Abstract
Objectives
To systematically examine the evidence guiding the use of implant therapy relative to glycemic control for patients with diabetes and to consider the potential for both implant therapy to support diabetes management and hyperglycemia to compromise implant integration.
Material and Methods
A systematic approach was used to identify and review clinical investigations directly assessing implant survival or failure for patients with diabetes. A MEDLINE (PubMED) database search identified potential articles for inclusion using the search strategy: (dental implants OR oral implants) AND (diabetes OR diabetic). Inclusion in this review required longitudinal assessments including at least 10 patients, with included articles assessed relative to documentation of glycemic status for patients.
Results
The initial search identified 129 publications, reduced to 16 for inclusion. Reported implant failures rates for diabetic patients ranged from 0–14.3%. The identification and reporting of glycemic control was insufficient or lacking in 13 of the 16 studies with 11 of these enrolling only patients deemed as having acceptable glycemic control, limiting interpretation of findings relative to glycemic control. Three of the 16 studies having interpretable information on glycemic control failed to demonstrate a significant relationship between glycemic control and implant failure, with failure rates ranging from 0–2.9%.
Conclusions
Clinical evidence is lacking for the association of glycemic control with implant failure while support is emerging for implant therapy in diabetes patients with appropriate accommodations for delays in implant integration based on glycemic control. The role for implants to improve oral function in diabetes management and the effects of hyperglycemia on implant integration remains to be determined.
Keywords: Diabetes, dental care, implant, glycemic control, literature review
Introduction
Diabetes mellitus is a chronic metabolic disorder that affects 25.6 million individuals or more than 11% of the adult US population. This prevalence represents a 28% increase in the number of patients with diabetes since 2005 (CDC 2005, 2008). Current projections of diabetes incidence suggest that as much as 33% of the US population may be diagnosed with diabetes by 2050, with type 2 diabetes mellitus accounting for 90 to 95% of all diabetes patients (Boyle, et al. 2010). World-wide over 150 million people were estimated as having diabetes in the year 1980, and that number had grown to over 350 million by 2008 (Danaei, et al. 2011). Taken together, these trends highlight the urgency for better understanding diabetes as well as for improving the care of patients with diabetes.
Diabetes mellitus has long been considered a relative contraindication to dental implant therapy and is increasingly becoming one of the most commonly encountered contraindications to dental implant therapy (Oikarinen, et al. 1995). Unfortunately, our understanding of diabetes mellitus as a relative contraindication based on the patient’s level of glycemic control has changed little since the 1988 NIH Consensus Conference on Dental Implants (National Institutes of Health Consensus Development Conference 1988, World Workshop in Periodontics 1996, Blanchaert 1998, Wilson & Higginbottom 1998, Beikler & Flemmig 2003, Kotsovilis, et al. 2006, Javed & Romanos 2009). As a result, well-controlled diabetic patients may be considered appropriate for implant therapy while diabetic patients lacking good glycemic control may be denied the benefits of implant therapy.
The overall goal of this review is to critically assess the evidence available for the use of implant therapy for patients with diabetes based on glycemic control. Importantly, clinical studies directly examining the relationship between diabetes and implant survival, and the potential for glycemic control to serve as an appropriate discriminator for the application of care, are evaluated using a systematic approach. Additionally, the use of implant therapy in special populations requires consideration of potential benefits to be gained from the therapy. In order to better appreciate this potential, the literature is reviewed relative to the benefit gained from implant therapy in a functionally debilitated situation, that is, for edentulous patients. Similarly, the literature is reviewed relative to the effects of hyperglycemia on implant integration as diabetes-related alterations in bone metabolism may have direct effects on osseointegration and implant survival in this patient population.
Material and Methods
A systematic approach was used to identify and review evidence guiding our use of implant therapy relative to glycemic control for patients with diabetes. Additionally, an overview of potential benefits of implant therapy and the risks associated with hyperglycemia on bone metabolism as critical to implant success were performed using a traditional narrative of relevant literature.
Study selection
To be eligible for inclusion in this review, clinical investigations needed to directly assess implant survival or failure for patients with diabetes. Where possible, failure rates were determined based on the total percentage of implants placed (implant failure rate) and based on the total percentage of patients experiencing at least 1 implant failure (patient failure rate). Studies had to be longitudinal in nature. Prospective and retrospective studies with at least 10 patients treated were considered for inclusion. Articles in French, German and English were considered for possible inclusion.
Search strategy
A search in the MEDLINE (PubMED) database up to and including July 2011 for articles published in the dental literature was performed. The search strategy applied was: (dental implants OR oral implants) AND (diabetes OR diabetic). A limit to “human” studies was applied to the search query.
In addition, the reference lists of publications selected for inclusion in this review were systematically screened.
Validity assessment
The screening of the search results for possible inclusion was conducted independently by two reviewers (T.W.O & G. H-B.). The discrepancies were resolved by discussion.
Results
The search resulted in the identification of 129 publications. Independent initial screening of the titles and summaries with respect to the question reviewed resulted in further consideration of 51 publications. If the abstract was not available, the full text was obtained for further screening. Based on available abstracts, 22 publications were further excluded.
Thus, out of the initial 129 titles, 100 were excluded based on screening of the titles and abstracts. The reasons for exclusion included:
Review article
Cases series with less than 10 patients treated
In vitro studies
No patient with diabetes included
No data on dental implant survival/failure rate
A total of 29 full texts were obtained and ultimately 16 full text articles were included in the present review. At the full text article level, 13 articles were excluded for the following reasons:
Review article (Garg 2010)
Cases series with less than 10 patients treated (Smith, et al. 1992, Alsaadi, et al. 2008b, Maximo, et al. 2008, Lee, et al. 2010)
No data on implant survival rate in diabetic patients (Ferreira, et al. 2006, Alsaadi, et al. 2007, Doyle, et al. 2007, Huynh-Ba, et al. 2008, Aloufi, et al. 2009, Lee, et al. 2010)
Same patient population as other study already included (Hamada, et al. 2001, Roumanas, et al. 2002, Oates, et al. 2009)
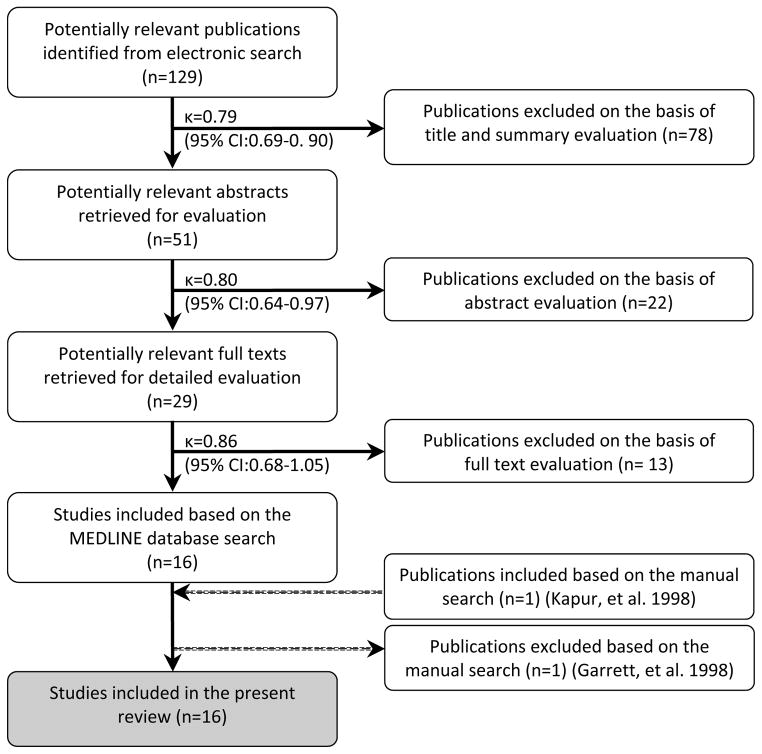
The manual search of the references of the included publications identified one article (Kapur, et al. 1998), which was included to the present review. However, this was the first article of subsequent publications based on the same patient population (Garrett, et al. 1998, Hamada, et al. 2001, Roumanas, et al. 2002) Therefore the publication by Garrett and coworkers (1998) which was previously included based on the electronic search was excluded and replaced by the publication by Kapur and coworkers (1998). Ultimately, a total of 16 publications were included for review following the selection process. The Kappa values for inter-reviewer agreement for inclusion of publications at the title, abstract and full text level were 0.79, 0.80, 0.86 with a 95% confidence interval (CI) of 0.69–0.90, 0.64–0.97, 0.68–1.05, respectively. These Kappa scores indicated “good” to “very good” inter-reviewer agreement (Fig. 1). The details and characteristics of these included studies are presented in Tables 1 and 2.
