Abstract
The rhythmic segmentation process of the presomitic mesoderm (PSM) orchestrates the formation of somites, the fundamental units for the vertebrate axial body plan. To aid the investigation of molecular components governing the conversion from PSM into somites, we generated a transgenic mouse line that expresses a tamoxifen (tmx) inducible CreERT2 under the control of a 2.5kb enhancer element of Tbx6, a gene essential for PSM formation and somite patterning. Combined with Cre reporters, this Tbx6;CreERT2 line displays robust tmx-inducible Cre activity in the PSM at various embryonic stages. This tool should be useful for studying gene function during somitogenesis by either conditional inactivation or mis-expression, and potentially coupled with cell marking.
Keywords: Tbx6, presomitic mesoderm, somite, segmentation, Cre, tamoxifen
The vertebrate segmented body plan is established during embryogenesis by a process known as somitogenesis. During this process, somites form periodically as bilateral epithelial spheres flanking the spinal cord, from the presomitic mesoderm (PSM) (Christ and Ordahl, 1995; Dequeant and Pourquie, 2008; Tam, 1981). Periodicity of somite formation is correlated with the “segmentation clock”, a molecular oscillator that pulses waves of gene expression throughout the PSM (Cooke and Zeeman, 1976; Palmeirim et al., 1997). Once the clock crosses a travelling determination front known as the “wavefront”, its activity ceases and synchronizes competent PSM cells to undergo somite formation (Cooke and Zeeman, 1976; Dubrulle et al., 2001). As somites are forerunners to the axial skeleton, their segmented organization prefigures the reiterated nature of vertebrae and ribs.
Certain genetic components controlling somite formation have been identified. For example, mutations in the Notch signaling pathway lead to defects in somitogenesis in a manner related to a disrupted segmentation clock (Bessho et al., 2001; Conlon et al., 1995; Evrard et al., 1998; Hrabe de Angelis et al., 1997; Wong et al., 1997; Zhang and Gridley, 1998). While most of these studies are based on germline mutations, recently conditional mutations and over-expression analysis have been conducted to tease out the mechanistic actions of selected players in the PSM (Aulehla et al., 2008; Feller et al., 2008; Wahl et al., 2007). To date there are only a handful of PSM-specific constitutive Cre expressing mouse lines and none have the advantage of temporal control for gene manipulation at different anterior and posterior (A/P) levels (Ji et al., 2006; Niwa et al., 2007; Perantoni et al., 2005; Wehn et al., 2009). Towards this goal, we sought to generate a mouse line expressing the CreERT2 gene specifically in the PSM for tmx-inducible Cre activity for recombining flanked loxP sites (Feil et al., 1996).
The T-box transcription factor Tbx6 interacts with the clock and wavefront and has a dual role in somitogenesis. First it is an essential mediator of the PSM fate for generating posterior somites (Chapman et al., 2003; Chapman and Papaioannou, 1998). Second, it has a critical role in A/P patterning of somites by synergizing with Notch signaling, a segmentation clock effector pathway (White and Chapman, 2005; White et al., 2003). Expression of Tbx6 commences on embryonic day 7.0 (E7.0) in the primitive streak and continues in the tailbud until the end of somitogenesis around E13.5 (Chapman et al., 1996). Extensive studies on the cis-regulatory sequences of Tbx6 identified a −2.3kb enhancer/promoter region that directs transgene expression in the PSM and newly formed somites (White et al., 2005). This regulatory region should theoretically be useful to drive CreERT2 expression in the PSM.
