticipate environmental changes and adapt its metabolism and behavior to attain a survival advantage.7 The circadian clock is composed of transcriptional and translational feedback loops that sustain ~24 h rhythm. This molecular clock is driven by Bmal1 and Clock (or their homologs), which heterodimerize and activate (positive limb) the transcription of Per1, Per2, Cry1 and Cry2 that form a complex to inhibit (negative limb) the transactivation by Bmal1/Clock, thus completing the feedback loop.7 The central and peripheral clocks regulate various behavioral and metabolic activities. This regulation has been convincingly demonstrated in mouse models, with deletion of genes driving the molecular clock, which display significant impairments in glucose and lipid metabolism.8,9
Bmal1, a bHLH-PAS domain containing transcription factor, is a non-redundant component of the positive limb of the core molecular clock. Disruption of Bmal1 leads to complete arrhythmia in the absence of light entrainment.10 In addition, Bmal1 deleted mice (Bmal1-/-) have metabolic perturbations, including hyperglycemia and hypoinsulinemia.5,11,12 However, the mechanisms underlying the regulation of β-cell function by the circadian clock and Bmal1 are poorly understood. In the present study, we show that a disruption of the molecular clock by deleting Bmal1 in the whole body results in profound defects in glucose-stimulated ATP production and consequently an abrogation of the glucose-simulated first-phase insulin secretory response in islets, leading to diabetes. We show that this effect is mediated by a disruption of the glucose-stimulated increase in mitochondrial membrane potential gradient due to uncoupling resulting from an increased expression of the uncoupling protein, Ucp2. We demonstrate further that inhibition of Ucp2 is sufficient to rescue the defective GSIS in Bmal1-/- islets.
Results
Altered glucose homeostasis in Bmal1-/- mice is secondary to impaired glucose-stimulated insulin secretion (GSIS).
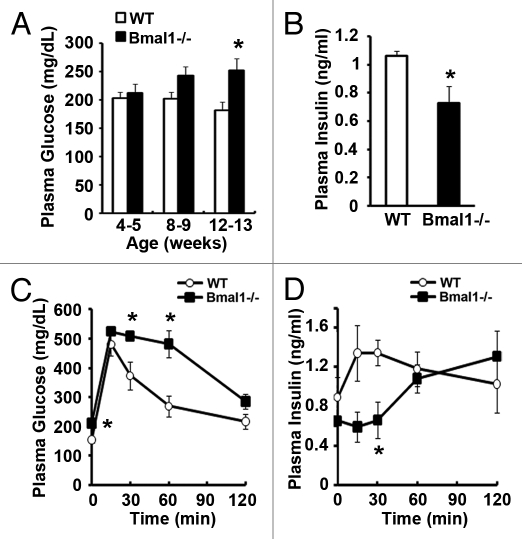
Global Bmal1-/- mice develop progressive increase in fasting plasma glucose levels (Fig. 1A) and have significant fasting hyperglycemia (Bmal1-/- 264 ± 8.6 mg/dl; WT 198.4 ± 7.2 mg/dl) accompanied by fasting hypoinsulinemia (Bmal1-/- 0.73 ± 0.12 ng/ml; WT 1.06 ± 0.03 ng/ml) by 12 weeks of age (Fig. 1B). A glucose tolerance test (GTT) revealed significant glucose intolerance starting at 8 weeks (Fig. S1A) that progressively worsened by 14 weeks of age (Fig. 1C). This impaired glucose tolerance is due to defective in vivo glucose-stimulated insulin secretion (GSIS) with no increase in insulin secretion during the first 30 min of the glucose tolerance test (Fig. 1D), and not due to changes in body weight (Fig. S1B) or peripheral insulin resistance (Fig. S1C). Indeed, on insulin tolerance testing, Bmal1-/- mice display a slower recovery from the insulin-induced hypoglycemia (Fig. S1C), which has been shown to result from impaired hepatic gluconeogenesis in these mice.11,12
Figure 1. Bmal1-/- mice develop diabetes with impaired GSIS. (A) Fasting plasma glucose at ZT-9. n = 11–12. (B) Fasting plasma insulin in 12 week old mice at ZT-9. n = 8. (C and D) Plasma glucose and insulin during glucose tolerance testing in 6 h fasted 14 week old mice at ZT-9. n = 4. All values are mean ± SEM *p ≤ 0.05.
Bmal1-/- mouse islets have normal insulin content.
