Abstract
Breastfeeding rates in the United States are low, and one possible reason may be the high prevalence of overweight/obesity among women of childbearing age. This analysis examined the association between pregravid body mass index and breastfeeding duration, and explored whether depressive symptoms, perceived stress and anxiety during pregnancy mediated this relationship. Participants (n = 550) in the Pregnancy, Infection and Nutrition Postpartum Study were recruited through prenatal clinics prior to 20 weeks gestation and followed to 12 months post‐partum. Duration of any breastfeeding was categorized as none, less than 4 months, 4–6 months, 7–12 months and more than 12 months (referent). Exclusive breastfeeding was categorized as less than 1 month, 1 to less than 4 months and 4 months or more (referent). Being overweight/obese before pregnancy (35.7% of 550) was inversely associated with the durations of any and exclusive breastfeeding. Women who entered pregnancy overweight or obese were more likely to not initiate breastfeeding [relative risk ratio (RRR) = 5.39 (95% confidence interval: 2.41, 12.04)] and to breastfeed less than 4 months [RRR = 2.38 (1.33, 4.27)] compared with women of normal weight status. Among women who initiated breastfeeding, being overweight or obese vs. normal weight was related to exclusively breastfeeding less than 1 month [RRR = 2.09 (1.24, 3.51)]. We did not find evidence to support mediation by depressive symptoms, perceived stress or anxiety during pregnancy. Future research needs to explore the reasons behind the association between overweight/obesity and breastfeeding duration.
Keywords: overweight, obesity, pregravid BMI, pregnancy, breastfeeding duration, depressive symptoms, stress, anxiety
Introduction
The American Academy of Pediatrics (AAP) promotes exclusive breastfeeding for the first 6 months of life followed by partial breastfeeding up to at least 1 year of age (World Health Organization 2003a; Gartner et al. 2005). However, adherence to these recommendations is low. Three‐quarters of women in the United States initiate breastfeeding, but only 13.3% of women exclusively breastfeed through 6 months post‐partum (Centers for Disease Control and Prevention 2010). In addition, the prevalence of any breastfeeding through 6 and 12 months is 43% and 22.4%, respectively (Centers for Disease Control and Prevention 2010). Low breastfeeding rates may be explained, in part, by the rise in overweight/obesity among women of childbearing age. There is evidence that women who enter pregnancy at a higher body mass index (BMI) are less likely to initiate breastfeeding and more likely to breastfeed for a shorter duration, but reasons behind this association are unclear (Li et al. 2003; 2004, 2007; Oddy et al. 2006). It may be that women who start pregnancy overweight or obese face more biological, physical, psychosocial and psychological barriers to breastfeeding than women of normal BMI (Rasmussen & Kjolhede 2004; Amir & Donath 2007).
Psychological factors present during pregnancy may account for part of the association between maternal overweight/obesity and breastfeeding duration, but few have researched the possibility of a mediatory pathway. Studies have linked obesity to poor mental health outcomes in the perinatal period (path a, Fig. 1) (Bodnar et al. 2009; Laraia et al. 2009). Further, psychological factors have been negatively associated with breastfeeding duration (path b, Fig. 1) (Hatton et al. 2005; Dunn et al. 2006; Dennis & McQueen 2007, 2009; O'Brien et al. 2009). To our knowledge, one epidemiologic study has accounted for the effect of psychological factors on pregravid BMI and breastfeeding duration. Hilson and colleagues (2004) adjusted for psychological factors such as maternal confidence in breastfeeding and body satisfaction, and found that they attenuated but did not eliminate the significant association between pregravid BMI and breastfeeding duration.
Figure 1.
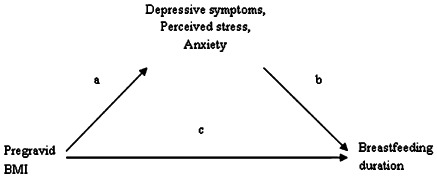
Mediation model of the associations between pregravid BMI, breastfeeding duration, and psychological factors.
We used data from the post‐partum component of the Pregnancy, Infection and Nutrition (PIN) study to examine whether women who started pregnancy overweight or obese were less likely to follow current breastfeeding guidelines. Further, we explored whether depressive symptoms, perceived stress and anxiety during pregnancy partially mediated the association between pregravid BMI and breastfeeding duration.
Key messages
-
•
Women who entered pregnancy overweight or obese were less likely to adhere to American Academy of Pediatrics guidelines for breastfeeding duration.
-
•
Depressive symptoms, perceived stress and anxiety did not help to explain the negative association between overweight/obesity and breastfeeding duration.
-
•
Future research needs to explore the reasons behind the association between overweight/obesity and breastfeeding duration.