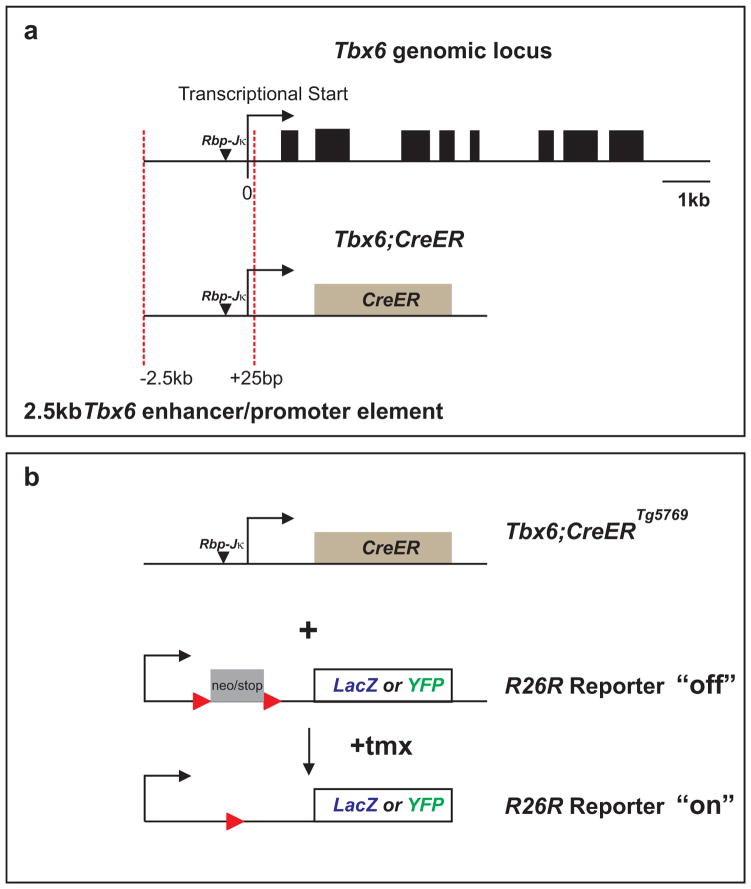
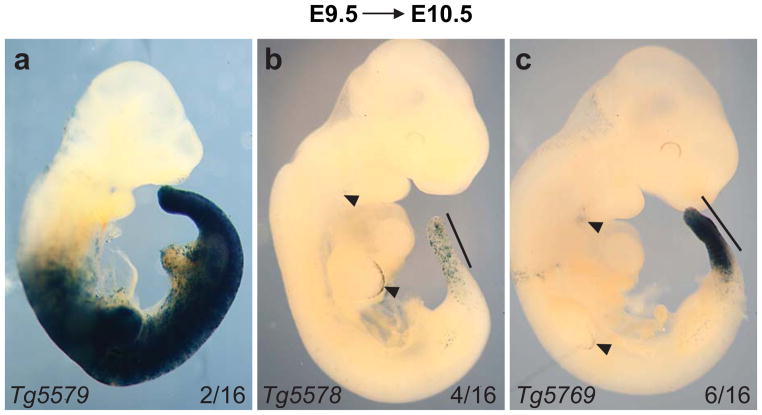
We created a transgene construct by cloning a −2.5kb to +25kb Tbx6 PSM-specific enhancer/promoter element which encompasses the −2.3kb region identified by White et al. (2005) upstream of a CreERT2 cassette (Fig.1a). Sixteen G0 lines were positive for the Tbx6;CreERT2 (referred to as Tbx6;CreER from here on) transgene as determined by polymerase chain reaction (PCR). To test for tmx-regulated Cre activity in the PSM, we crossed each transgenic line to R26RLacZ Cre reporter mice (Fig.1b). Cre activity was induced by a single intraperitoneal (IP) injection of tmx into pregnant R26RLacZ mice during mid-somitogenesis at E9.5. Embryos that were hemizygous for Tbx6;CreER and heterozygous for the reporter R26RLacZ (Tbx6;CreER;R26RLacZ/+) along with control littermates of various genotypic combinations were harvested 1 day later for β-galactosidase staining (using the X-gal substrate). We found that 6 transgenic lines were either sterile, negative for Cre activity, or ubiquitous for Cre activity in all mesodermal tissues (Fig.2a). In the remaining lines we observed low to high Cre activity in the PSM by this assay (compare Figs.2b and c). Line Tbx6;CreERTg5578;R26RLacZ/+ is representative of 4 lines that had highly mosaic Cre activity in the PSM (Fig.2b). Six lines similar to Tbx6;CreERTg5769;R26RLacZ/+ showed more uniform Cre activity in the PSM and its somite derivatives (Fig.2c). The Tbx6;CreERTg5769 line displayed tightly regulated tmx-inducible Cre activity as control injection of corn oil, without tmx did not result in positive β-galactosidase activity for Tbx6;CreERTg5769;R26RLacZ/+ embryos (not shown). We note however that tmx-induced Tg5578 and Tg5769 lines have small number of cells in the apical ectodermal ridge (AER) of the limb bud and 3rd brachial arch (Figs.2b,c) which may reflect either ectopic Cre activity due to insertion sites or differential sensitivities of using a permanent R26RLacZ reporter (in this study) versus a status-quo LacZ reporter driven by the enhancer element (White et al., 2005). Since Tbx6;CreERTg5769 was responsive to tmx-induction with PSM-specificity, we further characterized this transgenic founder line.
