Abstract
Fenoldopam is a selective dopamine-1 receptor agonist that causes peripheral arterial vasodilation, increased renal blood flow, and diuresis. Enthusiasm exists for the use of fenoldopam in non-polyuric kidney injury in dogs, though pharmacokinetic data is lacking. The purpose of this study was to collect basic pharmacokinetic and hemodynamic effect data for fenoldopam when administered to healthy awake dogs. Six healthy, awake beagles were given a 180 minute fenoldopam constant rate infusion at 0.8 μg/kg/min followed by a 120 minute washout period. Citrated blood was collected during and after infusion for plasma fenoldopam concentration measurement by HPLC with mass spectrometry. Heart rate and indirect systolic blood pressure were concurrently measured. Mean +/− SD steady state plasma fenoldopam concentrations of 20 +/− 17 ng/mL were achieved within 10 min of starting the infusion. Area under the plasma concentration-time curve was 3678 +/− 3030 ng/mL*min and plasma clearance was 66 +/− 43 mL/min/kg. Elimination was rapidly achieved in all dogs. Heart rate and systolic blood pressure were unaffected by the fenoldopam infusion. Based on the results of this study, further evaluation of the effects of fenoldopam in dogs at differing doses and in dogs with clinical conditions such as acute non-polyuric kidney injury is warranted.
Keywords: fenoldopam, dogs, pharmacokinetics, blood pressure, heart rate
INTRODUCTION
Fenoldopam is a selective DA-1(dopamine-1) receptor agonist which is used in human medicine in the treatment of acute hypertension and acute kidney injury (Aronson et al., 1991; Singer & Epstein, 1998; Mathur et al., 1999). DA-1 receptors are G-coupled protein receptors that activate adenylate cyclase, converting AMP(adenosine monophosphate) to cAMP (cyclic adenosine monophosphate) and leading to smooth muscle relaxation and vasodilation. These receptors are found in the central nervous system, on the proximal tubule and cortical collecting duct of the kidney, on post-synaptic smooth muscle, and in the heart in humans and in dogs. In the systemic circulation, activation of the DA-1 receptor on smooth muscle causes vasodilation. In the kidney, activation of DA-1 receptors in the renal proximal tubule causes renal vasodilation, promotes natriuresis and diuresis, increases renal blood flow, and preserves or improves glomerular filtration rate (Grund et al., 1979; Goldberg, 1988). Because of these effects, fenoldopam is used in human medicine to decrease blood pressure in acute hypertensive crises, and to improve renal blood flow and urine output in oliguric or anuric acute kidney injury (Mathur, 2003; Tuncel & Venkata, 2003; Haas & LeBlanc, 2004). It is also used to prevent acute kidney injury in situations where renal blood flow and glomerular filtration rate are expected to decrease, such as critically ill patients, patients undergoing cardiac surgery, and patients undergoing renal diagnostics with radiocontrast medium.
Fenoldopam has been extensively studied as a superior replacement for dopamine, which is a non-selective, dose-dependent agonist of DA-1, dopamine-2, α-1 adrenergic, and β-1 adrenergic receptors. Dopamine’s α-1 adrenergic effects, prominent at high doses, are contraindicated in patients with hypertension or acute kidney injury. Since the effects of fenoldopam are more specific to DA-1 receptors and less dose-dependent with respect to activation of non-DA-1 receptors than the effects of dopamine, fenoldopam carries a lower risk for side effects and has essentially replaced dopamine for prevention of acute kidney injury in human patients (Brienza et al., 2006; Bellomo et al., 2000). However, dopamine remains widely used in veterinary medicine in part because of a lack of information on fenoldopam pharmacokinetics in non-human species (Sigrist, 2007).
Consequently, the main objective of our study was to obtain basic pharmacokinetic and hemodynamic data for intravenously administered fenoldopam in healthy awake beagle dogs.
MATERIALS AND METHODS
This protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Tufts University (IACUC protocol #G930-07).
Experimental animals
Six mature beagle dogs of either sex, aged 4 – 5 years, and weighing 7.9 – 15.8 kg (mean weight 10.4 kg), were randomly selected from the Tufts University Department of Laboratory Animal Medicine beagle dog colony. All dogs were in good health based on normal physical examination, complete blood count, chemistry profile, and urinalysis prior to inclusion in the study. All dogs were fasted for 20 hours prior to use in the study. All dogs had free access to water prior to and during the study.