Figure 1.
Selection process of the included publications and inter-reviewer agreement measures
Table 1.
Studies with partial information on glycemic control
| Reference | Study type | Follow-up time | Patient Population | # of patients | # of implants | Glycemic Assessment | Qualitative glycemic control | Quantitative glycemic control | # of patient experiencing failure (rate) | # of implants failed (rate) | Findings and conclusions of the study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shernoff et al. 1994 | Multicenter prospective CS | 1y | T2D | 89 | 178 | FPG HbA1c |
acceptable | NR | 11 (12.4%) | 13 (7.3%) | “Implants can be considered for T2D patients” |
| Kapur et al. 1998 | Prospective CS | 2y | T1D T2D |
52 | 104 | GHb | acceptable | mean 9.1% | 0 (0.0%) | 0 (0%) | “…implants can be successfully used…in DM patients with even low to moderate levels of metabolic control.” |
| Balshi & Wolfinger 1999 | Retrospective CS | NR | NR | 34 | 227 | Health history | controlled | NR | 6 (17.6%) | 14 (6.2%) | “…high success rate is achievable when dental implants are placed in DM patients whose disease is under control.” |
| Morris et al. 2000 | Retrospective cohort | 3y | ND T2D |
NR | 2632 ND 255 T2D |
NR | NR | NR | NR | ND: 180 (6.8%) T2D: 20 (7.8%) |
“…the use of endosseous dental implants for T2D patients involves a marginal risk to long-term implant survival.” |
| Fiorellini et al. 2000 | Multicenter retrospective CS | mean 4y | T1D T2D |
40 | 215 | NR | controlled or uncontrolled | NR | NR | 31 (14.3%) | “[no]…relationship between failure and… level of diabetic control…” AND “…implants… in well-controlled DM patients…reduced success and survival…” |
| Olson et al. 2000 | Multicenter prospective CS | 5y | T2D | 89 | 178 | FP, HbA1c (normal not defined) | controlled to uncontrolled | FPG=154mg/dl HbA1c: normal or above |
14 (15.7%) | 16 (9.0%) | “…degree of diabetic control…did not make a significant difference in implant outcome.” “…endosseous dental implants…for…T2D patients…predictable procedure.” |
| Farzad et al. 2002 | Retrospective CS | 1y | T1D T2D |
25 | 136 | Health history | well-controlled | NR | 3 (12.0%) | 8 (5.9%) | “ Diabetics that undergo dental implant treatment do not encounter higher failure rate than the normal population if the patients’ plasma glucose level is normal or close to normal…” |
| van Steenberghe et al. 2002 | Prospective CS | NR | T1D T2D |
NR | ~ 30 | NR | controlled | NR | 0 (0.0%) | 0 (0%) | “…controlled T1D and T2D…did not lead to an increased incidence in the early-failure group.” |
| Peled et al., 2003 | Prospective CS | 3y | T2D | 41 | 141 | FP 2hPPG |
well-controlled | NR | NR | 4 (2.8%) | “…dental implants can be used in DM patients if…proper patient selection and…diabetes is well controlled.” |
| Abdulwassie & Dhanrajani 2002 | Prospective CS | NR | NR | 25 | 113 | FPG | controlled | ≤126 mgl/dl | NR | 5 (4.4%) | “Dental implants can be used successfully in patients who are diabetic provided that blood sugar levels are under control” |
| Moy et al. 2005 | Retrospective CS | ≤21 y | NR | 48 | NR | NR | adequate | NR | 15 (31.8%) | NR | “Significantly increased failure rates were seen in …diabetics.” |
| Alsaadiet al. 2008a | Prospective CS | up to loading | ND T2D |
283 | 682 ND 24T2D |
NR | controlled | NR | NR | ND: 13 (1.9%) T2D: 1 (4.2%) |
“Certain factors, such as… Controlled T2D, …did not lead to an increased incidence in the early failures.” |
| Anner et al. 2010 | Retrospective cohort | mean 30.8m | NR | 426 ND 49 DM |
1449 ND 177 DM |
NR | controlled | NR | ND: 54 (12.7%) DM:4 (8.2%) |
ND: 72 (5.0%) DM: 2 (2.8%) |
“This study found no evidence of diminished clinical success or significant early healing complications associated with implant therapy in patients with controlled T2D.” |
CS=case series; RCT=randomized controlled trial; ND=non-diabetic; DM=diabetes mellitus; T1/2D=type 1/2 diabetes; NR=not reported; GHB=Total glycated hemoglobin [9.1%=[est]HbA1c 7.5–8.1%]
Table 2.
Studies meeting glycemic control documentation criteria (including the report of the assessment method and the stratification of glycemic control from a qualitative and quantitative point of view)
| Reference | Study type | Follow-up time | Patient Population | # of patients | # of implants | Method for glycemic control assessment | Qualitative glycemic control | Quantitative glycemic control (HbA1c%) | # of patients | # of implants | # of patient experiencing failure (rate) | # of implants failed (rate) | Findings and conclusions of the study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dowell et al. 2007 | Prospective cohort | 4m | ND T2D |
10 ND 25 T2D |
11 ND 39 T2D |
HbA1c | ND | < 6 | 10 | 12 | 0 (0.0%) | 0 (0%) | “ …no evidence of diminished clinical success or significant early healing complications associated with implant therapy based on the glycemic control levels of patients with T2D.” |
| Well | 6.0–8.0 | 10 | 17 | 0 (0.0%) | 0 (0%) | ||||||||
| Moderate | 8.1–10.0 | 12 | 17 | 0 (0.0%) | 0 (0%) | ||||||||
| Poor | > 10.0 | 3 | 4 | 0 (0.0%) | 0 (0%) | ||||||||
| Tawilet al. 2008 | Prospective cohort | mean 42.4m (1–12 y) | ND T2D |
45 ND 45 T2D |
244 ND 255 T2D |
FPG HbA1c |
ND | NR | 45 | 244 | NR | 1 (0.4%) | “ Well-to fairly well-controlled DM patients…had the same overall survival rates as controls…” “…6 of 7 failures occurred [within] first year…” Note: study did include immediate loading of some implants. “HbA1c is the most important factor affecting implant complication rate.” |
| Well | < 7.0 | 22 | 103 | NR | 1 (1.0%) | ||||||||
| Moderate | 7.0–9.0 | 22 | 141 | NR | 5 (3.5%) | ||||||||
| Poor | > 9.0 | 1 | 11 | NR | 1 (9.1%) | ||||||||
| All T2D | - | 45 | 255 | NR | 7 (2.9%) | ||||||||
| Turkyilmaz 2010 | Prospective CS | 1 y | T2D | 10 | 23 | HbA1c | Well | <8.0 | 6 | 15 | 0 (0.0%) | 0 (0%) | “ …dental implant treatment can be offered to patients with well or moderately controlled T2D.” |
| Moderate | 8.1–10.0 | 4 | 8 | 0 (0.0%) | 0 (0%) |
CS=case series; ND=non-diabetic; DM=diabetes mellitus; T2D=type 2 diabetes; NR=not reported
Secondarily, the 16 reports identified were categorized based on their completeness in the consideration and reporting of methodology for assessments of glycemic control, as well as qualitative and quantitative stratifications of glycemic control. Those reports containing all 3 components were considered separately in the review (Table 2).
Discussion
Glycemic Control
Glycemic control is a primary consideration for patients with diabetes. There is a clear correlation between glycemic control and the development of microvascular and macrovascular complications (Cohen & Horton 2007). Glycated hemoglobin A1c, HbA1c, is a frequently used diagnostic and therapeutic measure of blood glucose control. This value represents the percent of glycated A1c hemoglobin in red blood cells. Because this value is based upon the average circulating time of a red blood cell, 60–90 days, it reflects longer-term or average blood glucose levels over two to three months. Elevated HbA1c levels correlate directly with morbidity and mortality in diabetes (Boltri, et al. 2005). Therefore, achieving low HbA1c levels serves as an important therapeutic target in the management of diabetes (Wysham 2010). Recent recommendations for strict glycemic control for persons with diabetes have targeted maximal HbA1c levels ranging from 6.5% up to 7.0% (Rodbard, et al. 2009, Standards of medical care in diabetes. 2010).