A blunted GSIS could arise from a decrease in insulin content, β-cell mass or defective glucose-induced stimulus-secretion coupling. The pancreatic insulin content, insulin content per β-cell, insulin content per equally-sized islet and islet morphology were all similar between Bmal1-/- and wild-type controls (Fig. 2A–C and Fig. S2) at 12 weeks of age, excluding all of these as contributing factors to defective GSIS. Therefore, we tested whether a defect in glucose-induced stimulus-secretion coupling underlies the β-cell dysfunction.
Figure 2. Bmal1-/- mice have normal insulin content. (A) Total pancreas insulin content expressed per mg protein. (B and C) Insulin content in isolated islets expressed per μg DNA or per equally sized islet. n = 3–5. All values are mean ± SEM; N.S., not significant.
Impaired stimulus-secretion coupling underlies the blunted GSIS in Bmal1-/- mice.
To assess stimulus-secretion coupling, which involves a cascade of events from glucose uptake, to its metabolism resulting in increased ATP/ADP ratio, closure of K+ATP channels with consequent depolarization-induced Ca2+ entry and exocytosis of pre-formed insulin granules from β-cells, we measured first-phase insulin secretion, in vivo. Bmal1-/- mice displayed a significantly blunted insulin secretion in the first few minutes after glucose stimulation (Fig. 3A) when compared with wild-type controls, similar to the absence of the increase in insulin during the first 30 min of the GTT (Fig. 1D). Interestingly, the loss of this first-phase insulin secretion in Bmal1-/- mice was with glucose, but not L-Arginine, a secretagogue that directly depolarizes the membrane and activates Ca2+ entry (Fig. 3B). This led us to conclude that there was an impairment in stimulus-secretion coupling specific to glucose, but not to secretagogues that acted downstream of K+ATP channels indicating that processes downstream of the K+ATP channels, leading up to insulin exocytosis, are largely intact in Bmal1-/- mice.
Figure 3. Bmal1-/- mice have impaired glucose-induced first-phase insulin secretion. (A and B) Acute insulin secretion after glucose (A) and L-Arginine (B) stimulation in 10 week old mice at ZT-9. n = 4–6. All values are mean ± SEM *p ≤ 0.05; N.S., not significant.
Insulin secretory defect in Bmal1-/- islets is secondary to defective mitochondrial function.
We then studied insulin secretion in response to glucose and other secretagogues in isolated islets to confirm the in vivo findings and pinpoint the defect in GSIS in Bmal1-/- mice. Bmal1-/- islets had a slightly lower basal insulin secretion that was not statistically different from wild-type islets on incubation with 2.8 mm glucose, ex vivo, (0.140 vs. 0.186 ng insulin/∝g DNA/h, respectively). However, consistent with our in vivo findings, Bmal1-/- islets displayed a defect in insulin secretion when challenged with increasing glucose concentrations (Fig. 4A). These ex vivo studies also suggested that this defect may be secondary to a loss-of-function of Bmal1 that is intrinsic to β-cells. To substantiate these findings, we generated stable shRNA-mediated Bmal1 knockdown in Ins-1 (832/13) cells (Fig. S3). These Bmal1 knockdown cells have normal basal insulin secretion (2.04 vs. 2.16 ng insulin/mg protein, respectively). On stimulation with glucose these Bmal1 knockdown cells displayed a significant loss of GSIS (Fig. 4B) as compared with the control scrambled shRNA cells, similar to the islets from Bmal1-/- mice.
Figure 4. Bmal1-/- islets have impaired insulin secretion with glucose but not depolarizing secretagogues. (A and B) Insulin secretion from (A) isolated islets (ten islets each from four individual mice of each genotype) and from (B) Bmal1 knockdown and scrambled control Ins-1 (832/13) cells (n = 6) on exposure to increasing glucose concentrations. Secretion is represented as a fold increase over basal insulin secretion at 2.8 mM glucose, assessed after normalization with total insulin content. (C) Insulin secretion from isolated islets (ten islets each from three individual mice of each genotype) on exposure to depolarizing secretagogues, 30 mM L-Arginine (L-Arg), 15 μM Glibenclamide (Glbn) and 30 mM KCl for 30 min in 2.8 mM glucose buffer. Y-axis represents secreted insulin in the presence of secretagogues as a fold increase over basal secretion in the absence of secretagogues displayed on a log-scale. (D) Insulin secretion during perifusion of isolated islets (n = 3–4 mice per genotype) with KRB containing glucose (2.8 G–2.8 mM glucose; 25 G–25 mM glucose) and KCl (30 mM in 2.8 mM glucose) from samples collected every minute. Results were normalized first to total insulin content of islets and then to the basal insulin secretion during the first 10 min under 2.8 mM glucose. **p ≤ 0.01 between the groups.