Materials and methods
The PIN Postpartum study (PINPost) was a prospective cohort study focused on post‐partum weight retention, infant feeding, diet, physical activity and psychological factors (Deierlein et al. 2008; Siega‐Riz et al. 2009). Women between 15 and 20 gestational weeks (n = 3203) were recruited at their second prenatal visit at University of North Carolina (UNC) hospitals between January 2001 and June 2005. Women ineligible to participate included those younger than 16, non‐English speaking, greater than or equal to 20 weeks' gestation on their second pre‐natal visit, not planning to continue care or deliver at the study site, and those carrying multiple gestations. Of the 2006 women followed through pregnancy in the PIN study, 1169 were eligible to participate in PINPost.
To participate in PINPost, women must have delivered a live‐born infant between October 2002 and December 2005, and have resided within a 2‐hour radius from UNC in order to facilitate home visits (Siega‐Riz et al. 2009). A total of 239 women were excluded from PINPost because of medical constraints (n = 24), they were unreachable (n = 153), they were more than 5 months post‐partum by the time they were contacted (n = 54) and, for eight women, study protocols were not in place at the time of their eligibility window. The remaining women (n = 930) were phoned 6 weeks after delivery with a description of the post‐partum component. Those who agreed to participate were interviewed in their homes by trained staff at 3 months (n = 688), 12 months (n = 550) and 36 months post‐partum (n = 409). This analysis examined those who consented and participated in both the 3‐ and 12‐month post‐partum visits (n = 550); data from the 36‐month interview was used to update time of breastfeeding cessation for those women who were still breastfeeding at the 12‐month interview. Protocols for the prenatal and post‐partum studies, as well as this analysis, were approved by the UNC School of Medicine Institutional Review Board.
The outcome variables for this analysis were any and exclusive breastfeeding duration, created using data collected during the 3‐, 12‐ and 36‐month post‐partum interviews. Any breastfeeding duration included women who exclusively or partially breastfed. Initiation of breastfeeding was determined at the 3‐month interview by asking women, ‘Did you ever breastfeed this baby?’ Those who initiated were then asked, ‘Are you still breastfeeding your baby?’, at the 3‐, 12‐ and 36‐month interviews. If women reported having stopped breastfeeding, they were asked how old the infant was when they stopped (reported in days/weeks/months). Duration of any breastfeeding was categorized as follows: none, those who breastfed less than 4 months, 4–6 months, 7–12 months and more than 12 months (referent).
Exclusive breastfeeding included infants fed only breast milk with the exception of drops or syrups consisting of vitamins, mineral supplements or medicines (World Health Organization 2003b). Exclusive breastfeeding duration was determined by comparing total duration of any breastfeeding with the age at which formula and complementary foods were introduced. Women were considered to have stopped exclusively breastfeeding when a non‐breast milk food (including formula) was introduced. At the 3‐ and 12‐month interviews, participants reported whether they fed their infant breast milk, breast milk substitutes and other foods such as cereals, tea, juice, fruits/vegetables and meats for each post‐partum month. A more detailed list of foods can be found in the questionnaires for the 3‐ and 12‐month interviews at http://www.cpc.unc.edu/pin. For this analysis, we categorized exclusive breastfeeding as follows: less than 1 month, 1 to less than 4 months and 4 months or more (referent). The cut point of 4 months was based on current AAP guidelines, which are conflicting. The AAP recommends that women should exclusively breastfeed for up to 6 months, but they also state that complementary foods may be introduced as early as 4 months based on the ‘unique needs or feeding behaviors of the individual infants’ (Gartner et al. 2005).
The main exposure variable was pregravid BMI (kg m−2) calculated from self‐reported pregravid weight and measured height collected during a prenatal clinic visit between 15 and 20 weeks gestation (screening), or at the 3‐month post‐partum visit. Weight was checked for implausible values, and three participants were excluded from analysis, leaving 547 women with complete information on pregravid BMI and breastfeeding duration. Pregravid BMI was a dichotomous variable created using the World Health Organization cut points for normal weight/underweight (≤24.9 kg m−2) and overweight/obese (≥25.0 kg m−2) (World Health Organization 2000).
Possible mediators
Depressive symptoms, perceived stress and state anxiety were measured at two time points in pregnancy. This analysis used the second measure, which was closer in time to the outcome of interest, breastfeeding duration. Measurements of depressive symptoms, perceived stress and state anxiety for both time points were reasonably well correlated (correlation coefficients = 0.66, 0.68 and 0.56, respectively).