In that glycemic control depends in large part on proper dietary management, it is the individuals with significant oral debilitation and elevated glycemic levels who may have the most to gain from improvements in oral function associated with implant therapy. In view of the increased prevalence of type 2 DM in ethnic minorities (including 14.7% in African Americans, 11% in Hispanics, and 18% in American Indians), coupled with barriers to care, these populations are at even greater risk for hyperglycemic complications (Peek, et al. 2007). Especially in the case of minority groups, for whom HbA1c levels are more commonly elevated, the adverse effects of compromised oral function may serve as an additional obstacle to optimal glycemic control (Boltri, et al. 2005, Choi, et al. 2011).
Masticatory Function and Diabetes
Periodontal disease frequently results in tooth loss, with the cumulative effects most significant in older patients (Albandar, et al. 1999). It is these older patients who are also particularly susceptible to type 2 diabetes and its comorbidities. Diabetes has been shown to significantly increase the levels of periodontal disease and tooth loss (Emrich, et al. 1991, Safkan-Seppala & Ainamo 1992, Oliver & Tervonen 1993, Collin, et al. 1998, Oliver, et al. 1998). Thus, one of the more subtle complications of diabetes may be a decrease in a patient’s health and quality of life due to tooth loss and compromised function (McGrath & Bedi 2001).
Edentulism represents a dramatic debilitation in oral health and function. Importantly, compromises in masticatory function that lead to alterations in dietary behaviors for diabetic patients may be an essential consideration in the overall disease management for these patients, directly impacting glycemic control (Kawamura, et al. 2001, Nuttall, et al. 2003, Roumanas, et al. 2003, Savoca, et al. 2010). Numerous studies have provided strong evidence of an association between diminished chewing function and the amount of fruits, vegetables, meats and breads that edentate individuals consume. These reductions of healthy food consumption lead to dietary deficiencies in vitamins, minerals, fiber and proteins. Edentate individuals then compensate calorically with a diet higher in fats and cholesterol (Osterberg & Steen 1982, Appollonio, et al. 1997, Ritchie, et al. 1997, Papas, et al. 1998, Mojon, et al. 1999, Sheiham, et al. 2001, Hutton, et al. 2002, Savoca, et al. 2010). In fact, the edentate condition and compromised masticatory function have been associated with malnutrition (Shigli & Hebbal 2010, Tsakos, et al. 2010). Difficulties in chewing and swallowing have also been identified as indicators of nutritional risk in older adults and ethnic minorities (Bailey, et al. 2004, Wu, et al. 2011). Therefore, oral health and, specifically, functional tooth replacement must be considered in the overall dietary and nutritional management of patients with diabetes (Quandt, et al. 2009).
Recent work has shown that complete denture wearers benefit greatly when even as few as 2 implants are used to retain their mandibular dentures, reporting significantly higher satisfaction and better oral health-related quality of life (Boerrigter, et al. 1995, Awad & Feine 1998, Awad, et al. 2003, Heydecke, et al. 2003, Thomason, et al. 2003, Meijer, et al. 2004). In a randomized trial, 60 independently living edentate males and females (aged 65–75 yrs) received either new conventional dentures or maxillary conventional dentures and mandibular 2-implant overdentures. Six months after wearing their new prostheses, those in the implant group reported being less limited in their choice of food and having less need to drink in order to swallow. They also reported significantly less difficulty chewing pieces of meat, as well as whole, hard vegetables (including carrots) and fruits (raw apples) than those who received the conventional dentures (Morais, et al. 2003). These results were recently replicated in a similar study with 283 edentate elders (Feine - unpublished results). The ability of edentate people to freely choose the foods they wish will allow them to eat fresher, healthier fare.
Our literature search revealed only one study investigating the impact of implant therapy on treatment satisfaction in a diabetic population (Kapur, et al. 1999). In a randomized trial, new maxillary and mandibular dentures were delivered to edentate diabetic patients. Of 89 subjects, 37 received maxillary and mandibular conventional dentures and 52 received a maxillary conventional denture and a mandibular 2-implant overdenture. While both groups showed improvements with their new prostheses, those patients with implant-retained overdentures had greater improvement in eating enjoyment, speech and general satisfaction. Also, a higher percentage of patients in the implant-retained overdenture group reported pre- to post-treatment improvements in chewing ability, chewing comfort, and denture security. However, this study failed to detect a difference in food choices, supporting the importance of dietary counseling as part of denture therapy (Oikarinen, et al. 1995, Roumanas, et al. 2003).
It is the aging population in which both tooth loss and type 2 diabetes mellitus coexist that the need may be greatest and for whom implant therapy may offer the greatest benefit. There is clearly the potential for implant-based oral rehabilitation to enhance the well-being of patients with diabetes. However, many of the benefits of implant therapy in patients with diabetes remain to be determined.
Bone Metabolism and Diabetes Mellitus
Dental implant survival is initially dependent upon successful osseointegration following placement. Subsequently, as an implant is restored and placed into function, bone remodeling becomes a critical aspect of implant survival in responding to the functional demands placed on the implant restoration and supporting bone. The critical dependence on bone metabolism for implant survival may be heightened in patients with diabetes.
Type 1 diabetes has been consistently associated with osteopathic outcomes. Numerous clinical assessments have shown decreased bone mass, alterations in bone turnover, and increased risk of bone fractures (Krakauer, et al. 1995, Hampson, et al. 1998, Christensen & Svendsen 1999, Campos Pastor, et al. 2000, Kemink, et al. 2000, Espallargues, et al. 2001, Valerio, et al. 2002, Heilman, et al. 2009). These findings are also consistent with numerous animal studies showing negative effects of hyperglycemia, not only on bone formation, but also on bone strength and fracture healing (Devlin, et al. 1996, Forsen, et al. 1999, Inaba, et al. 1999, Funk, et al. 2000, Gooch, et al. 2000, Alkan, et al. 2002, Amir, et al. 2002, Beam, et al. 2002, Gebauer, et al. 2002, Follak, et al. 2003, Lu, et al. 2003, Follak, et al. 2004, Liu, et al. 2006, Kayal, et al. 2007). Similarly, decreased levels of implant osseointegration have been consistently demonstrated in hyperglycemic animals consistent with untreated type 1 diabetes (Siqueira, et al. 2003, de Morais, et al. 2009)
In contrast to type 1 diabetes, the effects of type 2 diabetes on bone turnover remain uncertain. Several studies have identified bone mineral densities consistent with or greater than non-diabetic patients, and lower or no difference in fracture rates (Barrett-Connor & Holbrook 1992, Bauer, et al. 1993, van Daele, et al. 1995, Forsen, et al. 1999, Tuominen, et al. 1999, Nicodemus & Folsom 2001, Sosa, et al. 2009). However, in a large prospective study of osteoporotic fractures, it was found that women with type 2 diabetes had higher fracture rates than non-diabetic women, even with higher bone mineral density in the diabetes patients, suggesting qualitative differences in the bone of diabetic patients (Schwartz, et al. 2001). Additionally, two systematic reviews of risk factors for fractures identified type 2 diabetes as a moderate risk factor (RR=1.57–1.67), consistent with gender, smoking, and family history of osteoporotic fracture (Espallargues, et al. 2001, Ottenbacher, et al. 2002).
More recent meta-analyses similarly identified direct associations between type 2 diabetes and increased risk of fracture, but failed to find an association between HbA1c levels and fracture risk (Janghorbani, et al. 2006, Vestergaard 2007, Asano, et al. 2008). These findings are consistent with another study of type 2 diabetic patients that showed no association between bone density and HbA1c while duration of diabetes remained in question (Janghorbani, et al. 2006, Asano, et al. 2008). In contrast, in a population of initially poorly controlled patients (mean HbA1c>10%), biochemical markers of bone resorption were reduced in association with improved glycemic control, suggesting that hyperglycemia in patients with type 2 diabetes has adverse effects on bone metabolism (Okazaki, et al. 1997, Okazaki, et al. 1999).