Drug administration
A sterile cephalic intravenous catheter was placed in each dog. All dogs (6 studied in total) then received a 0.8 μg/kg/min fenoldopam intravenous continuous rate infusion for 180 min (IMED Gemini PC1TX single pump, Trident Medical, Texas USA). This dosage is within the reported clinical dose range for humans (0.03 – 1.6 μg/kg/min), although this is somewhat higher than the recommended starting dosage of 0.1 μg/kg/min for humans with hypertensive crisis, and in critical patients with early renal dysfunction (Tuncel & Venkata, 2003; Haas & LeBlanc, 2004; fda.gov). We used this relatively high dose (0.8 μg/kg/min) since plasma fenoldopam concentrations were mostly less than the assay detection limit (1 ng/mL) in an initial pilot study we had performed in one healthy beagle dog following a dose of 0.1 μg/kg/min for 180 min. Although a dose of 0.8 μg/kg/min is considered high enough to cause hypotension and reflex tachycardia in human patients with hypertensive crisis, it is considered well tolerated in patients with mild to moderate hypertension (Taylor, Shepherd, et al., 1999).
Blood Sample Collection
For blood collection, a sterile long-line right-sided lateral saphenous intravenous sampling catheter was placed in each dog. At each time point, 6 mL of blood was collected and transferred into a heparinized tube. Each dog received 12 mL intravenous sterile saline at each sample time to replace lost volume. Blood tubes were immediately placed on ice and centrifuged for 10 minutes at 3,000 g. Resultant plasma was immediately transferred to polypropylene tubes containing 0.1 mL (100 uL) of 10% (weight/volume percent) ascorbic acid solution (as recommended by Boppana et al., 1989), mixed, and stored immediately at − 20 °C. Samples were kept frozen prior to analysis.
Blood samples were collected prior to drug administration, and at minutes 5, 10, 20, 30, 60, 90, 120, and 150 minutes during infusion to ensure achievement of steady state. The infusion was stopped at 180 minutes, and additional samples were collected at minutes 182, 185, 190, 195, 210, 225, 240, 270, and 300. Approximately 114 mL of blood was removed for sampling purposes per dog, which was considered a safe volume to remove.
Physiological measurements
At baseline (time point 0 minutes for each dog), three samples of systolic blood pressure, heart rate, and respiratory rate were taken and averaged. At subsequent time points corresponding to the blood sample collection times, blood pressure (an average of 3 determinations) as well as heart rate (single) and respiratory rate (single) measurements were recorded. Blood pressure was measured indirectly via the Doppler technique using the left hind limb.
Determination of blood fenoldopam concentrations
Plasma samples were analyzed by high-performance liquid chromatography with mass spectrometric detection based on a prior published method (Boppana et al., 1989) with modification as follows. Polypropylene tubes (15 mL) were prepared containing one mL of plasma sample, 50 ng of SKF-38393 hydrochloride (internal standard; Sigma-Aldrich, St Louis, Mo) in 25 μL of 50 mM acetic acid and 0.5 mL of 0.5 M dibasic sodium phosphate. Samples were then extracted twice with 2.5 mL of ethyl acetate by vortexing for 10 min and centrifuging at 2000 g for 10 min with the supernatants transferred to a fresh 15 mL tube. The organic phase was back extracted by adding 250 μL of 0.5% formic acid in water, vortexing for 5 min, and centrifuging at 2000 g for 5 min. The organic supernatant was discarded and residual ethyl acetate was evaporated in a vacuum oven set at room temperature. Extracted samples were then injected into the high-performance liquid chromatography – mass spectrometric apparatus consisting of a Surveyor HPLC with Deca XP Plus ion trap detector and electrospray source operating in the positive ion mode (Thermo Fisher Scientific, Waltham, MA). Chromatographic separation was achieved using a 150 × 2 mm Synergi Fusion column (Phenomenex, Torrance, CA) with a mobile phase run at 0.3 mL/min and a 10 minute run time. Mobile phase was 0.5% formic acid in water with 10 % acetonitrile, which was increased linearly to 95% between 1 and 6 minutes of the run. For fenoldopam, positive ion mass-to-charge ratio (M/Z+) transitions monitored were 306 → 136, 171, 195, 212, and 289, while the parent M/Z+ ion of 256 was monitored for assay of SKF-38393. Calibration and quality control samples were constructed using fenoldopam (Sigma-Aldrich, St Louis, Mo) added to blank bovine serum, which had identical fenoldopam recovery (>90%) and calibration curve slopes as compared with dog plasma in preliminary studies. Fenoldopam calibration curves were linear (R2 > 0.98) over the range of 1 ng/mL to 50 ng/mL. At the lowest quantifiable concentration (1 ng/mL) the deviation from nominal concentration was consistently between −20 and +20%, while the coefficient of variation of duplicates was less than 15%. Quality control samples (5 ng/mL and 25 ng/mL) were run in duplicate with each assay and showed deviations of −15% to +15% from nominal concentration and less than 10% coefficient of variation between duplicates.
Data Analysis
Plots of fenoldopam concentration against time after start of drug infusion were made for each dog. Estimates of plasma clearance for each dog were made by dividing the drug administration rate and the measured fenoldopam concentration at 150 minutes after infusion start (C150), assuming that steady state had been achieved.
Heart rate, respiratory rate, and indirect systolic blood pressure data were averaged at each time point for all 6 dogs, and the standard deviation calculated.