Depressive symptoms during pregnancy were assessed using the Center for Epidemiologic Studies‐Depression Scale (Radloff 1977). Mail‐in questionnaires given at the second prenatal visit between 24 and 29 weeks' gestation were completed by 490 (89% of 550) participants. The 20‐item scale had Likert response categories that assessed the participant's feelings and activities in the previous week. A composite score was calculated, and scores greater than or equal to 17 indicated the presence of a higher level of depressive symptoms. Although a cut point of 16 or higher has been associated with a significant level of depressive symptoms in the general population, we used a slightly higher cut point to distinguish between depressive and pregnancy symptoms, which are often similar (Orr & Miller 1995). We compared our method of a higher cut point with that proposed by Hoffman & Hatch (2000), in which they used a cut point of 16 after removing items that overlapped with pregnancy and rescaling scores so that the range still lay between 0 and 60. There was no difference in how women were categorized between the two methods (data not shown). Internal consistency as indicated by Cronbach's alpha ranged from 0.83 to 0.92 (Neugebauer et al. 1992).
The Perceived Stress Scale (Cohen & Williamson 1988) measured the degree to which respondents found situations to be stressful. Of 550 participants, 527 (95.8% of 550) completed a modified 10‐item scale administered during a phone interview conducted between 27 and 30 gestational weeks. Questions were on a Likert scale, and higher overall scores indicated higher levels of perceived stress. After summing across items, the variable was categorized into three levels: 0 to <11 (referent), 11 to <17 and ≥17. Cronbach's alpha was 0.83 in three non‐pregnant samples tested by Cohen et al. (1983).
The State‐Trait Anxiety Inventory was used to assess state and trait anxiety during pregnancy (Spielberger 1983). For this analysis, we used the state anxiety measurement because it assessed ‘immediate’ feelings of anxiety, which better represented how women felt during pregnancy than the trait‐anxiety scale, a stable measure of anxiety. The majority of participants (88.5% of 550) completed the mail‐in questionnaire provided at the second prenatal visit (24–29 weeks). Scores from 20 questions on a 4‐point Likert scale were summed and categorized into three levels: 0 to <29 (referent), 29 to <39 and ≥39. Cronbach's alpha ranged from 0.90 to 0.94 for the state scale (Spielberger 1983).
Covariates
Although the PINPost study collected data on a wide range of variables, we discuss here only those that were tested for confounding. These covariates were chosen based on a directed acyclic graph, created from a review of the literature (Greenland et al. 1999), and on the strength of their relationship with exposure and outcome. Data were collected at screening (15–20 weeks' gestation) and through self‐reported questionnaires, telephone interviews and medical chart abstraction. Participants reported their race, age, parity, family income, household size, education, marital status and smoking status in the first 6 months of pregnancy. Information on family income and household size was used to create a variable representing percentage of the 2001 poverty index according to the US Bureau of the Census (Proctor & Dalaker 2001).
Statistical analyses
The models for pregravid BMI and duration of any breastfeeding were restricted to participants for whom we had complete information on pregravid BMI (n = 547). The models for pregravid BMI and duration of exclusive breastfeeding were limited to participants for whom we had complete information on pregravid BMI and who initiated breastfeeding (n = 509). There are several ways to analyse polytomous outcomes. We originally considered analysing the data using a cumulative logit ordinal regression model, which would have taken into account the natural order of the outcome categories. However, the proportional odds assumption was violated for the majority of our explanatory variables. Hence, we calculated relative risk ratios (RRRs) for the association between pregravid BMI and breastfeeding duration using multinomial logit models. Multinomial models include two sets of referents categories, one for the outcome category and one for the exposure.
We were unable to analyse effect measure modification because of low power. Backward elimination was used to build our adjusted model, and covariates were kept as confounders in the model if they changed the beta coefficients of the exposure categories by greater than 10%.
Mediation was examined using a series of regression analyses (Baron & Kenny 1986). To be considered a mediator, the exposure must be associated with the outcome (path c in Fig. 1), the exposure must be associated with the mediator (path a, Fig. 1), the mediator must be associated with the outcome while adjusting for the exposure (path b, Fig. 1) and the effect estimate of the exposure must fully or partially reduce while adjusting for the mediator. A third regression model was added to determine the relationship between the potential mediator and breastfeeding duration when pregravid BMI was not accounted for because few have researched these associations, specifically in regard to stress and anxiety. Each psychological factor was tested in separate mediation analyses. All statistical analyses were conducted using Stata software (version 9.2, College Station, TX, USA).
Results
The majority of participants initiated breastfeeding (92.6% of 550 women). Duration of any breastfeeding ranged from 0 to 38.6 months with a mean duration of 8.0 ± 6.3 months. Prevalence at 3 and 6 months was 67.5% and 56.7%, respectively. Women who did not initiate and those who breastfed less than 4 months were more likely to be overweight or obese, while those who breastfed longer were more likely to be of normal BMI (Table 1). Compared with normal weight women, those who entered pregnancy overweight or obese were more likely to breastfeed for a shorter duration (any and exclusive) and to introduce complementary foods earlier (P < 0.01).
Table 1.