However, in contrast to the impaired insulin secretion in response to glucose, Bmal1-/- islets displayed a normal insulin secretory response with depolarizing secretagogues that bypass mitochondrial ATP synthesis, such as, glibenclamide (binds to SUR1 and closes K+ATP channels), KCl and L-Arginine (both depolarize membrane and activate voltage-dependant calcium channels), indicating that the defect in Bmal1-/- islets involves events upstream of K+ATP channels (Fig. 4C). This is also consistent with the in vivo acute insulin secretion studies, described above (Fig. 3). These findings were confirmed on perfusion analysis with isolated islets from both groups which revealed that there was a significant blunting of glucose-stimulated insulin secretion, but there was no difference in insulin secretory response to stimulation with KCl between the Bmal1-/- and control islets (Fig. 4D).
Bmal1 is necessary for normal mitochondrial OXPHOS coupling in β-cells.
Since a defect in GSIS, but not with depolarizing agents such as KCl, strongly suggested a defect upstream of membrane depolarization, we next examined glucose- stimulated changes in mitochondrial membrane potential that drives ATP synthesis. Glucose stimulation led to ~6-fold increase in mitochondrial potential gradient (ψ)-dependent accumulation of the cationic JC-1 dye13 in wild-type islets that was completely abolished in Bmal1-/- islets (Fig. 5A and B), indicating that loss of Bmal1 resulted in abrogation of glucose-induced hyperpolarization and increase in potential gradient across the inner mitochondrial membrane.
Figure 5. Mitochondrial defects in OXPHOS coupling underlie GSIS defects in Bmal1-/- islets. (A) Red/green (590/530) fluorescence ratio of isolated islets (ten islets each from three individual mice for each genotype) after loading with JC-1 dye on exposure to 2.8 and 25 mM glucose concentrations. Values are represented as fold change over wild-type islets in 2.8 mM glucose. (B) Representative images of wild-type (WT) and Bmal1-/- mouse islets exposed to 2.8 mM glucose (top panels) or 25 mM glucose (bottom panels) after loading with JC-1 dye. Images taken are at emission 530 (green-indicating the cytosolic JC-1 dye) and at 590 nM (red-indicating the intra-mitochondrial dye). All values are mean ± SEM *p ≤ 0.05. (C) Representative image of immunohistochemistry of pancreas for Ucp2 showing intense brown staining only in Bmal1-/- islets but not in wild-type (WT) controls. Scale bar represents 20 μm. (D) Relative expression of Ucp2 transcript in isolated Bmal1-/- islets as compared with wild-type controls, after normalization to housekeeping genes. (E) Protein gel blot analysis of Upc2 and Bmal1 proteins from isolated islets (each lane represents 350–400 islets, pooled from two mice) in Bmal1-/- and wild-type control mice. (F) Relative expression of Ucp2 protein, as compared with wild-type control, after normalization to β-actin using ImageJ to quantitate the protein bands from (E).
We then investigated which steps of the glucose-induced stimulus-secretion coupling were impaired in Bmal1-/- mice that lead to a blunted glucose-induced increase in mitochondrial ψ. The expression of glut2 and glucokinase, which are involved in the rate-limiting glucose uptake and phosphorylation steps, respectively, were similar in Bmal1-/- and wild-type islets (Fig. S4A and B). We then assessed the expression of the major subunits of the mitochondrial electron transport system and found them to be unchanged in the Bmal1-/- islets (Fig. S5). The mitochondrial copy number and the activities of Complex I and II were not decreased with Bmal1 deletion or knockdown in islets and Ins-1 cells, respectively (Fig. S6A–C) indicating that these factors did not contribute to the defective GSIS in Bmal1-/- islets. Indeed there was a mild increase in SDH (Complex II) activity in Bmal1 knockdown cells (Fig. S6C). Hence, we turned our attention to coupling of OXPHOS in β-cells, as increased uncoupling due to an upregulation of uncoupling protein 2 (Ucp2) has been demonstrated to result in impaired glucose-induced increase in mitochondrial ψ and GSIS.14-17 We assessed the expression of Ucp2 protein in Bmal1-/- mice and found that there was indeed a significant increase of immunoreactive Ucp2 in Bmal1-/- islets (Fig. 5C). Though the Ucp2 transcript level in isolated Bmal1-/- islets was higher, it did not reach statistical significance (Fig. 5D), but the immunostaining result was confirmed on protein gel blotting for Ucp2 protein from isolated islet lysates (Fig. 5D).