RESULTS
Pharmacokinetic data
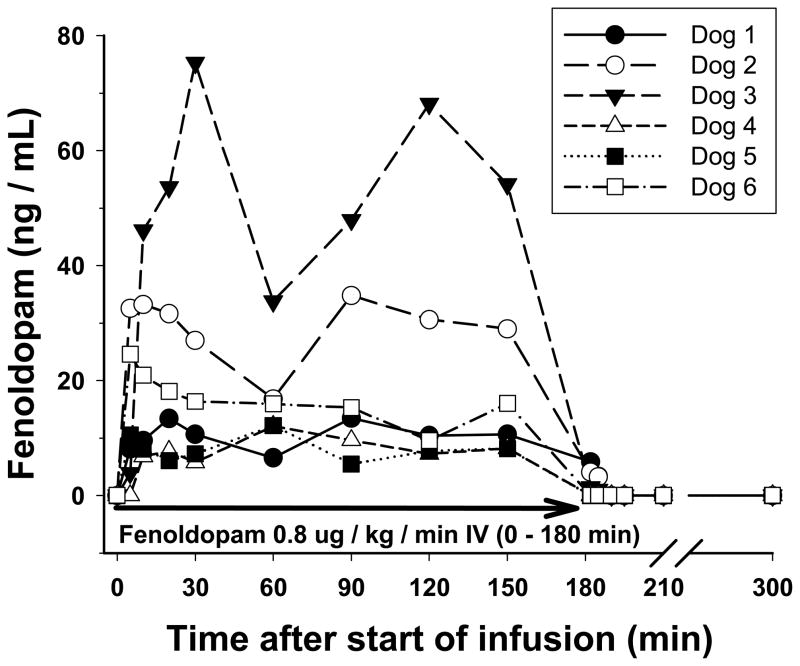
Figure 1 shows the disposition of fenoldopam in plasma for each of the 6 study dogs over the course of the study. Steady state concentrations appeared to have been reached rapidly in each dog by 30 minutes after infusion start. In 3 of 6 dogs a transient decrease in concentration was noted at 60 minutes after infusion start. Following discontinuation of the infusion, drug was rapidly eliminated such that fenoldopam levels were below the minimum quantifiable level (1 ng/mL) in 3 of 6 dogs (Dogs 4, 5, and 6) at 2 minutes, and in all dogs at 10 minutes after stopping infusion. C150 and plasma clearance estimates for individual dogs are given in Table 1. Both C150 and clearance estimates varied greatly ranging from 8 to 54 ng/mL and 15 to 98 mL/min/kg, respectively. Because of the rapid drug elimination, we were unable to accurately estimate elimination half-life or volume of distribution.
Figure 1.
Plot of fenoldopam plasma concentrations measured in 6 healthy dogs administered fenoldopam at 0.8 μg/kg/min intravenously for 180 minutes.
Table 1.
Pharmacokinetic parameter estimates derived for 6 healthy beagle dogs administered fenoldopam at 0.8 μg/kg/min intravenously for 180 minutes. Shown for each dog are the fenoldopam concentrations measured at 150 minutes after drug infusion start (C150) and the weight normalized clearance calculated from the C150 (assuming steady state had been reached) and drug infusion rate.
| DOG | C150 (ng/mL) | CLEARANCE (mL/min/kg) |
|---|---|---|
| 1 | 11 | 75 |
| 2 | 29 | 28 |
| 3 | 54 | 15 |
| 4 | 8 | 97 |
| 5 | 8 | 98 |
| 6 | 16 | 50 |
| Mean (SD) | 21 (18) | 60 (35) |
Cardiac, Respiratory, and Vascular data
Heart rate measurements over time and averaged for all 6 dogs at each time point are listed in Table 2. Both individual dog data (not shown) and averages for each time point were within normal range of 70–120 beats per minute. Respiratory rate measurements over time and averaged for all 6 dogs at each time point are given in Table 2. Both individual dog data (not shown) and averages for each time point were within normal range of 16–30 breaths per minute. Indirect systolic blood pressure measurements are listed in Table 2. Both individual dog data (not shown) and averages for each time point were within normal range of 80–160 mmHg.
Table 2.
Cardiac, respiratory, and vascular parameters measured from 6 healthy dogs administered fenoldopam at 0.8 μg/kg/min intravenously for 180 minutes. Data at each time point after infusion start were averaged. Normal heart rate: 70–120 bpm. Normal respiratory rate: 16–30 br/min. Normal systolic blood pressure: 80–160 mmHg.