Fig. 1.
Diagram depicting Tbx6;CreER transgene construction and strategy used to screen for Cre activity in embryonic mice, Tbx6 genomic diagram was adapted from (White et al., 2005). (a) A CreERT2 (CreER) cassette was cloned downstream to a 2.5kb enhancer and promoter element from the Tbx6 genomic locus (red dotted lines). (b) Transgenic candidates such as Tbx6;CreERTg5769 were screened by crossing to LacZ or YFP report lines (R26RLacZ/LacZ or R26RYFP/YFP), followed by a single dose of tmx to activate CreER to remove the floxed neo/stop cassette (Soriano, 1999; Srinivas et al., 2001). Arrows mark the Tbx6 promoter. Black arrowheads mark Rbp-Jκ binding sites. Red arrowheads represent the location of lox-P sites in reporter mice.
Fig. 2.
Identification of a Tbx6;CreER line that mediates efficient Cre activity in the presomitic mesoderm (PSM) after 24 hour tmx-induction (E9.5→E10.5). Three types of transgenic lines with distinct patterns of Cre activity are shown here as Tbx6;CreER;R26RLacZ/+ X-gal stained embryos (a–c) representative of each category found. (a) 2/16 lines were positive for Cre activity in most mesodermal structures. (b) 4/16 lines had mosaic Cre activity in the PSM and newly formed somites (solid black line). (c) 6/16 embryos such as Tg5769 had robust Cre activity in the PSM and nascent somites (solid black line). Arrowheads mark X-gal stained cells in the apical ectodermal ridge (AER) and 3rd brachial arch. The name of each transgenic line is located in the bottom left corner of each image.
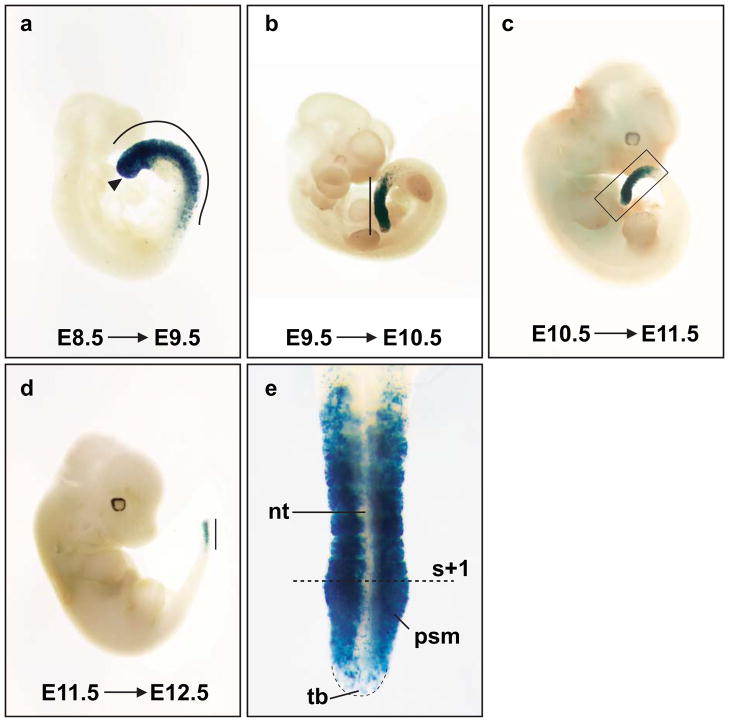
After backcrosses to the C57BL/6J background, the tmx-inducible PSM-specific Cre activity remained in the Tbx6;CreERTg5769 line when assayed at E9.5. As somitogenesis is a reiterated process that occurs over several days, we next wanted to ascertain the time window of Tbx6;CreERTg5769 directed Cre activity. We found that the Tbx6;CreERTg5769 transgene was active during most stages of somitogenesis by injecting tmx from E7.5 to E11.5 at daily intervals and examining reporter activity one day later (Figs.3 and 4). From E7.5 to E9.5, tmx-induced Cre activity was strongly active in the entire PSM and newly formed somites (Figs.3a and 4c). While tailbud stages (E10.5–12.5) had robust Cre activity in the anterior 3/4 of PSM and nascent somites, the tail bud was positive for only a few cells (Figs.3b–e). We have titrated the tmx dosage, and found the optimal dosage for PSM specific Cre activity to be ~2.25 mg tmx/35 gram body weight. Above this amount we noticed embryonic toxicity, while below this dosage, PSM and somites displayed mosaic reporter expression (data not shown). In mice, one pair of somites forms every two hours (Tam, 1981). Given this rate, fewer than the expected 12 somites were labeled for Cre activity in the 24 hours following tmx injection. We had expected a delay in Cre activity, as the precise time frames of tmx action and sufficient accumulation of reporter product for assay (from recombination, transcription to translation) are unknown. Importantly, Tbx6;CreERTg5769 confers tmx-inducible Cre activity in the PSM.