Inhibition of uncoupling rescues GSIS in Bmal1-/- islets.
To assess if this increased Ucp2 expression in Bmal1-/- islets was pathophysiologically significant and contributing to impaired GSIS, we performed rescue experiments using genipin, a potent and specific inhibitor of Ucp2.14 Bmal1-/- islets, when exposed to high glucose (16.7 mM), displayed a blunting of the glucose-induced increment in ATP/ADP ratio (Fig. 6A), as would be expected from the blunted increase in mitochondrial ψ, described earlier (Fig. 5A and B). This blunting in glucose-stimulated ATP/ADP ratio was rescued by inhibiting Ucp2 with genipin, attesting to the detrimental role of increased uncoupling in the Bmal1-/- islets (Fig. 6A). This was further confirmed in ex vivo insulin secretion experiments, wherein incubation of isolated Bmal1-/- islets with genipin restored GSIS to that seen with control islets (Fig. 6B).
Figure 6. Inhibition of Ucp2 rescues GSIS in Bmal1-/- islets. (A) Fold change over basal ATP/ADP ratio measured in isolated islets after exposure to increasing glucose concentrations and a rescue after exposure to 10 μM genipin for 30 min. n = 3–5 (ten islets each from three to five individual mice of each genotype). (B) Insulin secretion from isolated islets on exposure to increasing glucose concentrations and a rescue of insulin secretion after exposure to 10 μM genipin for 60 min. n = 3–5 (ten islets each from three to five individual mice of each genotype). All values are mean ± SEM *p ≤ 0.05.
Thus, the deletion of Bmal1 leads to Ucp2 upregulation with significant uncoupling of mitochondrial OXPHOS, resulting in a significant dissipation of the mitochondrial ψ leading to defective GSIS in islets.
Discussion
The largest fluctuations in plasma glucose, in the unstressed state, are due to feeding/fasting cycles that are intimately linked to the circadian-driven activity/behavioral rhythms. Hence, it is quite logical that the β-cells possess the ability to anticipate feeding-induced increase in plasma glucose and consequent requirement of insulin, by integrating mechanisms controlling circadian rhythm with its metabolism and stimulus-secretion coupling. In this study, we demonstrate this integration in vivo by demonstrating that Bmal1 is required for normal glucose-induced ATP synthesis by a coupled OXPHOS in the β-cell. We provide multiple lines of evidence to demonstrate the regulation of mitochondrial function in β-cells by Bmal1 and the circadian clock. First, deletion/knockdown of Bmal1 leads to a profound defect in glucose-induced insulin secretion in vivo, ex vivo and in vitro that is a result of defective stimulus-secretion coupling. Second, we show that the defect in stimulus-secretion coupling is specific to glucose and not to depolarizing secretagogues, pinpointing this to a defect upstream of the K+ATP channels. Third, we demonstrate that there is a defect in glucose-induced mitochondrial membrane hyperpolarization and impaired ATP synthesis, consequent to uncoupling of OXPHOS by an upregulated expression of the uncoupling protein, Ucp2.