| TIME (minute) | Mean (SD) HR (bpm) | Mean (SD) RR (br/min) | Mean (SD) systolic BP (mmHg) |
|---|---|---|---|
| 0 | 98.3 (13.4) | 22.3 (3.7) | 110.5 (16.7) |
| 5 | 98.7 (14.5) | 22.8 (5.2) | 119.4 (6.6) |
| 10 | 96.7 (17.2) | 25.0 (6.9) | 112.6 (11.3) |
| 20 | 100.0 (16.0) | 23.0 (6.7) | 115.6 (13.6) |
| 30 | 99.0 (20.4) | 26.0 (7.0) | 119.8 (10.7) |
| 60 | 93.3 (10.3) | 22.0 (5.5) | 119.6 (9.3) |
| 90 | 84.7 (8.2) | 22.0 (8.4) | 120.1 (10.4) |
| 120 | 93.3 (15.3) | 19.7 (5.4) | 112.6 (8.4) |
| 150 | 91.0 (6.4) | 16.7 (4.5) | 118.6 (10.6) |
| 180 | 86.3 (8.6) | 15.7 (4.8) | Not taken |
| 182 | 82.0 (10.7) | 18.7 (4.1) | Not taken |
| 185 | 76.7 (13.7) | 19.0 (5.9) | Not taken |
| 190 | 80.7 (12.2) | 18.7 (2.1) | 115.8 (12.3) |
| 195 | 76.7 (10.9) | 18.0 (4.9) | 115.3 (10.9) |
| 210 | 80.0 (8.4) | 22.0 (5.1) | 117.1 (12.8) |
| 225 | 76.7 (12.2) | 19.3 (4.3) | 115.8 (12.9) |
| 240 | 79.3 (13.5) | 18.0 (4.2) | 117.5 (13.4) |
| 270 | 83.3 (6.9) | 19.7 (2.7) | 116.9 (11.1) |
| 300 | 82 (12.8) | 17.0 (2.8) | 119.6 (11.3) |
bpm = beats per minute
br/min = breaths per minute
mmHg = millimeters of mercury
DISCUSSION
Fenoldopam is a DA-1 receptor agonist that causes systemic and renal vasodilation, increased renal blood flow, natriuresis, and diuresis. This drug has the potential to be of great use in veterinary medicine, particularly for prevention and treatment of acute kidney injury. Based on the Tufts University Cummings School of Veterinary Medicine FIRST database, we have seen 155 cases diagnosed with acute kidney injury in small animal patients between January 2007 and December 2009 (59% feline, 41% canine). In that same time period, 66 small animal patients received continuous rate infusions of dopamine, given in conjunction with the diuretic furosemide (73% canine, 27% feline). Acute kidney injury is a common presenting problem in our small animal patients, and fenoldopam may be an improvement over dopamine/furosemide continuous rate infusion, which is no longer standard of care in human medicine. Although we treat acute kidney injury more often than we have an opportunity to prevent it in veterinary medicine, we may also be able to apply this study to use fenoldopam to prevent kidney injury in critical patients, as it is used in human medicine. This study aims to take an important early step in understanding the use of fenoldopam in dogs.
The most common cause of acute kidney injury in dogs is ischemia, secondary to a variety of causes including pancreatitis, hypovolemia, hypotensive shock, and sepsis (Vaden et al., 1997). Prior studies have investigated the effects of fenoldopam on renal hemodynamics in dogs. A 2001 study monitored the effects of intravenous fenoldopam infusion on renal blood flow in healthy, awake dogs using surgically implanted blood flow probes around the renal artery, as well as quantitative Doppler ultrasonography. While primarily an imaging study, the authors did show that intravenous fenoldopam markedly increased blood flow through the kidney in healthy, awake dogs (Sehgal et al., 2001). A separate study used a canine model of normovolemia and hypovolemia to analyze the effects of fenoldopam on renal hemodynamics, by measuring urine output, creatinine clearance, sodium clearance, and renal blood flow. Measurements were taken in healthy anesthetized dogs given an intravenous fenoldopam infusion, and repeated when the dogs were partially exsanguinated to mimic acute hypovolemia. Intravenous fenoldopam infusion at 0.1 – 0.2 μg/kg/min lead to increased urine output, creatinine clearance, and renal blood flow in 4 healthy, anesthetized dogs, but these changes did not reach statistical significance. The study did find, however, that fenoldopam infusion at the above rates significantly maintained urine output, creatinine clearance, sodium clearance, and renal blood flood in the face of partial exsanguinations (Halpenny et al., 2001). Similar findings are reported in an aortic cross-clamping model of renal ischemia. These studies suggest that fenoldopam infusion may be renoprotective in the face of acute ischemic injury, the most common cause of acute kidney injury in dogs (Halpenny et al., 2000).
Despite these promising data on the renal hemodynamic effects of fenoldopam in dogs, there remains a paucity of data on the pharmacokinetics of fenoldopam in veterinary patients, and to the authors’ knowledge there have been no published studies of fenoldopam pharmacokinetics in dogs. This study aimed to look at both clearance and elimination of fenoldopam, without distinguishing enantiomers or major metabolites. It has been shown in dogs that the renal and systemic vasodilator activities of fenoldopam are properties of the R-enantiomer, and that the S-enantiomer is essentially inactive (Kinter et al., 1990). Consequently it is possible that if there is differential elimination of the enantiomers, the measured fenoldopam (combined R- and S- form) concentrations may not reflect the active drug level.