Diversity within type 1 and type 2 diabetic patient populations has been proposed to account for these differences in results (Krakauer, et al. 1995, Masse, et al. 2010). These differences may include the timing of onset of the two diseases relative to the development of peak bone mass, patient characteristics typical of these two diseases (e.g., obesity, levels of glycemic control), or the regulatory interplay between insulin and bone metabolism (Basu, et al. 2011, Clemens & Karsenty 2011). In addition, measures frequently used in assessing the effects of diabetes on bone metabolism (i.e., fracture rate) may also be susceptible to confounding factors such as differences in risk of falling due to hypoglycemic episodes. Variation in the duration or severity of the diseases leading to the onset of diabetic complications, such as retinopathy, nephropathy, or neuropathy, may also affect the likelihood of falling and, subsequently, fracture rates (Ivers, et al. 2001, Patel, et al. 2008). Furthermore, it has been proposed that diabetes leads to decreased bone turnover, with reductions in both resorption and formation and that it is the difference in ages of onset of types 1 and 2 diabetes relative to bone growth patterns that lead to these distinctions in outcomes (Krakauer, et al. 1995). For example, decreased bone turnover during the period of skeletal growth in type 1 diabetes patients may attenuate formation during this period of bone growth, resulting in a decreased peak bone mass in these patients. In contrast, decreased bone turnover in older, type 2 diabetes patients may actually be protective by maintaining bone density during periods of net bone loss in older non-diabetic individuals. While bone density may be protected, bone metabolism and healing associated with implant placement may be adversely affected.
The osteopathic potential of diabetes has been documented. However, the effects of diabetes are confounded by many aspects of the conditions, ranging from differences inherent between type 1 and type 2 diabetes to the role of insulin in bone metabolism. The extrapolation of these concerns with bone metabolism and diabetes to dental implant therapy, while indirect, certainly supports caution.
Taken together, there are numerous studies that offer indirect evidence for diabetes patients to benefit from oral rehabilitation using dental implant therapy. There is also considerable evidence documenting compromises in bone metabolism associated with hyperglycemic conditions with the potential for these risks to mitigate benefits gained from implant therapy. Biologic studies suggest diabetes-related effects on bone metabolism; however, true differences in metabolic effects between type 1 and type 2 diabetes remain unclear. Similarly, the translation of results from hyperglycemic animal studies to patients remains to be elucidated. Further clarification of the impacts of diabetes and glycemic control with implant therapy in diabetic patients ultimately requires direct assessment provided through clinical investigations.
Diabetes and implant integration (based on the systematic search)
The goal of this systematic review is to critically evaluate clinical studies directly related to the use of dental implant therapy for patients with diabetes. As glycemic control is viewed primary to the application of implant therapy in patients with diabetes, this review further considers the evidence available specific to glycemic control as an indicator for implant therapy.
The majority of clinical investigations of implant survival identified in this review, 11 of 16 reports, were undertaken with the prevailing view that good glycemic control is critical to the successful use of implant therapy for patients with diabetes. All eleven of these studies only included patients considered as having acceptable glycemic control in order to receive implant therapy.
On closer evaluation of the documentation of glycemic control, 13 of the 16 studies identified were found to not meet methodologic completeness criteria for consideration of glycemic control (Table 1). This evaluation identified great variability in the way glycemic control was evaluated, reported or defined. Overall, eight of these studies did not report having a quantitative assessment of glycemic control (Shernoff, et al. 1994, Balshi & Wolfinger 1999, Fiorellini, et al. 2000, Morris, et al. 2000, Farzad, et al. 2002, van Steenberghe, et al. 2002, Peled, et al. 2003, Moy, et al. 2005). Several studies mentioned that the patients were well-controlled but did not report how this information was gained or how it was defined (Morris, et al. 2000, van Steenberghe, et al. 2002, Moy, et al. 2005). In some studies, patients were “encouraged” to have a good glycemic control and, for some patients, the diabetic status was determined by interview, but without any specific validation of these patient reports (Balshi & Wolfinger 1999, Farzad, et al. 2002). Further studies assessed the controlled diabetic status by means of glucose level testing (Fasting Plasma Glucose and 2-hour Postprandial Glucose) at one time point pre-operatively (Abdulwassie & Dhanrajani 2002) or 1 week before and after surgery and one time on the day of surgery (Peled, et al. 2003). While these tests have important diagnostic value, their ability to describe long-term levels of glycemic control is minimal, thus preventing these studies from discriminating effects of glycemic control on implant survival (see Table 1).
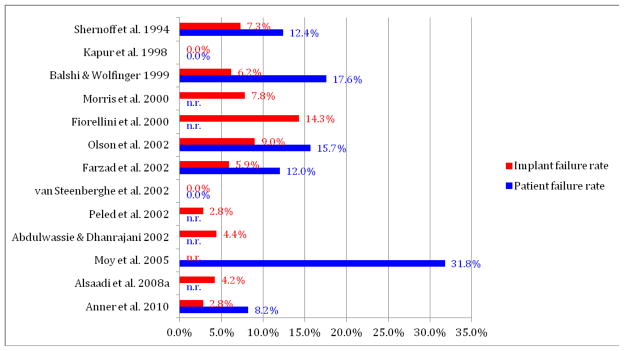
Evaluation of implant failure rates for these 13 studies demonstrated considerable variability in the rate of implant failure in patients with diabetes (0 to 14.3%; Figure 2). Additionally, the rate at which diabetic patients receiving one or more implants experienced at least one failed implant was highly variable (0 to 31.3%, Figure 2).
Figure 2.
Implant failure rate and patient failure rate (%) for studies on implant outcomes in patient with diabetes with partial information on level of glycemic control.
n.r.= not reported
It is critical to our use of implant therapy in diabetic patients to recognize the lack of a clear definition of diabetes status and glycemic control in the interpretation of the results from these studies. This deficiency limits the application of their results toward the development of specific evidence-based guidelines for care for patients with diabetes. Additionally, in only 3 of these 13 studies was a comparative non-diabetic control population assessed (Morris, et al. 2000, Anner et al. 2010, Alsaadi et al. 2008a). Interestingly, consideration of the findings from these 3 of 13 studies also fails to demonstrate a difference in implant failure between diabetic (ranging from 2.8–7.8%) and non-diabetic patients (ranging from 1.9–6.8%). It is clear that these discordant results demonstrate our continuing need to clarify the parameters of diabetes impacting successful implant therapy.
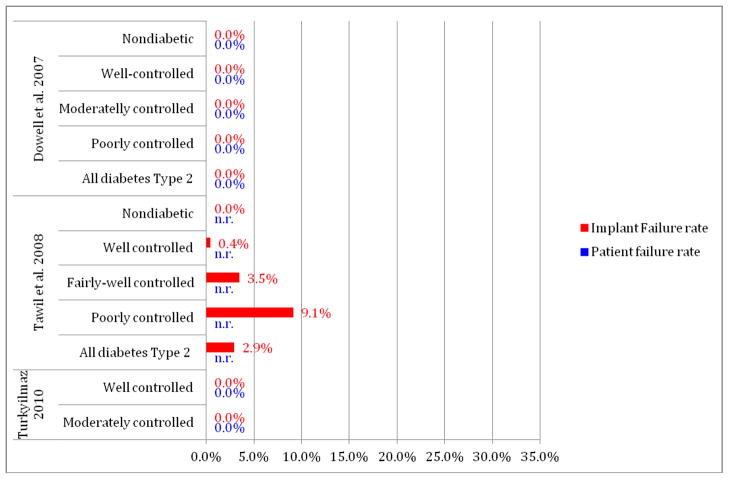
The systematic search of the literature also identified 4 of the 16 studies in which patients lacking acceptable glycemic control were included, and 3 of these 4 studies in which all three methodologic components, i.e., methodology for assessments of glycemic control, as well as qualitative and quantitative stratifications of glycemic control, were considered (Tables 1 and 2).