The role of the circadian clock in the regulation of metabolism has been demonstrated in mouse knockout models of the core clock genes. Significant abnormalities were seen in glucose metabolism (Bmal1-/-, ClockΔ19/Δ19, Cry1&2 double KO) and lipid metabolism (Rev-erb α-/-, ClockΔ19/Δ19 and Per2-/- mice).11,18-24 In addition, the circadian clock also regulates key metabolic switches such as Srebp1,20 NAD,25,26 Sirt1,27,28 PGC1a,29 PPARα,30 PPARγ.23 In addition, human studies have also associated perturbations of the circadian rhythm with metabolic abnormalities, especially diabetes and metabolic syndrome.31-34 Furthermore, two haplotypes of BMAL1 were shown to be associated with an increased risk of diabetes and hypertension in a population study.35
Our results, showing defective GSIS in Bmal1-/- mice, are in agreement with two recent reports in reference 5 and 6. While Sadacca et al. did not report a mechanism for the defective GSIS, Marcheva et al.5 observed a defect in insulin release with KCl suggesting a late-stage exocytosis defect. In contrast, our results demonstrate a normal L-Arginine-stimulated insulin secretion, in vivo and a robust KCl-induced GSIS and normal arginine- and glibenclamide-induced GSIS in Bmal1-/- islets ex vivo, suggesting a defect upstream of the depolarization-inducing K+ATP channels. While we cannot exclude a contributory role of disrupted exocytosis, our studies implicate mitochondrial dysfunction as a primary mechanism underlying the impaired insulin secretion in β-cells of Bmal1-/- mice. Though, mitochondrial function was not assessed in the report by Marcheva et al.5 other studies have also suggested a mitochondrial defect with disruptions of the circadian clock. Bray et al.36 utilized a heart-specific transgenic overexpression of ClockΔ19/Δ19 and reported a decrease in State III respiration with pyruvate, glutamate and palmitoyl carnitine in cardiac sub-sarcolemmal mitochondria. Andrews et al.37 showed a decreased mitochondrial volume associated with a slightly decreased expression of PGC1-α and -β along with a decrease in state III respiration in skeletal muscle of Bmal1-/- mice. These studies also support our results that Bmal1 and the circadian clock regulate mitochondrial energy metabolism.
Ucp2 has been implicated in β-cell dysfunction38 that is associated with glucolipotoxicity39 and its overexpression leads to impaired GSIS16,17 while its knockdown or deletion leads to improvement in GSIS.14,15,40,41 There is still uncertainty42,43 as to the pathophysiological role of Ucp2, as under normal circumstances it is expressed at a very low level and is increased only in pathological states associated with impaired GSIS.38 In the current study, we show that Ucp2 is significantly increased in expression in the absence of Bmal1, contributing to impaired GSIS. Though the exact mechanism by which the circadian clock regulates Ucp2 expression still needs further study, there are many possible candidates to mediate this effect. For instance, Bmal1/Clock have been shown to upregulate NAD+-dependant Sirt1 activity,25,26 while Sirt1 has been shown to be a potent suppressor of Ucp2 in β-cells.44,45 Other potential mediators of this effect on Ucp2 could include Srebp1c, PGC1α and oxidative stress dependent pathways all of which, not only directly regulate Ucp2 promoter activity directly, but also are regulated by the circadian clock in other tissues.46-48 It is also possible that the stability and activity of Ucp2 protein may also be regulated by post-translational modification,49,50 especially since there appears to be a more striking increase in protein expression on immunostaining of islets than in mRNA expression of Ucp2 in isolated islets from Bmal1-/- mice. Nevertheless, the restoration of glucose-induced ATP production and insulin secretion by specifically inhibiting Ucp2 with genipin in Bmal1-/- islets demonstrates the central role this uncoupling of OXPHOS plays in the β-cell dysfunction in Bmal1-/- mice.
In summary, our study demonstrates that Bmal1 regulates mitochondrial ATP synthesis, a critical step in glucose-stimulated insulin secretion in β-cells. We show that disruption of Bmal1 leads to inefficient glucose-induced ATP production due to impaired substrate-driven membrane potential generation resulting from uncoupling of OXPHOS in the mitochondria. Circadian control of mitochondrial bioenergetics has implications not only for β-cell function and diabetes, as demonstrated in this study, but also for other tissues that depend primarily on mitochondrial OXPHOS. This study reveals a novel layer of integration of the circadian clock machinery with cellular and energy metabolism in β-cells that could be applicable to disease processes.
Materials and Methods
Animals.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Global Bmal1-/- mice were generated by heterozygous breeding of Bmal1+/- on a C57Bl/6J background.10 Mice were maintained under standard 12:12 Light/Dark cycles, with lights on at 7 AM—Zeitgeber Time 0 (ZT-0) under ad lib access to food and water.
In vivo experiments.
Glucose tolerance testing was performed at ZT-9 after a 6 h fast with Intra-Peritoneal (IP) injection of D-glucose of 1.5 g/kg body weight. For acute insulin secretion studies, a dose of 3 g/kg of D-glucose IP was used. For L-Arginine studies, L-Arginine was injected at 1.5 g/kg IP. For insulin tolerance testing regular insulin 1 unit/kg was used IP.
Islet isolation and insulin secretion.