Fig. 3.
Tbx6;CreERTg5769 drives robust Cre activity during most stages of somitogenesis. Tbx6;CreERTg5769;R26RLacZ/+ embryos were tmx-induced (E8.5–E11.5) and harvested one day later for β–galactosidase staining. (a–d) presomitic mesoderm (PSM) and somite derivatives were positive for X-gal in all stages examined. (a) Cre activity is positive in the entire PSM, nascent somites (curved line) and primitive streak (black arrowhead). (b–e) Cre activity is down-regulated in the tailbud (tb) by stage E10.5 and onward. (e) Higher magnification of the boxed area in (c), the tb has few X-gal positive cells, while the anterior PSM and newly formed somites contains high levels of β-galactosidase activity. The dotted line marks the boundary in between the first budded somite (s+1) and the PSM. All solid lines mark the length of Cre activity in embryos. The blue tint in the neural tube (nt) (e) is a result from light scattering of the X-gal positive somites.
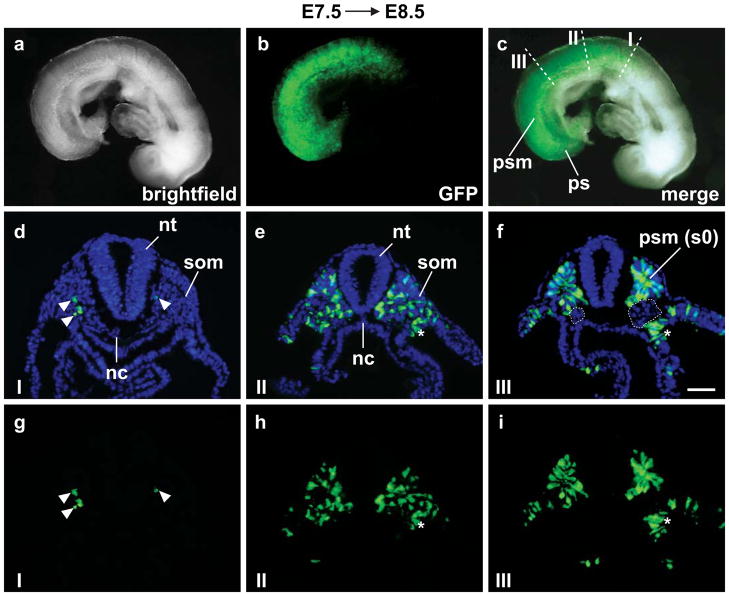
Fig. 4.
Tbx6;CreERTg5769 is active on the first day of somitogenesis (E7.5→E8.5). (a–c) Live Tbx6;CreERTg5769;R26RYFP/+ embryos exhibit fluorescent reporter activity along the A/P axis of the embryo including the presomitic mesoderm (psm), somites (som) and primitive streak (ps). (c) White dotted lines mark sections (I, II and III) merged for GFP and DAPI staining (d–f). (g–i) GFP only. (d and g) Section I has few +YFP cells (white arrowheads). Reporter activity increases posteriorly along the A/P axis of the embryo from moderate (e and h) to high in the presumptive somite (s0) of the psm (f and i). Cre activity was mosaic in the lateral plate mesoderm (white asterisks), labeling a few cells (e,h and f,i). (d–f) Cre activity was absent in the neural tube (nt), notochord (nc), intermediate mesoderm (white dotted circles), head and cardiac mesoderm (b,c). Scale bar=50 μm.