In humans, total body clearance of fenoldopam has been reported as 30 mL/min/kg in adults, and 51 mL/min/kg in pediatric patients (Weber et al., 1988; Hammer et al., 2008). Therefore, the plasma fenoldopam clearance value estimate we obtained for adult dogs of 60 +/− 35 mL/min/kg is closest to pediatric human values and about twice that of reported adult human values. In humans, fenoldopam is eliminated by conjugation in the liver and excretion in the urine (90%) and feces (10%), with only 4% of the drug excreted unchanged (fda.gov). The fenoldopam clearance for dogs exceeds predicted hepatic blood flow (up to approximately 51 mL/min/kg). Consequently, we suspect that fenoldopam probably undergoes complete first-pass hepatic elimination, and may also undergo additional extrahepatic clearance such as metabolism and/or excretion unchanged via the kidneys. Additional studies on the presence of fenoldopam and its metabolites in plasma and urine are warranted to test this hypothesis.
The mean plasma fenoldopam concentration measured at 150 minutes after infusion start (21 ng/mL) was similar to reported adult human mean fenoldopam concentration at steady state of approximately 28 ng/mL in patients receiving fenoldopam CRI of 0.8 μg/kg/min (Taylor, Shepherd, et al., 1999). Css has been shown in humans to be proportional to infusion rate. Further pharmacokinetic studies in dogs at varying fenoldopam infusion rates are warranted to verify dose-concentration proportionality in dogs.
Of the six dogs in this study, three dogs (50%) had plasma fenoldopam concentrations below the limit of quantification (< 1 ng/mL) by 2 minutes after discontinuation of the infusion, and levels were unmeasurable levels at 10 minutes after infusion discontinuation. The reported half-life of fenoldopam in humans is approximately 5 minutes in adults and 3–5 minutes in pediatric patients (fda.gov; Weber et al., 1988). Although we were not able to obtain accurate estimates of half-life in dogs, it appears likely that it is shorter than values in humans. Consequently, further studies on elimination pharmacokinetics of fenoldopam in dogs would require rapid (greater than once per minute), perhaps automated blood sampling methods. The rapid elimination of fenoldopam from plasma suggests that drug effects (beneficial or adverse) would be quickly responsive to changes in drug infusion rate assuming rapid equilibration between plasma and effect sites. These are desirable characteristics for drugs used in the emergency and critical care setting.
We observed relatively high variability in plasma fenoldopam concentrations and clearance rates between dogs with coefficients of variation (SD/mean expressed as a percentage) of 86% and 59%, respectively. Adult human studies have also showed relatively high variation in steady state plasma fenoldopam levels at the 0.8 ug/kg/min dosage level with coefficient of variation estimates up to 50% (Taylor, Shepherd, et al., 1999). However, variation was much lower at lower dose rates (<10%). Although a study in 77 human pediatric patients did not report variation in drug concentration values at the different drug dosages evaluated, derived clearance estimates (using data from all doses evaluated) had variabilities ranging from 10% to over 100% (coefficient of variation) depending on the method of parameter estimation (Hammer et al., 2008). Further studies in dogs using lower dosage levels are needed to determine whether dosing needs to be adjusted for individual dogs.
The cardiovascular effects of fenoldopam have been studied in humans. In normotensive human pediatric patients, a relatively high average fenoldopam dose of 0.8 – 1.2 μg/kg/min was required to induce hypotension (Hammer et al., 2008). In normotensive human adults, systolic blood pressure and heart rate are unchanged at fenoldopam doses up to 0.3 μg/kg/min, although diastolic blood pressure begins to drop mildly at that dose (Mathur et al., 1999; Brienza et al., 2006). Conversely, in mildly to moderately hypertensive human adults, fenoldopam doses as low as 0.1 μg/kg/min induced significant and dose-dependent reductions in blood pressure (Taylor, Shepherd, et al., 1999; Taylor, Mangoo-Karim, et al., 1999). The cardiovascular effects of fenoldopam also have been studied in several veterinary species. In healthy cats, a fenoldopam dose of 0.5 μg/kg/min had no effect on systolic blood pressure (Simmons et al., 2006). In neonatal foals, lower doses of fenoldopam (0.04 μg/kg/min) did not affect hemodynamic parameters, while at higher doses (0.4 μg/kg/min), mean, systolic, and diastolic blood pressure decreased, and heart rate increased significantly (Hollis et al., 2006). In adult horses, fenoldopam doses as low as 0.01 μg/kg/min significantly increased heart rate without changing blood pressure, while doses above 0.05 μg/kg/min significantly increased heart rate and significantly decreased average carotid arterial pressure (Clark & Moore, 1989). In neonatal pigs, adult rabbits, and adult rats, high doses of fenoldopam (> 5, 10, or 125 μg/kg/min, respectively) significantly reduced blood pressure (Pearson et al., 1996; Yakazu et al., 2001; Quevedo et al., 1999). Previous studies in healthy adult dogs have not agreed upon a fenoldopam dose that leads to hypotension and reflex tachycardia. Studies agree that lower doses of fenoldopam (0.1 μg/kg/min) do not cause hypotension or tachycardia in normotensive dogs (Lass et al., 1988; Halpenny et al., 2000; Halpenny et al., 2001). Very high doses of fenoldopam (3.4 +/− 2 μg/kg/min and >=10 μg/kg/min) do cause significant hypotension or tachycardia (Aronson et al., 1990; Damase-Michel et al., 1995). In one study in normotensive dogs, a fenoldopam dose of 0.2 μg/kg/min caused hypotension in 3 out of 4 dogs, while in another study using pentobarbital-anesthetized dogs, the same dose of fenoldopam showed no effect on blood pressure or heart rate (Halpenny et al., 2001; Lass et al., 1988).