Islets were isolated as previously described in reference 51. Briefly, 1% collagenase P (Roche) was injected into the common bile duct of anesthetized mice and the pancreas removed, minced and incubated in collagenase solution at 37°C for 7–8 min. After three washes with 10% FBS, the islets were pelleted at 200 g and then were handpicked to purity. Islets from each mouse were individually purified for all experiments. The islets were allowed to incubate in 11.1 mM RPMI-1640 with 10% FBS and 1% penicillin/streptomycin/
L-Glutamine overnight. GSIS studies were performed as described previously in reference 51, using ten similar sized islets from individual mice with at least three mice from each genotype under each experimental condition. Incubation times for ex vivo insulin secretion studies were for 30 min for each condition and the results were normalized to the DNA content or to the total insulin content of the islets measured after acid ethanol extraction at the end of the experiment, as indicated. For genipin rescue studies, the islets were incubated with 10 µm genipin (Sigma) dissolved in DMSO. All insulin assays were performed using mouse insulin ELISA kit (Mercodia). Insulin content was assayed after acid-ethanol extraction from whole pancreas and isolated islets and normalized to DNA and protein measured from the same extract.
Perifusion experiments.
Sixty islets were handpicked and loaded into a perifusion chamber (BioRep) with acrylamide gel column beads (Bio-Gel P4G; Bio-Rad) and perfusion buffer (Krebs-Ringer buffer with 2.8 mM glucose, pre-gassed with 95% O2/5% CO2). Perifusion was achieved with twin syringe pumps (Harvard Apparatus) that allowed precise flow control. A solution in-line heater (Harvard Apparatus) was used to keep the perifusate at 37∞C. Perfusion was done at 300 ∝l/min with the indicated secretagogues after a 60 min equilibration period. The perifusate samples was collected every 1 min into glass vials using a refrigerated fraction collector (Honey comb, Harvard Apparatus). At the end of the perifusion, total insulin was extracted from the islets and used for normalization. Insulin from the samples was measured using the ultra-sensitive mouse ELISA kit (Mercodia).
Gene expression.
RNA was isolated by Trizol (Invitrogen) and all RNA was DNased prior to cDNA synthesis using standard protocols. qPCR was performed using gene specific primers with SYBR Green mix using a Stratagene Mx3000p machine and normalized to the expression of housekeeping genes (β-actin, Gapdh, Hmbs, ppia) using the Genorm method.52 Melting curve analysis and gel electrophoresis was performed to verify specific product amplification. The primer sequences are shown in Table S1.
Immunostaining.
Immunostaining was performed as previously described in reference 51, using formalin-fixed paraffin-embedded tissues. Primary antibodies used were Glut2 (Millipore, rabbit polyclonal 1:200), Insulin (Abcam, guinea pig ployclonal 1:500), Glucagon (Dako rabbit polyclonal 1:200), Ucp2 (Santa Cruz Biotechnology, goat polyclonal 1:25).
Protein gel blotting.
Islets were isolated from each mouse individually as described above and 175–200 islets were frozen immediately. Total protein was extracted from 350–400 islets, pooled from two mice using a protein lysis buffer with a cocktail of protease inhibitors and separated by a standard SDS-PAGE and transferred to a PVDF membrane, as described before in reference 53. Ucp2 (Santa Cruz, goat polyclonal, 1:1,000), Bmal1 (abcam, rabbit polyclonal, 1:3,000) and β-actin (cell signaling, rabbit monoclonal, HRP Conjugate, 1:5,000, 1 h RT) as a house keeping control, were visualized by enhanced chemiluminescence (Pierce) after protein gel blotting. Expression was quantitated using ImageJ software and normalized to β-actin.
In vitro experiments.
Lentiviral shRNA against Bmal1 and scrambled shRNA were obtained from Thermo Scientific, and used to generate stable cell lines with rat insulinoma Ins-1 cell line (832/13 line, a gift from Dr. C. Newgard, Duke University) after puromycin selection. The knockdown efficiency was confirmed by RT-qPCR from isolated RNA and using Bmal1 specific primers. Gene expression of clock related genes was performed using gene specific primers as described above. GSIS was performed as described above.
Mitochondrial experiments.