We further characterized tissue specificity of Tbx6;CreERTg5769 by using the R26RYFP Cre reporter and examined live tmx-induced E7.5 Tbx6;CreERTg5769;R26RYFP/+ embryos at E8.5 (Figs.4a–c). We found a gradual wave of YFP signal intensity along the A/P axis indicative of Cre activity in the somites and PSM as with the LacZ reporter. To pinpoint the labeled cells, we performed immunofluorescence analysis on transverse sections (Figs.4d–i). In rostral somites (section I), we found few YFP positive cells (Figs.4d,g). In more caudal somites (section II), YFP labeling occurred in approximately ~42% of cells (Figs.4e,h). At the most anterior PSM level, ~68% are YFP positive (Figs.4f,i). Few scattered YFP positive cells were present in the lateral plate mesoderm, possibly through expression of the transgene in the primitive streak (Figs.4e,h and f,i). On the other hand, YFP positive cells were absent from the notochord, intermediate mesoderm, head and cardiac mesoderm.
Here, we describe a new genetic tool that provides conditional manipulation of gene function in the PSM and somites at any time during somitogenesis. The Tbx6;CreERTg5769 line is unique among Cre lines that are currently used in the somite field (Ji et al., 2006; Niwa et al., 2007; Perantoni et al., 2005; Wehn et al., 2009). For example, the transgenic T-Cre exhibits high Cre activity in all mesodermal tissues, in addition to notochord, head and cardiac mesoderm (Perantoni et al., 2005). Similar to T-Cre, Dll1-msd Cre is widely expressed in the intermediate and lateral plate mesoderm, although Cre activity does overlap with Tbx6;CreERTg5769 in the anterior PSM and somites (Wehn et al., 2009). Aside from greater tissue specificity, Tbx6;CreERTg5769 provides temporal control that does not exist in constitutively active Cre lines in use to study somitogenesis. This genetic tool has the potential to test the function of segmentation clock or somite patterning genes at the desired A/P level of an embryo by either inactivation or mis-expression. Furthermore, since we observed mosaic Cre activity at lower tmx dosages, we envision that titrating tmx concentrations will enable the study of cell autonomous gene function at the single cell level. This would require the concomitant labeling of genetically modified cells by a cis-linked Cre reporter provided by the investigator.
Materials and Methods
Vertebrate animals
All experimental procedures involving live mice complied with institutional and national animal welfare laws, guidelines and policies, and are approved by Institutional Animal Care and Use Committee of Carnegie Institution of Washington.
Generation of Tbx6;CreERT2 transgenic mice
To obtain the Tbx6 enhancer and promoter fragment, we amplified a region −2.5 kb upstream and +25bp downstream from the transcriptional start site of Tbx6 using polymerase chain reaction (PCR) on a BAC template (CHORI BAC ID: RP24-239G12): primers 5′-ATAGCGGCCGCTGGAT GCCCCATTGCAAAGACAGTC-3′ and 5′-GCGTCTAGAGTTGTAGTTTCTTCTGGCCTTGTGTCC-3′. This product was digested with Not1 and Xba1, cloned 5′ to the CreERT2 expression cassette with a 3′ SalI site (Feil et al., 1997) in the pBluescript SK+ vector (Stratagene), and sequence verified. This plasmid was Not1-SalI digested and the transgene fragment was purified for pronuclear injection into C57BL/6 × DBA/2 F1-hybrid embryos. Of 150 injected embryos, 54 grew to adulthood, and 16 were positive for Cre by PCR, (Feil et al., 1996). The Tbx6;CreERTg5769 founder line was backcrossed to the C57BL/6J (JAX) background for eight generations.
Preparation of tamoxifen
A 20 mg/ml tmx citrate (Sigma T5648) solution dissolved in corn oil (Sigma C8267) was used in this study. After solubilization, 10 ml aliquots were flash frozen and were stored up to 6 months at −80°C.
Characterization of Tbx6;CreER transgenic mice for PSM-specific Cre activity
Tbx6;CreERT2(Tbx6;CreER) transgenic lines were crossed to R26RLacZ/LacZ or R26RYFP/YFP Cre reporter mice (Soriano, 1999; Srinivas et al., 2001). Pregnant mice were intraperitoneal (IP) injected with a single dose of ~2.25 mg tmx/35 gram of body weight at stages specified in the text (E7.5–E11.5; E=embryonic day; the vaginal plug date was designated as E0.5). Embryos were harvested 24 hours later in L-15 media (Gibco #11415064) and the yolk sac was used for genotyping by PCR. β-galactosidase staining followed standard procedures using the X-gal substrate (Hogan, 1994). Live fluorescence of Tbx6;CreER;R26RYFP embryos (in PBS) was visualized under a Leica fluorescent dissecting scope and images were captured with a SPOT camera. For immunofluorescence, embryos were fixed in 4% paraformaldehyde (PFA)/PBS at room temperature for 2 hours, equilibrated in 20% sucrose/PBS, followed by quick freeze-embedding in OCT. 10 μm transverse sections were taken, post-fixed for 5 minutes in 4% PFA/PBS, blocked with 10% bovine serum albumin (Sigma A9418) in 0.1% TritonX-100/PBS for 1 hour, then blocked in 10% goat serum in 0.1% TritonX-100/PBS for another hour. Chick anti-GFP (1:200; Aves labs #GFP-1020) was used for overnight incubation at 4°C. After washing, Goat anti-chick Alexa fluor 488 (1:1000; Molecular Probes #A-11039) was incubated for 2 hours. After wash, slides were counterstained with DAPI, rinsed, and mounted in Fluoromount-G (Southern Biotech #0100-01). Fluorescent images were taken with an AxioCam. Digital images were processed by Metamorph and Photoshop software for merging and brightness/contrast adjustments respectively.