Maternal characteristics by pregravid BMI and breastfeeding status among women in the Pregnancy, Infection and Nutrition Postpartum study (n = 550)
| Pregravid BMI | ||
|---|---|---|
| Normal weight (≤24.9 kg m−2) | Overweight/obese (≥25.0 kg m−2) | |
| n = 355 | n = 192 | |
| Pregravid BMI (kg m−2) | 21.4 ± 1.9* | 32.1 ± 6.8 † |
| Age (years) | 30.2 ± 5.2 | 29.4 ± 5.8 |
| Race: white (%) | 86.8 ‡ | 64.1 |
| Married (%) | 90.7 ‡ | 70.8 |
| Education (years) | 16.6 ± 2.5 | 14.9 ± 2.7 † |
| Percent of the 2001 poverty level | 479.2 ± 204.1 | 351.7 ± 219.4 † |
| Primiparous (%) | 51.6 | 42.2 † |
| Smoked during pregnancy (%) | 4.7 | 13.8 † |
| Breastfeeding duration (months) | 9.2 ± 6.2 | 5.9 ± 6.1 † |
| No breastfeeding (%) | 3.4 ‡ | 15.1 |
| >0 to <4 (%) | 20.9 | 37.5 |
| 4 to 6 (%) | 10.1 | 6.3 |
| >6 to <12 (%) | 32.7 | 24.0 |
| >12 (%) | 33.0 | 17.2 |
| Exclusive breastfeeding (months) | 3.5 ± 2.2 | 2.5 ± 2.3 † |
| <1 (%) | 17.2 ‡ | 31.9 |
| >0 to <4 (%) | 23.9 | 30.7 |
| ≥4 (%) | 58.9 | 37.4 |
| Age of complementary food introduction (months) | 4.7 ± 1.3 | 4.0 ± 1.7 † |
| Depressive symptoms (% with high levels) | 17.4 | 29.5 † |
| Perceived stress | ||
| Low (%) | 37.9 ‡ | 32.0 |
| Moderate (%) | 40.8 | 33.2 |
| High (%) | 21.3 | 34.8 |
| State anxiety | ||
| Low (%) | 46.4 ‡ | 43.3 |
| Moderate (%) | 35.8 | 26.8 |
| High (%) | 17.8 | 29.9 |
BMI, body mass index. *X ± standard deviation (all such values). †Significantly different from normal weight women: t‐test P < 0.01. ‡Pearson's chi‐squared test P < 0.05.
Of the 509 women who breastfed, more than half (51.7%) exclusively breastfed for 4 months or longer. Duration of exclusive breastfeeding ranged from 0 to 9 months with a mean duration of 3.2 ± 2.3 months. Women who exclusively breastfed less than 1 month and 1 to <4 months had mean BMIs of 26.7 kg m−2 and 25.4 kg m−2, respectively. Women who exclusively breastfed for 4 months or longer were more likely to be white (90.0%), be married (94.3%), of higher income (mean = 492.3% ± 186.0 of the poverty line) and have more years of education completed (mean = 17.1 ± 2.1 years).
Women with low levels of depressive symptoms, stress and anxiety tended to be white, be married, of higher income and have more years of education completed. They were also more likely to enter pregnancy at a BMI ≤ 24.9 kg m−2 and to breastfeed (any or exclusively) longer than women with high levels of these factors.
Crude multinomial regression showed a strong negative association between pregravid BMI and duration of any breastfeeding (Table 2). After adjusting for race, education, marital status and smoking in the first 6 months of pregnancy, the effect estimate for the overweight/obese BMI category was attenuated. However, overweight/obese women remained at higher risk of not breastfeeding [RRR = 5.39 (95% confidence interval: 2.41, 12.04)] and of breastfeeding less than 4 months [RRR = 2.38 (1.33, 4.27)] compared with normal/underweight women.
Table 2.
Adjusted results for multinomial logit regression* models of the association between pregravid BMI and breastfeeding duration
| Breastfeeding duration | Pregravid BMI | |
|---|---|---|
| Overweight/obese | ||
| Crude RRR (95% CI) | Adjusted RRR (95% CI) | |
| Any breastfeeding (months) † | ||
| No breastfeeding | 8.57 (3.94, 18.63) | 5.39 (2.41, 12.04) |
| >0 to <4 | 3.45 (2.08, 5.73) | 2.38 (1.33, 4.27) |
| 4 to 6 | 1.18 (0.55, 2.53) | 0.91 (0.39, 2.14) |
| >6 to 12 | 1.41 (0.83, 2.37) | 1.34 (0.77, 2.32) |
| >12 | 1.00 | 1.00 |
| Exclusive breastfeeding (months) [Link] , [Link] | ||
| <1 | 2.92 (1.82, 4.67) | 2.09 (1.24, 3.51) |
| <4 | 2.02 (1.29, 3.17) | 1.41 (0.86, 2.33) |
| ≥4 | 1.00 | 1.00 |
BMI, body mass index; RRR, relative risk ratio; CI, confidence interval. *There are two referent categories in multinomial logit regression models, one for the exposure (normal weight/underweight) and one for the outcome variable (any breastfeeding >12 months; exclusive breastfeeding ≥4 months). †Model of the association between pregravid BMI and any breastfeeding was adjusted for race, maternal education, marital status and smoking in the first 6 months of pregnancy (n = 524). ‡Model of the association between pregravid BMI and exclusive breastfeeding was adjusted for race, maternal education and percent of the 2001 poverty index (n = 493). §Does not include women who did not initiate breastfeeding (n = 41).