Mitochondrial copy number was determined by assessing the ratio of mitochondrial DNA-encoded mt-16S and nuclear-encoded hexokinase using specific primers by qPCR. NADH oxidase (Complex I) staining was performed using frozen sections of pancreas and incubated with 0.05 M TRIS-HCl (pH 7.4), 1 mg/ml of Nitro blue tetrazoilum and 2.4 mg/ml of β-NADH for 30 min and washed with 60, 90, 60% acetone for 30 sec each and mounted after washing. SDH (Complex II) enzyme activity was measured by MTT assay. A day before assay, 105 cells (Bmal1 knockdown and scrambled control) were seeded in a 48 well plate. After 2 h incubation with 0.5 mg/ml methyltetrazolium (Sigma), cells were lysed with DMSO and OD measured at 570 nm. ATP/ADP assay was performed using BioVision apoSENSOR kit as per manufacturer’s instructions using ten isolated islets per well. For determining mitochondrial membrane potential, isolated islets were incubated overnight in 11.1 mM glucose RPMI. After washing in 2.8 mM glucose KRB buffer (119 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4 and 0.25% BSA) solution, they were then incubated in 2.8 mM glucose with 2 ∝M JC-1 dye in DMSO for 20 min. After washing three times with 2.8 mM glucose KRB solution, they were imaged using 530 and 590 nm emission filters on an Axiovert microscope.13 Following this, the glucose concentration of the solution was increased to 25 mM, and after 30 min, the same islets were reimaged at the two wavelengths. The images were processed using ImageJ software to obtain intensities and a ratio of the 590 nm/530 nm fluorescence was obtained and normalized to that seen with the wild-type islets in 2.8 mM glucose.
Statistical methods.
All statistical testing was performed either by two-tailed Student’s t-test, assuming unequal variance for two groups or ANOVA for multiple samples with p ≤ 0.05 considered significant between the groups.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgments
The work was supported by grants from the NIH: R56 DK089061-01 (V.K.Y.); Pilot and Feasibility grant (Diabetes and Endocrinology Research Center-P30DK079638) (V.K.Y.); Caroline Wiess Law Fund for Molecular Medicine (V.K.Y.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/18157
References
- 1.Schibler U. The 2008 Pittendrigh/Aschoff lecture: peripheral phase coordination in the mammalian circadian timing system. J Biol Rhythms. 2009;24:3–15. doi: 10.1177/0748730408329383. [DOI] [PubMed] [Google Scholar]
- 2.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 3.Mühlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–6. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- 4.Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, et al. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226:59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–4. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–56. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–42. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16:462–7. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology. 1999;140:3422–8. doi: 10.1210/en.140.8.3422. [DOI] [PubMed] [Google Scholar]
- 14.Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–27. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–55. doi: 10.1016/S0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 16.Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–10. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- 17.Chan CB, MacDonald PE, Saleh MC, Johns DC, Marbàn E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–6. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- 18.Inoue I, Shinoda Y, Ikeda M, Hayashi K, Kanazawa K, Nomura M, et al. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J Atheroscler Thromb. 2005;12:169–74. doi: 10.5551/jat.12.169. [DOI] [PubMed] [Google Scholar]
- 19.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–6. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–9. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda H, Yong Q, Kurose T, Todo T, Mizunoya W, Fushiki T, et al. Clock gene defect disrupts light-dependency of autonomic nerve activity. Biochem Biophys Res Commun. 2007;364:457–63. doi: 10.1016/j.bbrc.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 23.Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–20. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–9. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–7. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–4. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–28. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 28.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–81. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 30.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J. 2005;386:575–81. doi: 10.1042/BJ20041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–54. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–30. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–83. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 35.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–7. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–47. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 37.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A. 2010;107:19090–5. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006;34:802–5. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- 39.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph JW, Koshkin V, Saleh MC, Sivitz WI, Zhang CY, Lowell BB, et al. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem. 2004;279:51049–56. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- 41.Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–9. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- 42.Produit-Zengaffinen N, Davis-Lameloise N, Perreten H, Bécard D, Gjinovci A, Keller PA, et al. Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia. 2007;50:84–93. doi: 10.1007/s00125-006-0499-6. [DOI] [PubMed] [Google Scholar]
- 43.Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, et al. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009;150:3040–8. doi: 10.1210/en.2008-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 47.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany NY) 2009;1:979–87. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011;286:21865–75. doi: 10.1074/jbc.M111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106–15. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, et al. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell. 2009;16:358–73. doi: 10.1016/j.devcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:H0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rauniyar R, et al. Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci U S A. 2004;101:16525–30. doi: 10.1073/pnas.0407574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.