In the current study, systolic blood pressure remained within normal limits at all time periods in all dogs suggesting that, at least in normotensive adult dogs, a fenoldopam dose of 0.8 μg/kg/min should not result in significant hypotension. However, given the small number of animals studied (n=6) and the possibility of variable responses between dogs, we acknowledge that heart rate and blood pressure in some dogs may be more readily affected by fenoldopam than in other dogs. This possibility is reinforced by the inter-dog variability in systolic blood pressure, which even at baseline (time 0 minutes) varied from 80.7 to 131.3 mmHg among the 6 dogs. Further studies on the hemodynamic effects of fenoldopam in healthy, normotensive adult dogs as well as clinically affected dogs, at various doses above and below 0.8 μg/kg/min, are warranted.
In the current study, a fenoldopam constant rate infusion at 0.8 μg/kg/min was given to healthy, normotensive adult dogs. It is important to note that clinically affected dogs may react differently to fenoldopam. The major indication for use of fenoldopam in veterinary medicine is likely to be acute kidney injury. It has been shown that 80% of dogs with acute uremia are hypertensive (Cowgill & Francey, 2005). This is thought to be due to reduced sodium excretion, upregulation of the renin-angiotensin-aldosterone pathway, and activation of the sympathetic nervous system, as well as a result of fluid overload (Syme, 2011). Because fenoldopam causes peripheral arterial vasodilation, increased renal blood flow, and diuresis, it is possible that fenoldopam may have two beneficial effects in dogs with acute kidney injury: increase in renal blood flow as well as reduction of systemic hypertension. However, it is not possible to know how clinically affected dogs will respond to fenoldopam infusion based on the current study in healthy dogs. Further study in dogs with acute, non-polyuric kidney injury is warranted to evaluate the cardiovascular and renal hemodynamic effects of fenoldopam on clinically affected dogs.
In humans, fenoldopam CRI above 0.8 μg/kg/min has been linked to an increased risk of adverse clinical signs, including headache, dizziness, diaphoresis, nausea, vomiting, and restlessness (Taylor, Mangoo-Karim, et al., 1999). While some of these signs would not be readily noticeable in dogs, we did not notice any signs of nausea or restlessness, nor did any dogs vomit during the study.
In conclusion, fenoldopam administration to 6 normotensive adult dogs at a dose of 0.8 μg/kg/min for up to 3 hours did not cause systolic hypotension nor reflex tachycardia, and did not cause apparent adverse clinical effects. The pharmacokinetics of fenoldopam in healthy beagle dogs is similar to that described in adult and pediatric humans, with high inter-dog variability. Fenoldopam shows promise in human medicine in the treatment and prevention of acute hypertensive crisis and acute non-polyuric kidney injury. Consequently, further evaluation of the effects of fenoldopam in dogs at differing doses, and in dogs with clinical conditions such as non-polyuric kidney failure, is warranted. Further studies should focus on both the cardiovascular and renal hemodynamic effects of larger numbers of healthy and clinically affected dogs, as their responses may be different.
Acknowledgments
This work was funded by the Companion Animal Health Fund, Tufts University (North Grafton, MA). Dr Court was supported by grant R01GM061834 from the National Institute of General Medical Sciences, National Institutes of Health (Bethesda, MD). This work is the sole the responsibility of the authors and do not necessarily represent the official views of the National Institute of General Medical Sciences, or the National Institutes of Health.
List of Abbreviations
- μ
micro
- <
less than
- >
greater than
- α
alpha
- β
beta
- %
percent
- °C
degrees Celsius
- m
milli
- mm
millimeter
- M
molar
- R2
coefficient of determination of a linear regression
- mmHg
millimeters of mercury
Contributor Information
Carly Anne Bloom, Tufts University Cummings School of Veterinary Medicine, North Grafton, MA.
Mary Anna Labato, Tufts University Cummings School of Veterinary Medicine, North Grafton, MA.
Suwagmani Hazarika, Department of Molecular Physiology and Pharmacology, Tufts University School of Medicine, Boston, MA.