Being overweight or obese before pregnancy was associated with shorter duration of exclusive breastfeeding in crude analysis (Table 2). After adjusting for race, education and poverty status, the association between pregravid BMI and exclusive breastfeeding duration decreased. Being overweight/obese remained associated with exclusively breastfeeding less than 1 month [RRR = 2.09 (1.24, 3.51)], but there was no longer an association with exclusive breastfeeding less than 4 months.
Mediation
The association between pregravid BMI and any breastfeeding duration was not explained by depressive symptoms, perceived stress or state anxiety status during pregnancy. All three psychological factors were significantly predicted by pregravid BMI (path a in Fig. 1; model 2 in Supporting Information Tables S1–S3). Higher levels of depressive symptoms were related to breastfeeding less than 4 months (path b in Fig. 1; model 3 in Supporting Information Table S1), but this association no longer remained significant once pregravid BMI was in the model (model 4, Supporting Information Table S1). Thus, depressive symptoms did not fulfil the criteria to be a mediator. In addition, once we adjusted for confounders (race, education, marital status and smoking in the first 6 months of pregnancy), pregravid BMI no longer predicted the psychological factors (path a, Fig. 1).
When examining mediation of the pregravid BMI‐exclusive breastfeeding relationship, higher levels of depressive symptoms and perceived stress were predicted by pregravid BMI (path a in Fig. 1; model 2 in Supporting Information Tables S4–S6). The presence of high depressive symptoms during pregnancy was associated with exclusive breastfeeding duration of less than 1 month and 1 to <4 months; high stress predicted exclusive breastfeeding less than 1 month (path b in Fig. 1; model 3 in Supporting Information Tables S4–S6). Depressive symptoms and stress remained associated with the outcome once pregravid BMI was added to the model (model 4 in Supporting Information Tables S4,S5), but they accounted for only a small part of the association between pregravid BMI and duration of exclusive breastfeeding (i.e. <10%). Further, adjusting for race, education and poverty status greatly attenuated, and made non‐significant, the association between depressive symptoms/stress and exclusive breastfeeding. The association between pregravid BMI and psychological factors also disappeared in the adjusted models.
Discussion
In these analyses, we found an inverse association between pregravid BMI and breastfeeding duration. Specifically, our results suggest that women who enter pregnancy overweight or obese are less likely to initiate breastfeeding and to breastfeed the amount of time recommended by current AAP guidelines; these associations were not explained by depressive symptoms, stress and anxiety during pregnancy. We find that our results of a negative association between pregravid BMI and any breastfeeding duration are consistent with those of studies conducted worldwide, despite differences in population and statistical methodology. Studies in Danish and Australian populations found that overweight and obese women are at greater risk of earlier termination of any breastfeeding than normal BMI women (Donath et al. 2003; Oddy et al. 2006; Baker et al. 2007), while studies conducted among US populations (Li et al. 2003; Hilson et al. 2004) found that being obese, but not overweight, was related to shorter duration of any breastfeeding. Although we found that being overweight or obese before pregnancy was inversely related to exclusive breastfeeding, one study in the United States found no association between pregravid BMI and exclusive breastfeeding, but this may have been because of small sample size (n = 151) (Hilson et al. 2004). Another reported higher risk of cessation for overweight/obese women up to 16 weeks post‐partum (Baker et al. 2007).
This study is unique in that women who did not initiate breastfeeding were included in the analyses. Previous studies conducted analyses only among women who initiated, which limits interpretation of the effects of obesity on breastfeeding duration to those who breastfeed and may be a source of selection bias (Donath et al. 2003; Li et al. 2003; Hilson et al. 2004; Oddy et al. 2006; Baker et al. 2007). From a prior analysis (Mehta et al. 2011), we know that being overweight or obese before pregnancy strongly predicts not initiating breastfeeding in our study population. Excluding non‐breastfeeders in our analyses, then, would have produced an artificially attenuated effect estimate of the association between obesity and breastfeeding duration. For example, an obese woman may choose not to initiate breastfeeding in the current pregnancy because she experienced obesity‐related mechanical difficulties breastfeeding a previous child. In our study, overweight and obese women were more likely to be multiparous (P < 0.05).