Acknowledgments
The authors thank the Fan lab members for reading the manuscript, Evan Siple and Samantha Satchell for genotyping assistance. The Harvard transgenic facility performed the pronuclei injection as a paid service. This work is funded by the Carnegie Institution Endowment and NIH grants to CMF and TPL (HD035596 and HD035596-S).
Footnotes
Author contribution: Both authors contributed to experimental designs and manuscript writing.
Competing interest: The authors declare no potential conflict of interests, financially or otherwise.
References
- Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, Lewandoski M, Pourquie O. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat Cell Biol. 2008;10:186–193. doi: 10.1038/ncb1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001;15:2642–2647. doi: 10.1101/gad.930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Cooper-Morgan A, Harrelson Z, Papaioannou VE. Critical role for Tbx6 in mesoderm specification in the mouse embryo. Mech Dev. 2003;120:837–847. doi: 10.1016/s0925-4773(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Cooke J, Zeeman EC. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol. 1976;58:455–476. doi: 10.1016/s0022-5193(76)80131-2. [DOI] [PubMed] [Google Scholar]
- Dequeant ML, Pourquie O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature. 1998;394:377–381. doi: 10.1038/28632. [DOI] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Feller J, Schneider A, Schuster-Gossler K, Gossler A. Noncyclic Notch activity in the presomitic mesoderm demonstrates uncoupling of somite compartmentalization and boundary formation. Genes Dev. 2008;22:2166–2171. doi: 10.1101/gad.480408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BBR, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Hrabe de Angelis M, McIntyre J, 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- Ji SJ, Zhuang B, Falco C, Schneider A, Schuster-Gossler K, Gossler A, Sockanathan S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev Biol. 2006;297:249–261. doi: 10.1016/j.ydbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Masamizu Y, Liu T, Nakayama R, Deng CX, Kageyama R. The initiation and propagation of Hes7 oscillation are cooperatively regulated by Fgf and notch signaling in the somite segmentation clock. Dev Cell. 2007;13:298–304. doi: 10.1016/j.devcel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP. The control of somitogenesis in mouse embryos. J Embryol Exp Morphol. 1981;65(Suppl):103–128. [PubMed] [Google Scholar]
- Wahl MB, Deng C, Lewandoski M, Pourquie O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development. 2007;134:4033–4041. doi: 10.1242/dev.009167. [DOI] [PubMed] [Google Scholar]
- Wehn AK, Gallo PH, Chapman DL. Generation of transgenic mice expressing Cre recombinase under the control of the Dll1 mesoderm enhancer element. Genesis. 2009;47:309–313. doi: 10.1002/dvg.20503. [DOI] [PubMed] [Google Scholar]
- White PH, Chapman DL. Dll1 is a downstream target of Tbx6 in the paraxial mesoderm. Genesis. 2005;42:193–202. doi: 10.1002/gene.20140. [DOI] [PubMed] [Google Scholar]
- White PH, Farkas DR, Chapman DL. Regulation of Tbx6 expression by Notch signaling. Genesis. 2005;42:61–70. doi: 10.1002/gene.20124. [DOI] [PubMed] [Google Scholar]
- White PH, Farkas DR, McFadden EE, Chapman DL. Defective somite patterning in mouse embryos with reduced levels of Tbx6. Development. 2003;130:1681–1690. doi: 10.1242/dev.00367. [DOI] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Zhang N, Gridley T. Defects in somite formation in lunatic fringe-deficient mice. Nature. 1998;394:374–377. doi: 10.1038/28625. [DOI] [PubMed] [Google Scholar]