Michael H. Court, Department of Molecular Physiology and Pharmacology, Tufts University School of Medicine, Boston, MA
References
- Aronson S, Goldberg LI, Glock D, Moss J, Roizen MF. Preservation of renal blood flow during hypotension induced with fenoldopam in dogs. Canadian Journal of Anaesthesiology. 1990;37(3):380–4. doi: 10.1007/BF03005596. [DOI] [PubMed] [Google Scholar]
- Aronson S, Goldberg LI, Glock D, Roth S, Moss J, Roizen MF. Effects of fenoldopam on renal blood flow and systemic hemodynamics during isoflurane anesthesia. Journal of Cardio-thoracic Vascular Anesthesiology. 1991;5(1):29–32. doi: 10.1016/1053-0770(91)90089-c. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Chapman M, Finfer S. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. ANZICS Clinical Trials Group. Lancet. 2000;356:2139–2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- Boppana VK, Lynn RK, Ziemkiak JK. Simplified procedures for the determination of fenoldopam and its metabolites in human plasma by high-performance liquid chromatography with electrochemical detection: comparison of manual and robotic sample preparation methods. Journal of Chromatography. 1989;487(2):285–399. doi: 10.1016/s0378-4347(00)83046-7. [DOI] [PubMed] [Google Scholar]
- Brienza N, Malcangi V, Dalfino L, Trerotoli P, Guagliardi C, Bortone D, Faconda G, Ribezzi M, Ancona G, Bruno F, Fiore T. A comparison between fenoldopam and low-dose dopamine in early renal dysfunction of critically ill patients. Critical Care Medicine. 2006;34(3):910–911. doi: 10.1097/01.CCM.0000201884.08872.A2. [DOI] [PubMed] [Google Scholar]
- Clark ES, Moore JN. Effects of fenoldopam on cecal blood flow and mechanical activity in horses. American Journal of Veterinary Research. 1989;50(11):1926–30. [PubMed] [Google Scholar]
- Cowgill LD, Francey T. Acute Uremia. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6. Elsevier Saunders; St/Louis, Missouri: 2005. pp. 1731–1751. [Google Scholar]
- Damase-Michel C, Montastruc JL, Tran MA. Effects of dopaminergic drugs on the sympathoadrenal system. Hypertension Research. 1995;18(Suppl 1):S119–24. doi: 10.1291/hypres.18.supplementi_s119. [DOI] [PubMed] [Google Scholar]
- Goldberg LI. Dopamine and new dopamine analogs: Receptors and clinical applications. Journal of Clinical Anesthesia. 1988;1:66–74. doi: 10.1016/0952-8180(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Grund E, Müller-Ruchholtz ER, Hauer F, Lapp ER. Effects of dopamine on total peripheral resistance and integrated systemic venous blood volume in dogs. Basic Research in Cardiology. 1979;75(5):623–34. doi: 10.1007/BF01907692. [DOI] [PubMed] [Google Scholar]
- Haas CE, LeBlanc JM. Acute postoperative hypertension: A review of therapeutic options. American Journal of Health-System Pharmacy. 2004;61(16):1661–73. [PubMed] [Google Scholar]
- Halpenny M, Markos F, Snow HM, Duggan PF, Gaffney E, O’Connell DP, Shorten GD. The effects of fenoldopam on renal blood flow and tubular function during aortic cross-clamping in anaesthetized dogs. European Journal of Anaesthesiology. 2000;17(8):491–8. doi: 10.1046/j.1365-2346.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- Halpenny M, Markos F, Snow HM, Duggan PF, Gaffney E, O’Connell DP, Shorten GD. Effects of prophylactic fenoldopam infusion on renal blood flow and renal tubular function during acute hypovolemia in anesthetized dogs. Critical Care Medicine. 2001;29(4):855–860. doi: 10.1097/00003246-200104000-00034. [DOI] [PubMed] [Google Scholar]
- Hammer GB, Verghese ST, Drover DR, Tobin JR. Pharmacokinetics and pharmacodynamics of fenoldopam for blood pressure control in pediatric patients. BMC Anesthesiology. 2008;8(6) doi: 10.1186/1471-2253-8-6. open access online article 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis AR, Ousey JC, Palmer L, Stoneham SJ, Corley KTT. Effects of fenoldopam mesylate on systemic hemodynamics and indices of renal function in normotensive neonatal foals. Journal of Veterinary Internal Medicine. 2006;20:595–600. doi: 10.1892/0891-6640(2006)20[595:eofmos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kinter LB, Horner E, Mann WA, Weinstock J, Ruffolo RR. Characterization of the hemodynamic activities of fenoldopam and its enantiomers in the dog. Chirality. 1990;2(4):219–25. doi: 10.1002/chir.530020405. [DOI] [PubMed] [Google Scholar]