In contrast to the studies lacking methodologic completeness, evaluation of the 3 recent studies meeting these methodologic criteria in reporting diabetes status of patients (Table 2) had implant failure rates ranging from 0 to 9.1%. These 3 reports extend the study of the effects of diabetes to clearly include patients having only moderate or poor glycemic control (Dowell, et al. 2007, Tawil, et al. 2008, Turkyilmaz 2010). The first of these studies evaluated implant healing over a 4 month evaluation period prior to implant restoration. Importantly, this study did so for diabetic patients having an HbA1c at the time of surgery up to 12% and with HbA1c levels extending as high as 13.8% over the 4 month evaluation period (Dowell, et al. 2007, Oates, et al. 2009). The 25 diabetes patients in this study included 12 patients (17 implants) who would not be considered as well-controlled, having HbA1c levels between 8.1–10.0%, and 3 patients (4 implants) with HbA1c levels over 10.0%. For all patients, this study failed to identify any implant failures over the initial 4-month healing period. However, consistent with animal studies of the effects of hyperglycemia on bone metabolism, this study did identify significant compromises in implant integration in direct relation to HbA1c levels (Oates, et al. 2009). Specifically, delays in implant integration were identified for patients having HbA1c levels over 8.0%, but not for patients below this level of glycemic control. This study’s findings suggest that the effects of hyperglycemia on implant integration, if clinically significant, were successfully accommodated with the extended healing period from 2 months to 4 months prior to functional loading as utilized in this study.
In a second study, 45 diabetes patients having an initial HbA1c below 7.2% received 255 implants. They were followed over a period ranging from 1 to 12 years (Tawil, et al. 2008). The HbA1c levels for these patients varied over the follow-up period, with 44 patients recording HbA1c levels up to 9%, and 1 patient recording an HbA1c level over 9%. This latter patient received 11 implants and had one failure, giving the study its seemingly high failure rate (9.1% implant failure rate) for this level of glycemic control. However, when this patient’s results are combined with the other 22 patients having only moderate glycemic control over the course of their evaluation period, the cumulative implant failure rate is 3.9%. As all these patients initiated implant therapy with an HbA1c <7.2% and the changes and duration of changes in HbA1c levels are unknown, the cumulative 2.9% failure rate for all diabetic patients remains most relevant. This study is limited by the lack of clarity as to when the HbA1c levels were obtained for the patients during the follow-up period and when implant failures were identified. This study also failed to find a statistically significant difference in implant survival based on HbA1c levels, yet interestingly concluded that HbA1c is the most important factor affecting implant complication rate. Presumably, this conclusion is based on the trend observed in the study data, however patient numbers and limitations in HbA1c reporting as noted limit the value of this interpretation.
The third of these studies was a recent prospective case series of 10 patients with diabetes that included 4 patients having HbA1c levels between 8.1% and 10.0%. This study evaluated implant survival after 1 year of restoration and reported no implant failures (Turkyilmaz 2010).
Taken together, it appears that more recent studies of implant success with better-defined parameters of glycemic control support the use of dental implants for patients with diabetes mellitus, independent of glycemic status (Figure 3). It is important to note that while these studies were designed to examine the role for glycemic control in implant survival, these findings must be viewed as preliminary in that they include relatively small numbers of patients having elevated glycemic levels and offer only limited information on the longer term effects of diabetes on implant survival. It is also important to consider the potential for many other factors such as technologic advances in implant designs to enhance survival rates for implants in patients with diabetes. These preliminary findings do strongly support the continued exploration and translation of the effects of elevated glycemic levels in implant survival utilizing larger study populations and longer follow-up periods.
Figure 3.
Implant failure rate and patient failure rate (%) for studies on implant outcomes in patients with diabetes meeting glycemic control documentation criteria (including the report of the assessment method and the stratification of glycemic control from a qualitative and quantitative point of view)
n.r.= not reported
* represents 1 patient having 1 implant fail out of 11 placed
Conclusion
Oral health is an integral part of nutritional well-being and systemic health. Chronic diseases such as diabetes have oral sequelae that may lead to compromises in function, and oral function may importantly modulate dietary interventions critical to the overall management of diabetes (Touger-Decker & Mobley 2003). From a medical standpoint, there is no doubt that long-term good glycemic control is critical to the patient’s minimizing diabetes related co-morbidities. However, good glycemic control may be dependent in part upon proper masticatory function. With diabetes contributing to oral pathologies and tooth loss, tooth replacement as can be provided with implant therapy may be an important contributor to the patient’s overall well-being. Based on available literature to date, there are no clear clinical data supporting increased implant failures for patients lacking good glycemic control and, in fact, more recent studies support the use of dental implant therapy for patients in the absence of good glycemic control with appropriate accommodations for delays in implant integration. Therefore, with the potential benefit implant therapy has to offer, it may be in the diabetic patient’s best interest to consider implant therapy, even in the absence of proper glycemic control. While this represents a shift in attitude toward diabetic patient care, it is one that requires careful consideration of both the risks and benefits of care, as well as our current limitations in our understanding of these relationships.
Acknowledgments
This study was supported by NIDCR R01 DE017882.
References
- Abdulwassie H, Dhanrajani PJ. Diabetes mellitus and dental implants: A clinical study. Implant Dentistry. 2002;11:83–86. doi: 10.1097/00008505-200201000-00019. [DOI] [PubMed] [Google Scholar]
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the united states, 1988–1994. Journal of Periodontology. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- Alkan A, Erdem E, Gunhan O, Karasu C. Histomorphometric evaluation of the effect of doxycycline on the healing of bone defects in experimental diabetes mellitus: A pilot study. Journal of Oral and Maxillofacial Surgery. 2002;60:898–904. doi: 10.1053/joms.2002.33859. [DOI] [PubMed] [Google Scholar]
- Aloufi F, Bissada N, Ficara A, Faddoul F, Al-Zahrani MS. Clinical assessment of peri-implant tissues in patients with varying severity of chronic periodontitis. Clin Implant Dent Relat Res. 2009;11:37–40. doi: 10.1111/j.1708-8208.2008.00087.x. [DOI] [PubMed] [Google Scholar]
- Alsaadi G, Quirynen M, Komarek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. Journal of Clinical Periodontology. 2007;34:610–617. doi: 10.1111/j.1600-051X.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- Alsaadi G, Quirynen M, Michiles K, Teughels W, Komarek A, van Steenberghe D. Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. Journal of Clinical Periodontology. 2008a;35:51–57. doi: 10.1111/j.1600-051X.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- Alsaadi G, Quirynen M, Komarek A, van Steenberghe D. Impact of local and systemic factors on the incidence of late oral implant loss. Clinical Oral Implants Research. 2008b;19:670–676. doi: 10.1111/j.1600-0501.2008.01534.x. [DOI] [PubMed] [Google Scholar]
- Amir G, Rosenmann E, Sherman Y, Greenfeld Z, Ne’eman Z, Cohen AM. Osteoporosis in the cohen diabetic rat: Correlation between histomorphometric changes in bone and microangiopathy. Laboratory Investigation. 2002;82:1399–1405. doi: 10.1097/01.lab.0000032378.19165.e2. [DOI] [PubMed] [Google Scholar]
- Appollonio I, Carabellese C, Frattola A, Trabucchi M. Influence of dental status on dietary intake and survival in community-dwelling elderly subjects. Age and Ageing. 1997;26:445–456. doi: 10.1093/ageing/26.6.445. [DOI] [PubMed] [Google Scholar]