Although we found that depressive symptoms, perceived stress and anxiety did not mediate the association between pregravid BMI and breastfeeding duration, there existed differences in the crude associations between these psychological factors and breastfeeding duration. For instance, all three factors were predictive of exclusively breastfeeding less than 1 month. However, only the presence of a high level of depressive symptoms during pregnancy was associated with the duration of any breastfeeding, specifically, a higher risk of breastfeeding less than 4 months. Previous studies support an association between high levels of depressive symptoms during pregnancy and shorter duration of any breastfeeding for women (Cooper et al. 1993; Dunn et al. 2006; Pippins et al. 2006). Depression during pregnancy is a strong predictor of post‐partum depression (American Psychiatric Association 2000), which can influence parenting practices (McLearn et al. 2006; Hurley et al. 2008). There is evidence that women with high levels of depressive symptoms, stress and anxiety in the post‐partum are less likely to engage in responsive infant feeding styles (Hurley et al. 2008) and in parenting practices that require active interactions with their infant, such as breastfeeding (McLearn et al. 2006). It is possible, then, that women who experience high levels of depressive symptoms during pregnancy are more likely to provide formula along with breast milk in early post‐partum. Providing formula makes it possible for a partner or family member to bottle‐feed the child, thus reducing the time that a woman experiencing depressive symptoms is forced to spend breastfeeding, an activity that requires a lot of active interaction with their child. In our study population, significantly greater proportions of women with high levels of depressive symptoms, stress and anxiety during pregnancy gave their infants formula in the first month compared with women with low levels of these factors (P < 0.05).
This study has several limitations that influence its interpretation and generalizability. Our study population is different from the general US population in that 92.6% initiated breastfeeding, and of those who initiated, almost 50% exclusively breastfed 4 months or more, which is much higher than national rates (Centers for Disease Control and Prevention 2009). We also have a much lower prevalence of overweight/obese women (35.7%) compared with the average for women of childbearing age in the United States (59.5%) (Flegal et al. 2010). In addition, out of the 1169 that were eligible, 480 did not to participate in PINPost. A comparison of the 480 to the 688 women who completed the 3‐month visit revealed that women who did not participate in PINPost were more likely to be overweight or obese before pregnancy, be non‐white, be younger, of lower income, less educated and have smoked during pregnancy. Further, they had significantly higher levels of depressive symptoms and anxiety during pregnancy than women who completed the 3‐month interview. This was also true of the 138 women who did not participate in the 12‐month post‐partum interview (20% of 688). Additionally, those lost between the 3‐ and 12‐month post‐partum interviews were less likely to have initiated breastfeeding compared with women who completed the 12‐month interview.
Nationally representative data show that there is a higher proportion of overweight/obesity and a lower proportion of breastfeeders among African‐American women compared with Caucasian women (Centers for Disease Control and Prevention 2009; Flegal et al. 2010). However, we were restricted in our ability to examine potential effect measure modification by race because of small sample size and a primarily Caucasian population. And although we have data collected prospectively, it is possible that there existed a reciprocal relationship between pregravid BMI and the psychological factors. For example, women with higher levels of the psychological factors during pregnancy may also have had higher levels before pregnancy, which placed them at risk of beginning pregnancy overweight or obese. Consequently, their risk of continuing in or developing a poor mental health state during pregnancy was increased. The literature supports evidence of bidirectionality between psychological factors and obesity, and, as an observational study, we cannot be certain that it does not exist in our data, thus limiting our ability to make causal inferences (Luppino et al. 2010). Additionally, it is possible that our method of determining exclusive breastfeeders captured women who were not exclusively breastfeeding. However, PINPost participants were mainly Caucasian, of high income and of high education, which are all predictors of better breastfeeding outcomes. Thus, the prevalence of exclusive breastfeeding, although high, is not unreasonable for this population. Finally, pregravid BMI was calculated from weight that was self‐reported during pregnancy. Women of childbearing age tend to underestimate their weight, which would result in some BMI values being artificially low (Brunner Huber 2007). However, we checked pregravid BMI values for implausibility and used a categorized pregravid BMI variable, thus minimizing any potential misclassification.
These analyses show that women who enter pregnancy overweight or obese are less likely to follow current breastfeeding guidelines. To our knowledge, this is the first study to examine whether psychological factors such as depressive symptoms, perceived stress and anxiety help explain the association between pregravid BMI and breastfeeding duration. Although we found that the psychological factors did not explain this association, it may be that clinically relevant assessment tools would show a different relationship with pregravid BMI and breastfeeding duration even after adjusting for confounders. Future studies should confirm the associations among pregravid BMI, psychological factors and breastfeeding duration in a larger and more diverse sample using clinically relevant assessment tools.
Sources of funding
This study received support from the National Institute of Child Health and Human Development, National Institutes of Health (HD37584, HD39373), the National Institute of Diabetes and Digestive and Kidney Diseases (DK61981, DK56350) and the Carolina Population Center.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
UJM was responsible for the analysis and writing of the manuscript. AMSR was the principal investigator and guided the statistical analysis and writing of the paper. AHH, a co‐investigator of the study, and LSA provided guidance for the statistical analysis. AHH, LSA and MEB provided significant advice in the writing of the manuscript.