- Lass NA, Glock D, Goldberg LI. Cardiovascular and renal hemodynamic effects of intravenous infusions of the selective DA1 agonist, fenoldopam, used alone or in combination with dopamine and dobutamine. Circulation. 1988;78:1310–1315. doi: 10.1161/01.cir.78.5.1310. [DOI] [PubMed] [Google Scholar]
- Mathur VS, Swan SK, Lambrecht LJ, Anjum S, Fellman J, McGuire D, Epstein M, Luther RR. The effects of fenoldopam, a selective dopamine receptor agonist, on systemic and renal hemdynamics in normotensive subjects. Critical Care Medicine. 1999;27(9):1832–1837. doi: 10.1097/00003246-199909000-00021. [DOI] [PubMed] [Google Scholar]
- Mathur VS. The role of the DA1 receptor agonist fenoldopam in the management of critically ill, transplant, and hypertensive patients. Reviews in Cardiovascular Medicine. 2003;4(Suppl 1):35–40. [PubMed] [Google Scholar]
- Pearson RJ, Barrington KJ, Jirsch DW, Cheung PY. Dopaminergic receptor-mediated effects in the mesenteric vasculature and renal vasculature of the chronically instrumented newborn piglet. Critical Care Medicine. 1996;24(10):1706–1712. doi: 10.1097/00003246-199610000-00018. [DOI] [PubMed] [Google Scholar]
- Quevedo M, Prieto JG, Perez-Olea J. The effects of fenoldopam on the blood pressure of the rat. General Pharmacology. 1999;32(1):123–125. doi: 10.1016/s0306-3623(98)00065-2. [DOI] [PubMed] [Google Scholar]
- Sehgal CM, Arger PH, Silver AC, Patton JA, Saunders HM, Bhattacharyya A, Bell CP. Renal blood flow changes induced with Endothelin-1 and Fenoldopam Mesylate at quantitative Doppler US: Initial results in a canine study. Radiology. 2001;219:419–426. doi: 10.1148/radiology.219.2.r01ma13419. [DOI] [PubMed] [Google Scholar]
- Sigrist NE. Use of dopamine in acute renal failure. Journal of Veterinary Emergency and Critical Care. 2007;17(2):117–126. [Google Scholar]
- Simmons JP, Wohl JS, Schwartz DD, Edwards HG, Wright JC. Diuretic effects of fenoldopam in healthy cats. Journal of Veterinary Emergency and Critical Care. 2006;16(2):96–103. [Google Scholar]
- Singer I, Epstein M. Potential of dopamine A-1 agonists in the management of acute renal failure. American Journal of Kidney Disease. 1998;31:743–755. doi: 10.1016/s0272-6386(98)70043-5. [DOI] [PubMed] [Google Scholar]
- Syme H. Vet Clin North Am Small Anim Pract. 1. Vol. 41. Elsevier Saunders; St/Louis, Missouri: 2011. Hypertension in small animal kidney disease; pp. 63–89. [DOI] [PubMed] [Google Scholar]
- Taylor AA, Mangoo-Karim R, Ballard KD, Luther RR, Pool JL. Sustained hemodynamic effects of the selective dopamine-1 agonist, fenoldopam, during 48-hour infusions in hypertensive patients: a dose-tolerability study. Journal of Clinical Pharmacology. 1999;39(5):471–9. [PubMed] [Google Scholar]
- Taylor AA, Shepherd AM, Polvino W, Mangoo-Karim R, Ballard K, Sunthornyothin S, Luther RR, Pool JL. Prolonged Fenoldopam infusions in patients with mild to moderate hypertension: Pharmacodynamic and Pharmacokinetic effects. American Journal of Hypertension. 1999;12:906–914. doi: 10.1016/s0895-7061(99)00068-0. [DOI] [PubMed] [Google Scholar]
- Tuncel M, Venkata CS. Hypertensive Emergencies: Etiology and Management. American Journal of Cardiovascular Drugs. 2003;3(1):21–31. doi: 10.2165/00129784-200303010-00003. [DOI] [PubMed] [Google Scholar]
- Vaden SL, Levine J, Breitschwerdt EB. A retrospective case-control study of acute renal failure in 99 dogs. Journal of Veterinary Internal Medicine. 1997;11(2):58–64. doi: 10.1111/j.1939-1676.1997.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Weber RR, McCoy CE, Ziemniak JA, Frederickson ED, Goldberg LI, Murphy MB. Pharmacokinetic and pharmacodynamic properties of intravenous fenoldopam, a dopamine-1 receptor agonist, in hypertensive patients. British Journal of Clinical Pharmacology. 1988;25:17–21. doi: 10.1111/j.1365-2125.1988.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakazu Y, Iwasawa K, Narita H, Kinscher JD, Benson KT, Goto H. Hemodynamic and sympathetic effects of fenoldopam and sodium nitroprusside. Acta Anaesthesiologica Scandinavica. 2001;45(9):1176–80. doi: 10.1034/j.1399-6576.2001.450920.x. [DOI] [PubMed] [Google Scholar]
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/19922se5-005_colopam_lbl.pdf