- Asano M, Fukui M, Hosoda H, Shiraishi E, Harusato I, Kadono M, Tanaka M, Hasegawa G, Yoshikawa T, Nakamura N. Bone stiffness in men with type 2 diabetes mellitus. Metabolism: Clinical and Experimental. 2008;57:1691–1695. doi: 10.1016/j.metabol.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Awad MA, Feine JS. Measuring patient satisfaction with mandibular prostheses. Community Dentistry and Oral Epidemiology. 1998;26:400–405. doi: 10.1111/j.1600-0528.1998.tb01978.x. [DOI] [PubMed] [Google Scholar]
- Awad MA, Lund JP, Shapiro SH, Locker D, Klemetti E, Chehade A, Savard A, Feine JS. Oral health status and treatment satisfaction with mandibular implant overdentures and conventional dentures: A randomized clinical trial in a senior population. International Journal of Prosthodontics. 2003;16:390–396. [PubMed] [Google Scholar]
- Bailey RL, Ledikwe JH, Smiciklas-Wright H, Mitchell DC, Jensen GL. Persistent oral health problems associated with comorbidity and impaired diet quality in older adults. Journal of the American Dietetic Association. 2004;104:1273–1276. doi: 10.1016/j.jada.2004.05.210. [DOI] [PubMed] [Google Scholar]
- Balshi TJ, Wolfinger GJ. Dental implants in the diabetic patient: A retrospective study. Implant Dentistry. 1999;8:355–359. doi: 10.1097/00008505-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Holbrook TL. Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA. 1992;268:3333–3337. [PubMed] [Google Scholar]
- Basu R, Peterson J, Rizza R, Khosla S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. Journal of Clinical Endocrinology and Metabolism. 2011;96:1450–1455. doi: 10.1210/jc.2010-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, Tao JL, Cummings SR. Factors associated with appendicular bone mass in older women. The study of osteoporotic fractures research group. Annals of Internal Medicine. 1993;118:657–665. doi: 10.7326/0003-4819-118-9-199305010-00001. [DOI] [PubMed] [Google Scholar]
- Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the bb wistar rat with diabetes mellitus. Journal of Orthopaedic Research. 2002;20:1210–1216. doi: 10.1016/S0736-0266(02)00066-9. [DOI] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. Implants in the medically compromised patient. Critical Reviews in Oral Biology and Medicine. 2003;14:305–316. doi: 10.1177/154411130301400407. [DOI] [PubMed] [Google Scholar]
- Blanchaert RH. Implants in the medically challenged patient. Dental Clinics of North America. 1998;42:35–45. [PubMed] [Google Scholar]
- Boerrigter EM, Stegenga B, Raghoebar GM, Boering G. Patient satisfaction and chewing ability with implant-retained mandibular overdentures: A comparison with new complete dentures with or without preprosthetic surgery. Journal of Oral and Maxillofacial Surgery. 1995;53:1167–1173. doi: 10.1016/0278-2391(95)90627-4. [DOI] [PubMed] [Google Scholar]
- Boltri JM, Okosun IS, Davis-Smith M, Vogel RL. Hemoglobin a1c levels in diagnosed and undiagnosed black, hispanic, and white persons with diabetes: Results from nhanes 1999–2000. Ethnicity and Disease. 2005;15:562–567. [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the us adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos Pastor MM, Lopez-Ibarra PJ, Escobar-Jimenez F, Serrano Pardo MD, Garcia-Cervigon AG. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: A prospective study. Osteoporosis International. 2000;11:455–459. doi: 10.1007/s001980070114. [DOI] [PubMed] [Google Scholar]
- CDC - Centers for disease control and prevention. National diabetes fact sheet: General information and national estimates on diabetes in the united states, 2005. Atlanta, ga: U.S. Department of health and human services, centers for disease control and prevention; 2005. Http://www.Cdc.Gov/diabetes/pubs/estimates05.Htm. [Google Scholar]
- CDC - Centers for disease control and prevention. National diabetes fact sheet: General information and national estimates on diabetes in the united states, 2007. Atlanta, ga: U.S. Department of health and human services, centers for disease control and prevention; 2008. Http://www.Cdc.Gov/diabetes/pubs/estimates07.Htm. [Google Scholar]
- Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT. Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care. 2011;34:381–386. doi: 10.2337/dc10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JO, Svendsen OL. Bone mineral in pre- and postmenopausal women with insulin-dependent and non-insulin-dependent diabetes mellitus. Osteoporosis International. 1999;10:307–311. doi: 10.1007/s001980050232. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Karsenty G. The osteoblast: An insulin target cell controlling glucose homeostasis. Journal of Bone and Mineral Research. 2011;26:677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- Cohen A, Horton ES. Progress in the treatment of type 2 diabetes: New pharmacologic approaches to improve glycemic control. Current Medical Research and Opinion. 2007;23:905–917. doi: 10.1185/030079907x182068. [DOI] [PubMed] [Google Scholar]
- Collin HL, Uusitupa M, Niskanen L, Kontturi-Narhi V, Markkanen H, Koivisto AM, Meurman JH. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. Journal of Periodontology. 1998;69:962–966. doi: 10.1902/jop.1998.69.9.962. [DOI] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- de Morais JA, Trindade-Suedam IK, Pepato MT, Marcantonio E, Jr, Wenzel A, Scaf G. Effect of diabetes mellitus and insulin therapy on bone density around osseointegrated dental implants: A digital subtraction radiography study in rats. Clinical Oral Implants Research. 2009;20:796–801. doi: 10.1111/j.1600-0501.2009.01716.x. [DOI] [PubMed] [Google Scholar]
- Devlin H, Garland H, Sloan P. Healing of tooth extraction sockets in experimental diabetes mellitus. Journal of Oral and Maxillofacial Surgery. 1996;54:1087–1091. doi: 10.1016/s0278-2391(96)90166-4. [DOI] [PubMed] [Google Scholar]
- Dowell S, Oates TW, Robinson M. Implant success in people with type 2 diabetes mellitus with varying glycemic control: A pilot study. Journal of the American Dental Association. 2007;138:355–361. doi: 10.14219/jada.archive.2007.0168. quiz 397–358. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Hodges JS, Pesun IJ, Baisden MK, Bowles WR. Factors affecting outcomes for single-tooth implants and endodontic restorations. Journal of Endodontics. 2007;33:399–402. doi: 10.1016/j.joen.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. Journal of Periodontology. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, Setoain J, Granados A. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: A systematic review of the literature. Osteoporosis International. 2001;12:811–822. doi: 10.1007/s001980170031. [DOI] [PubMed] [Google Scholar]
- Farzad P, Andersson L, Nyberg J. Dental implant treatment in diabetic patients. Implant Dentistry. 2002;11:262–267. doi: 10.1097/00008505-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in brazilian subjects. Journal of Clinical Periodontology. 2006;33:929–935. doi: 10.1111/j.1600-051X.2006.01001.x. [DOI] [PubMed] [Google Scholar]
- Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. International Journal of Periodontics and Restorative Dentistry. 2000;20:366–373. [PubMed] [Google Scholar]
- Follak N, Kloting I, Ganzer D, Merk H. Scanning electron microscopic examinations on retarded bone defect healing in spontaneously diabetic bb/o(ttawa)k(arlsburg) rats. Histology and Histopathology. 2003;18:111–120. doi: 10.14670/HH-18.111. [DOI] [PubMed] [Google Scholar]
- Follak N, Kloting L, Wolf E, Merk H. Delayed remodeling in the early period of fracture healing in spontaneously diabetic bb/ok rats depending on the diabetic metabolic state. Histology and Histopathology. 2004;19:473–486. doi: 10.14670/HH-19.473. [DOI] [PubMed] [Google Scholar]
- Forsen L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: Results from the nord-trondelag health survey. Diabtologia. 1999;42:920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. Biomechanical evaluation of early fracture healing in normal and diabetic rats. Journal of Orthopaedic Research. 2000;18:126–132. doi: 10.1002/jor.1100180118. [DOI] [PubMed] [Google Scholar]
- Garg A. Dental implants in the diabetic patient. Dental Implantology Update. 2010;21:33–39. [PubMed] [Google Scholar]
- Garrett NR, Kapur KK, Hamada MO, Roumanas ED, Freymiller E, Han T, Diener RM, Levin S, Chen T. A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part ii. Comparisons of masticatory performance. Journal of Prosthetic Dentistry. 1998;79:632–640. doi: 10.1016/s0022-3913(98)70069-1. [DOI] [PubMed] [Google Scholar]