Supporting information
Table S1. Mediation analysis of the association between pregravid BMI and duration of any breastfeeding by depressive symptoms in the Pregnancy, Infection, and Nutrition study.
Table S2. Mediation analysis of the association between pregravid BMI and duration of any breastfeeding by perceived stress in the Pregnancy, Infection, and Nutrition study.
Table S3. Mediation analysis of the association between pregravid BMI and duration of any breastfeeding by state anxiety in the Pregnancy, Infection, and Nutrition study.
Table S4. Mediation analysis of the association between pregravid BMI and exclusive breastfeeding duration by depressive symptoms in the Pregnancy, Infection, and Nutrition study.
Table S5. Mediation analysis of the association between pregravid BMI and exclusive breastfeeding duration by perceived stress in the Pregnancy, Infection, and Nutrition study.
Table S6. Mediation analysis of the association between pregravid BMI and exclusive breastfeeding duration by state anxiety in the Pregnancy, Infection, and Nutrition study.
Supporting info item
Acknowledgements
We would like to thank all of the PIN Postpartum investigators: Nancy Dole, Kelly Evenson, David Savitz, June Stevens and John Thorp for obtaining funding and designing the study. Additionally, we would like to thank Kathryn Carrier for managing the PIN study.
References
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Publishing: Washington, DC. [Google Scholar]
- Amir L.H. & Donath S. (2007) A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.L., Michaelsen K.F., Rasmussen K.M. & Sørensen T. (2004) Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. American Journal of Clinical Nutrition 80, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Michaelsen K.F., Sørensen T. & Rasmussen K.M. (2007) High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. American Journal of Clinical Nutrition 86, 404–411. [DOI] [PubMed] [Google Scholar]
- Baron R.M. & Kenny D.A. (1986) The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Bodnar L.M., Wisner K.L., Moses‐Kolko E., Sit D.K. & Hanusa B.H. (2009) Prepregnancy body mass index, gestational weight gain, and the likelihood of major depressive disorder during pregnancy. Journal of Clinical Psychiatry 70, 1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner Huber L.R. (2007) Validity of self‐reported height and weight in women of reproductive age. Maternal and Child Health Journal 11, 137–144. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (5 October 2010. – last update) Breastfeeding Report Card, United States – 2009: Outcome Indicators Available at: http://www.cdc.gov/breastfeeding/data/report_card2.htm
- Cohen S., Kamarck T. & Mermelstein R. (1983) A global measure of perceived stress. Journal of Health and Social Behavior 24, 385–396. [PubMed] [Google Scholar]
- Cohen S. & Williamson G.M. (1988) Perceived stress in a probability sample of the United States In: The Social Psychology of Health (eds Spacapam S. & Oscamp S.), pp 31–67. Sage: Newbury Park, CA. [Google Scholar]
- Cooper P.J., Murray L. & Stein A. (1993) Psychosocial factors associated with the early termination of breast‐feeding. Journal of Psychosomatic Research 37, 171–176. [DOI] [PubMed] [Google Scholar]
- Deierlein A.L., Siega‐Riz A.M. & Herring A. (2008) Dietary energy density but not glycemic load is associated with gestational weight gain. American Journal of Clinical Nutrition 88, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C.L. & McQueen K. (2007) Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatrica 96, 590–594. [DOI] [PubMed] [Google Scholar]
- Dennis C.L. & McQueen K. (2009) The relationship between infant‐feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics 123, e736–e751. [DOI] [PubMed] [Google Scholar]
- Donath S.M., Amir L.H. & ALSPAC Study Team (2003) Relationship between prenatal infant feeding intention and initiation and duration of breastfeeding: a cohort study. Acta Paediatrica 92, 352–356. [PubMed] [Google Scholar]
- Dunn S., Davies B., McCleary L., Edwards N. & Gaboury I. (2006) The relationship between vulnerability factors and breastfeeding outcome. Journal of Obstetric, Gynecologic, and Neonatal Nursing 35, 87–97. [DOI] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Ogden C.L. & Curtin L.R. (2010) Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association 303, 235–241. [DOI] [PubMed] [Google Scholar]
- Gartner L.M., Morton J., Lawrence R.A., Naylor A.J., O'Hare D., Schanler R.J. et al (2005) Breastfeeding and the use of human milk. Pediatrics 115, 496–506. [DOI] [PubMed] [Google Scholar]
- Greenland S., Pearl J. & Robins J.M. (1999) Causal diagrams for epidemiologic research. Epidemiology 10, 37–48. [PubMed] [Google Scholar]
- Hatton D.C., Harrison‐Hohner J., Coste S., Dorato V., Curet L.B. & McCarron D.A. (2005) Symptoms of postpartum depression and breastfeeding. Journal of Human Lactation 21, 444–449. [DOI] [PubMed] [Google Scholar]