- Gebauer GP, Lin SS, Beam HA, Vieira P, Parsons JR. Low-intensity pulsed ultrasound increases the fracture callus strength in diabetic bb wistar rats but does not affect cellular proliferation. Journal of Orthopaedic Research. 2002;20:587–592. doi: 10.1016/S0736-0266(01)00136-X. [DOI] [PubMed] [Google Scholar]
- Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connective Tissue Research. 2000;41:81–91. doi: 10.3109/03008200009067660. [DOI] [PubMed] [Google Scholar]
- Hamada MO, Garrett NR, Roumanas ED, Kapur KK, Freymiller E, Han T, Diener RM, Chen T, Levin S. A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part iv: Comparisons of dietary intake. Journal of Prosthetic Dentistry. 2001;85:53–60. doi: 10.1067/mpr.2001.112491. [DOI] [PubMed] [Google Scholar]
- Hampson G, Evans C, Petitt RJ, Evans WD, Woodhead SJ, Peters JR, Ralston SH. Bone mineral density, collagen type 1 alpha 1 genotypes and bone turnover in premenopausal women with diabetes mellitus. Diabtologia. 1998;41:1314–1320. doi: 10.1007/s001250051071. [DOI] [PubMed] [Google Scholar]
- Heilman K, Zilmer M, Zilmer K, Tillmann V. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum icam-1 and urinary isoprostane levels. Journal of Bone and Mineral Metabolism. 2009;27:598–604. doi: 10.1007/s00774-009-0076-4. [DOI] [PubMed] [Google Scholar]
- Heydecke G, Locker D, Awad MA, Lund JP, Feine JS. Oral and general health-related quality of life with conventional and implant dentures. Community Dentistry and Oral Epidemiology. 2003;31:161–168. doi: 10.1034/j.1600-0528.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Hutton B, Feine J, Morais J. Is there an association between edentulism and nutritional state? Journal/Canadian Dental Association Journal de l Association Dentaire Canadienne. 2002;68:182–187. [PubMed] [Google Scholar]
- Huynh-Ba G, Friedberg JR, Vogiatzi D, Ioannidou E. Implant failure predictors in the posterior maxilla: A retrospective study of 273 consecutive implants. Journal of Periodontology. 2008;79:2256–2261. doi: 10.1902/jop.2008.070602. [DOI] [PubMed] [Google Scholar]
- Inaba M, Nishizawa Y, Mita K, Kumeda Y, Emoto M, Kawagishi T, Ishimura E, Nakatsuka K, Shioi A, Morii H. Poor glycemic control impairs the response of biochemical parameters of bone formation and resorption to exogenous 1,25-dihydroxyvitamin d3 in patients with type 2 diabetes. Osteoporosis International. 1999;9:525–531. doi: 10.1007/s001980050180. [DOI] [PubMed] [Google Scholar]
- Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: The blue mountains eye study. Diabetes Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: The nurses’ health study. Diabetes Care. 2006;29:1573–1578. doi: 10.2337/dc06-0440. [DOI] [PubMed] [Google Scholar]
- Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: A systematic literature review. Journal of Periodontology. 2009;80:1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- Kapur KK, Garrett NR, Hamada MO, Roumanas ED, Freymiller E, Han T, Diener RM, Levin S, Ida R. A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part i: Methodology and clinical outcomes. Journal of Prosthetic Dentistry. 1998;79:555–569. doi: 10.1016/s0022-3913(98)70177-5. [DOI] [PubMed] [Google Scholar]
- Kapur KK, Garrett NR, Hamada MO, Roumanas ED, Freymiller E, Han T, Diener RM, Levin S, Wong WK. Randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part iii: Comparisons of patient satisfaction. Journal of Prosthetic Dentistry. 1999;82:416–427. doi: 10.1016/s0022-3913(99)70028-4. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Tsurumoto A, Fukuda S, Sasahara H. Health behaviors and their relation to metabolic control and periodontal status in type 2 diabetic patients: A model tested using a linear structural relations program. Journal of Periodontology. 2001;72:1246–1253. doi: 10.1902/jop.2000.72.9.1246. [DOI] [PubMed] [Google Scholar]
- Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. Journal of Bone and Mineral Research. 2007;22:560–568. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. Journal of Endocrinological Investigation. 2000;23:295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- Kotsovilis S, Karoussis IK, Fourmousis I. A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clinical Oral Implants Research. 2006;17:587–599. doi: 10.1111/j.1600-0501.2005.01245.x. [DOI] [PubMed] [Google Scholar]
- Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim YK, Park JY, Kim SG, Kim MJ, Yun PY. Short-term clinical retrospective study of implants in geriatric patients older than 70 years. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2010;110:442–446. doi: 10.1016/j.tripleo.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M, Luan Q, Graves DT. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. Journal of Dental Research. 2006;85:510–514. doi: 10.1177/154405910608500606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144:346–352. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- Masse PG, Pacifique MB, Tranchant CC, Arjmandi BH, Ericson KL, Donovan SM, Delvin E, Caissie M. Bone metabolic abnormalities associated with well-controlled type 1 diabetes (iddm) in young adult women: A disease complication often ignored or neglected. Journal of the American College of Nutrition. 2010;29:419–429. doi: 10.1080/07315724.2010.10719859. [DOI] [PubMed] [Google Scholar]
- Maximo MB, de Mendonca AC, Alves JF, Cortelli SC, Peruzzo DC, Duarte PM. Peri-implant diseases may be associated with increased time loading and generalized periodontal bone loss: Preliminary results. Journal of Oral Implantology. 2008;34:268–273. doi: 10.1563/1548-1336(2008)34[269:PDMBAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McGrath C, Bedi R. Can dentures improve the quality of life of those who have experienced considerable tooth loss? Journal of Dentistry. 2001;29:243–246. doi: 10.1016/s0300-5712(00)00063-4. [DOI] [PubMed] [Google Scholar]
- Meijer HJ, Batenburg RH, Raghoebar GM, Vissink A. Mandibular overdentures supported by two branemark, imz or iti implants: A 5-year prospective study. Journal of Clinical Periodontology. 2004;31:522–526. doi: 10.1111/j.1600-051X.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- Mojon P, Budtz-Jorgensen E, Rapin CH. Relationship between oral health and nutrition in very old people. Age and Ageing. 1999;28:463–468. doi: 10.1093/ageing/28.5.463. [DOI] [PubMed] [Google Scholar]
- Morais JA, Heydecke G, Pawliuk J, Lund JP, Feine JS. The effects of mandibular two-implant overdentures on nutrition in elderly edentulous individuals. Journal of Dental Research. 2003;82:53–58. doi: 10.1177/154405910308200112. [DOI] [PubMed] [Google Scholar]
- Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: Placement to 36 months. Annals of Periodontology. 2000;5:157–165. doi: 10.1902/annals.2000.5.1.157. [DOI] [PubMed] [Google Scholar]
- Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. International Journal of Oral and Maxillofacial Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24:1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- NIH. National institutes of health consensus development conference statement on dental implants june 13–15, 1988. Journal of Dental Education. 1988;52:824–827. [PubMed] [Google Scholar]
- Nuttall FQ, Gannon MC, Saeed A, Jordan K, Hoover H. The metabolic response of subjects with type 2 diabetes to a high-protein, weight-maintenance diet. Journal of Clinical Endocrinology and Metabolism. 2003;88:3577–3583. doi: 10.1210/jc.2003-030419. [DOI] [PubMed] [Google Scholar]
- Oates TW, Dowell S, Robinson M, McMahan CA. Glycemic control and implant stabilization in type 2 diabetes mellitus. Journal of Dental Research. 2009;88:367–371. doi: 10.1177/0022034509334203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikarinen K, Raustia AM, Hartikainen M. General and local contraindications for endosseal implants--an epidemiological panoramic radiograph study in 65-year-old subjects. Community Dentistry and Oral Epidemiology. 1995;23:114–118. doi: 10.1111/j.1600-0528.1995.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Okazaki R, Miura M, Toriumi M, Taguchi M, Hirota Y, Fukumoto S, Fujita T, Tanaka K, Takeuchi A. Short-term treatment with troglitazone decreases bone turnover in patients with type 2 diabetes mellitus. Endocrine Journal. 1999;46:795–801. doi: 10.1507/endocrj.46.795. [DOI] [PubMed] [Google Scholar]
- Okazaki R, Totsuka Y, Hamano K, Ajima M, Miura M, Hirota Y, Hata K, Fukumoto S, Matsumoto T. Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. Journal of Clinical Endocrinology and Metabolism. 1997;82:2915–2920. doi: 10.1210/jcem.82.9.4258. [DOI] [PubMed] [Google Scholar]