- Hilson J.A., Rasmussen K.M. & Kjolhede C.L. (2004) High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. Journal of Human Lactation 20, 18–29. [DOI] [PubMed] [Google Scholar]
- Hoffman S. & Hatch M.C. (2000) Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychology 19, 535–543. [PubMed] [Google Scholar]
- Hurley K.M., Black M.M., Papas M.A. & Caufield L.E. (2008) Maternal symptoms of stress, depression, and anxiety are related to nonresponsive feeding styles in a statewide sample of WIC participants. Journal of Nutrition 138, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia B.A., Siega‐Riz A.M., Dole N. & London E. (2009) Pregravid weight is associated with prior dietary restraint and psychosocial factors during pregnancy. Obesity 17, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Jewell S. & Grummer‐Strawn L. (2003) Maternal obesity and breast‐feeding practices. American Journal of Clinical Nutrition 77, 931–936. [DOI] [PubMed] [Google Scholar]
- Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W. et al (2010) Overweight, obesity, and depression: a systematic review and meta‐analysis of longitudinal studies. Archives of General Psychiatry 67, 220–229. [DOI] [PubMed] [Google Scholar]
- McLearn K.T., Minkovitz C.S., Strobino D.M., Marks E. & Hou W. (2006) Maternal depressive symptoms at 2 to 4 months post partum and early parenting practices. Archives of Pediatrics and Adolescent Medicine 160, 279–284. [DOI] [PubMed] [Google Scholar]
- Mehta U.J., Siega‐Riz A.M., Herring A.H., Adair L.S. & Bentley M.E. (2011) Maternal obesity, psychological factors and breastfeeding initiation. Breastfeeding Medicine doi:10.1089/bfm.2010.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer R., Kline J., O'Connor P., Shrout P., Johnson J., Skodol A. et al (1992) Determinants of depressive symptoms in the early weeks after miscarriage. American Journal of Public Health 82, 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M., Buikstra E., Fallon T. & Hegney D. (2009) Exploring the influence of psychological factors on breastfeeding duration, phase 1: perceptions of mothers and clinicians. Journal of Human Lactation 25, 55–63. [DOI] [PubMed] [Google Scholar]
- Oddy W.H., Li J., Landsborough L., Kendall G.E., Henderson S. & Downie J. (2006) The association of maternal overweight and obesity with breastfeeding duration. Journal of Pediatrics 149, 185–191. [DOI] [PubMed] [Google Scholar]
- Orr S.T. & Miller C.A. (1995) Maternal depressive symptoms and the risk of poor pregnancy outcome. Review of the literature and preliminary findings. Epidemiologic Reviews 17, 165–171. [DOI] [PubMed] [Google Scholar]
- Pippins J.R., Brawarsky P., Jackson R.A., Fuentes‐Afflick E. & Haas J.S. (2006) Association of breastfeeding with maternal depressive symptoms. Journal of Women's Health 15, 754–762. [DOI] [PubMed] [Google Scholar]
- Proctor B.D. & Dalaker J. (2001) Poverty in the United States: 2001. US Government Printing Office: Washington, DC. [Google Scholar]
- Radloff L.S. (1977) The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Rasmussen K.M. & Kjolhede C.L. (2004) Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 113, e465–e471. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A.M., Herring A., Carrier K., Evenson K.R., Dole N. & Deierlein A. (2009) Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity 18, 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. (1983) Manual for the State‐Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA. [Google Scholar]
- World Health Organization (2000) Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organization: Geneva, Switzerland. [PubMed] [Google Scholar]
- World Health Organization (2003a) Global Strategy for Infant and Young Child Feeding. World Health Organization: Singapore. [Google Scholar]
- World Health Organization (2003b) Infant and Young Child Feeding: A Tool for Assessing National Practices, Policies and Programmes. World Health Organization: Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mediation analysis of the association between pregravid BMI and duration of any breastfeeding by depressive symptoms in the Pregnancy, Infection, and Nutrition study.
Table S2. Mediation analysis of the association between pregravid BMI and duration of any breastfeeding by perceived stress in the Pregnancy, Infection, and Nutrition study.
Table S3. Mediation analysis of the association between pregravid BMI and duration of any breastfeeding by state anxiety in the Pregnancy, Infection, and Nutrition study.
Table S4. Mediation analysis of the association between pregravid BMI and exclusive breastfeeding duration by depressive symptoms in the Pregnancy, Infection, and Nutrition study.
Table S5. Mediation analysis of the association between pregravid BMI and exclusive breastfeeding duration by perceived stress in the Pregnancy, Infection, and Nutrition study.
Table S6. Mediation analysis of the association between pregravid BMI and exclusive breastfeeding duration by state anxiety in the Pregnancy, Infection, and Nutrition study.
Supporting info item