- Oliver RC, Brown LJ, Loe H. Periodontal diseases in the united states population. Journal of Periodontology. 1998;69:269–278. doi: 10.1902/jop.1998.69.2.269. [DOI] [PubMed] [Google Scholar]
- Oliver RC, Tervonen T. Periodontitis and tooth loss: Comparing diabetics with the general population. Journal of the American Dental Association. 1993;124:71–76. doi: 10.14219/jada.archive.1993.0247. [DOI] [PubMed] [Google Scholar]
- Osterberg T, Steen B. Relationship between dental state and dietary intake in 70-year-old males and females in goteborg, sweden: A population study. Journal of Oral Rehabilitation. 1982;9:509–521. doi: 10.1111/j.1365-2842.1982.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Ostir GV, Peek MK, Goodwin JS, Markides KS. Diabetes mellitus as a risk factor for hip fracture in mexican american older adults. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2002;57:M648–653. doi: 10.1093/gerona/57.10.m648. [DOI] [PubMed] [Google Scholar]
- Papas AS, Palmer CA, Rounds MC, Russell RM. The effects of denture status on nutrition. Special Care in Dentistry. 1998;18:17–25. doi: 10.1111/j.1754-4505.1998.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Hyer S, Tweed K, Kerry S, Allan K, Rodin A, Barron J. Risk factors for fractures and falls in older women with type 2 diabetes mellitus. Calcified Tissue International. 2008;82:87–91. doi: 10.1007/s00223-007-9082-5. [DOI] [PubMed] [Google Scholar]
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: A systematic review of health care interventions. Medical Care Research and Review. 2007;64:101S–156S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled M, Ardekian L, Tagger-Green N, Gutmacher Z, Machtei EE. Dental implants in patients with type 2 diabetes mellitus: A clinical study. Implant Dentistry. 2003;12:116–122. doi: 10.1097/01.id.0000058307.79029.b1. [DOI] [PubMed] [Google Scholar]
- Quandt SA, Chen H, Bell RA, Anderson AM, Savoca MR, Kohrman T, Gilbert GH, Arcury TA. Disparities in oral health status between older adults in a multiethnic rural community: The rural nutrition and oral health study. Journal of the American Geriatrics Society. 2009;57:1369–1375. doi: 10.1111/j.1532-5415.2009.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie CS, Burgio KL, Locher JL, Cornwell A, Thomas D, Hardin M, Redden D. Nutritional status of urban homebound older adults. American Journal of Clinical Nutrition. 1997;66:815–818. doi: 10.1093/ajcn/66.4.815. [DOI] [PubMed] [Google Scholar]
- Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, Handelsman Y, Horton ES, Lebovitz H, Levy P, Moghissi ES, Schwartz SS. Statement by an american association of clinical endocrinologists/american college of endocrinology consensus panel on type 2 diabetes mellitus: An algorithm for glycemic control. Endocr Pract. 2009;15:540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- Roumanas ED, Garrett NR, Hamada MO, Diener RM, Kapur KK. A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part v: Food preference comparisons. Journal of Prosthetic Dentistry. 2002;87:62–73. doi: 10.1067/mpr.2002.121025. [DOI] [PubMed] [Google Scholar]
- Roumanas ED, Garrett NR, Hamada MO, Kapur KK. Comparisons of chewing difficulty of consumed foods with mandibular conventional dentures and implant-supported overdentures in diabetic denture wearers. International Journal of Prosthodontics. 2003;16:609–615. [PubMed] [Google Scholar]
- Safkan-Seppala B, Ainamo J. Periodontal conditions in insulin-dependent diabetes mellitus. Journal of Clinical Periodontology. 1992;19:24–29. doi: 10.1111/j.1600-051x.1992.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Savoca MR, Arcury TA, Leng X, Chen H, Bell RA, Anderson AM, Kohrman T, Frazier RJ, Gilbert GH, Quandt SA. Severe tooth loss in older adults as a key indicator of compromised dietary quality. Public Health Nutr. 2010;13:466–474. doi: 10.1017/S1368980009991236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Older women with diabetes have an increased risk of fracture: A prospective study. Journal of Clinical Endocrinology and Metabolism. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- Sheiham A, Steele JG, Marcenes W, Lowe C, Finch S, Bates CJ, Prentice A, Walls AW. The relationship among dental status, nutrient intake, and nutritional status in older people. Journal of Dental Research. 2001;80:408–413. doi: 10.1177/00220345010800020201. [DOI] [PubMed] [Google Scholar]
- Shernoff AF, Colwell JA, Bingham SF. Implants for type ii diabetic patients: Interim report. Va implants in diabetes study group. Implant Dentistry. 1994;3:183–185. doi: 10.1097/00008505-199409000-00009. [DOI] [PubMed] [Google Scholar]
- Shigli K, Hebbal M. Does prosthodontic rehabilitation change the eating patterns among completely edentulous patients? Gerodontology. 2010 doi: 10.1111/j.1741-2358.2010.00404.x. [DOI] [PubMed] [Google Scholar]
- Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. Bone formation around titanium implants in the rat tibia: Role of insulin. Implant Dentistry. 2003;12:242–251. doi: 10.1097/01.id.0000074440.04609.4f. [DOI] [PubMed] [Google Scholar]
- Smith RA, Berger R, Dodson TB. Risk factors associated with dental implants in healthy and medically compromised patients. International Journal of Oral and Maxillofacial Implants. 1992;7:367–372. [PubMed] [Google Scholar]
- Sosa M, Saavedra P, Jodar E, Lozano-Tonkin C, Quesada JM, Torrijos A, Perez-Cano R, Nogues X, Diaz-Curiel M, Moro MJ, Gomez C, Mosquera J, Alegre J, Olmos J, Munoz-Torres M, Guanabens N, Del Pino J, Hawkins F. Bone mineral density and risk of fractures in aging, obese post-menopausal women with type 2 diabetes. The giumo study. Aging Clin Exp Res. 2009;21:27–32. doi: 10.1007/BF03324895. [DOI] [PubMed] [Google Scholar]
- Standards of medical care in diabetes. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawil G, Younan R, Azar P, Sleilati G. Conventional and advanced implant treatment in the type ii diabetic patient: Surgical protocol and long-term clinical results. International Journal of Oral and Maxillofacial Implants. 2008;23:744–752. [PubMed] [Google Scholar]
- Thomason JM, Lund JP, Chehade A, Feine JS. Patient satisfaction with mandibular implant overdentures and conventional dentures 6 months after delivery. International Journal of Prosthodontics. 2003;16:467–473. [PubMed] [Google Scholar]
- Touger-Decker R, Mobley CC. Position of the american dietetic association: Oral health and nutrition. Journal of the American Dietetic Association. 2003;103:615–625. doi: 10.1053/jada.2003.50130. [DOI] [PubMed] [Google Scholar]
- Tsakos G, Herrick K, Sheiham A, Watt RG. Edentulism and fruit and vegetable intake in low-income adults. Journal of Dental Research. 2010;89:462–467. doi: 10.1177/0022034510363247. [DOI] [PubMed] [Google Scholar]
- Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I. One-year clinical outcome of dental implants placed in patients with type 2 diabetes mellitus: A case series. Implant Dentistry. 2010;19:323–329. doi: 10.1097/ID.0b013e3181e40366. [DOI] [PubMed] [Google Scholar]
- Valerio G, del Puente A, Esposito-del Puente A, Buono P, Mozzillo E, Franzese A. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Hormone Research. 2002;58:266–272. doi: 10.1159/000066441. [DOI] [PubMed] [Google Scholar]
- van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, Birkenhager JC, Pols HA. Bone density in non-insulin-dependent diabetes mellitus. The rotterdam study. Annals of Internal Medicine. 1995;122:409–414. doi: 10.7326/0003-4819-122-6-199503150-00002. [DOI] [PubMed] [Google Scholar]
- van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M. The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clinical Oral Implants Research. 2002;13:617–622. doi: 10.1034/j.1600-0501.2002.130607.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporosis International. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Jr, Higginbottom FL. Periodontal diseases and dental implants in older adults. Journal of Esthetic Dentistry. 1998;10:265–271. doi: 10.1111/j.1708-8240.1998.tb00367.x. [DOI] [PubMed] [Google Scholar]
- World Workshop in Periodontics: Consensus report. Implant therapy ii. Annals of Periodontology. 1996;1:816–820. doi: 10.1902/annals.1996.1.1.816. [DOI] [PubMed] [Google Scholar]
- Wu B, Plassman BL, Liang J, Remle RC, Bai L, Crout RJ. Differences in self-reported oral health among community-dwelling black, hispanic, and white elders. Journal of Aging and Health. 2011;23:267–288. doi: 10.1177/0898264310382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysham CH. New perspectives in type 2 diabetes, cardiovascular risk, and treatment goals. Postgraduate Medicine. 2010;122:52–60. doi: 10.3810/pgm.2010.05.2142. [DOI] [PubMed] [Google Scholar]