Abstract
Background
Invasive candidiasis is a leading cause of mortality and morbidity in neonatal intensive care units. Treatment recommendations are limited by a lack of comparative outcomes data.
Methods
We identified all infants ≤120 days of age with positive blood, urine, or cerebrospinal fluid cultures for Candida sp. who received amphotericin B deoxycholate, fluconazole, amphotericin B lipid products, or combination therapy admitted to 1 of 192 neonatal intensive care units in the United States between 1997 and 2003. Primary outcome measures included overall mortality and therapeutic failure (combined outcome of duration of infection >7 days, need for additional antifungal therapy, or death prior to discharge). We compared outcomes by antifungal therapy using logistic regression, controlling for gestational age, day of life at start of antifungal therapy, delay in therapy, and site of infection.
Results
Overall, 138/730 (19%) infants died. On multivariable logistic regression, we observed higher overall mortality for infants receiving amphotericin B lipid products compared with infants receiving amphotericin B deoxycholate (OR 1.96 [95% CI: 1.16, 3.33]; p=0.01) or fluconazole (OR 2.39 [1.18, 4.83]; p=0.02).
Conclusions
Infants treated with amphotericin B lipid products had higher mortality than infants treated with either amphotericin B deoxycholate or fluconazole. This finding may be related to inadequate penetration of amphotericin B lipid products into the kidneys, inappropriate dosing in premature infants, or unknown differences in acuity of illness in infants treated with amphotericin B lipid products.
Keywords: invasive candidiasis, infants, amphotericin B deoxycholate, fluconazole, amphotericin B lipid products
Invasive candidiasis is a leading cause of mortality and morbidity in neonatal intensive care units (NICUs).1-3 In extremely low-birth-weight infants (ELBW, <1000 g birth weight), the cumulative incidence of candidemia is 7% with an associated mortality of 30%.3 Among survivors, invasive candidiasis frequently leads to significant morbidities including neurodevelopmental impairment, chronic lung disease, and severe retinopathy of prematurity.1,3 There are currently no FDA-approved antifungal therapies for infants <6 months of age with invasive candidiasis. As a result, management of Candida infections in the NICU varies widely. A survey of neonatologists and pediatric infectious disease specialists found that 88% prefer amphotericin B deoxycholate for treatment of invasive candidiasis, but both fluconazole and amphotericin B lipid products (liposomal amphotericin B, amphotericin B lipid complex, and amphotericin B colloidal dispersion) are widely used.4
Previous studies of outcomes following antifungal treatments in infants are limited to small cohort or underpowered randomized trials.5-17 The largest cohort to date included 118 infants with invasive candidiasis, and, of the 2 randomized trials, the largest included 23 infants.5,6,15 Here we report clinical outcomes, including mortality, by antifungal therapy from a large cohort of infants with invasive candidiasis.
MATERIALS AND METHODS
Data Source
We assembled a cohort of infants from a national electronic medical record database managed by the Pediatrix Medical Group. The group coordinates prospective data collection from NICUs using a computer-assisted tool that generates daily clinical progress notes. Data are prospectively collected each day from admission until death or discharge. Collected data are de-identified and sent to a common database, and on a monthly basis a subset of data from a sample of 10–20% of the patients is reviewed and checked for greater than 95% accuracy.
Study Design and Setting
We performed a retrospective cohort study of infants admitted to 192 NICUs in the United States between 1997 and 2003 using a database managed by the Pediatrix Medical Group. Analysis was limited to the years prior to 2004 as, after this point, the Pediatrix database no longer distinguished amphotericin B lipid products from amphotericin B deoxycholate. We gathered information on all Candida cultures from normally sterile body fluids (blood obtained from central or peripheral sites, cerebrospinal fluid [CSF], and urine obtained by sterile in-and-out catheterization) for each infant during the first 120 days of life. Infants eligible for inclusion in this study had 1 or more positive Candida cultures and received an antifungal agent for at least 1 day. Infants for whom treatment was started >7 days after the first positive culture were excluded from the analysis. Multiple positive Candida cultures within 21 days of each other were considered a single episode of candidiasis. We examined only the first episode of candidiasis for all infants.
Definitions
Monotherapy was defined as use of a single antifungal on the day on which the first positive Candida culture was obtained. Combination therapy was defined as use of >1 antifungal on the day on which the first positive Candida culture was obtained. For instance, if both fluconazole and caspofungin were started 1 day prior to the culture and continued through the day the culture was obtained, we considered this combination therapy. In these data, 3 antifungals were used as monotherapy: amphotericin B deoxycholate, amphotericin B lipid products, and fluconazole. Caspofungin and flucytosine were used only in combination with other antifungals and, therefore, were categorized as combination therapy. Overall mortality was defined as death prior to discharge. Duration of candidiasis was defined by the time between the first positive blood, urine, or CSF culture obtained and the last positive culture for that episode of candidiasis. Prolonged duration of candidiasis was defined as duration of candidiasis >7 days. Additional antifungal therapy added was defined as addition or change in antifungal therapy during the time that cultures were positive for Candida. Therapeutic failure was defined as the combined outcome of prolonged duration of candidiasis, additional therapy added, or death prior to discharge.
Statistical Methods
The unit of observation for this analysis was the infant. Statistical significance for unadjusted comparisons was calculated based on linear regression for continuous variables and logistic regression for binary variables. We used logistic regression to evaluate the effect of the antifungal agent on overall mortality and therapeutic failure. Negative binomial regression was used to compare the impact of alternative treatments on the duration of candidiasis. Cox regression was used to calculate hazard ratios for each therapy, and the Schoenfeld residuals test was used to evaluate the assumption of proportional hazards. For all regressions, we controlled for gestational age at birth (categorical: <26, 26–29, 30–33, and >33 weeks), day of life at onset of infection (categorical: 0–7, 8–30, and >30 days), delay in therapy (continuous: days) and site of infection (categorical: any blood, any urine, and any CSF positive cultures).
STATA 11 (College Station, TX) was used to perform the statistical analysis. Significance for all tests was established at p<0.05. Permission to conduct this analysis was provided by the Duke University Institutional Review Board.
RESULTS
Demographics
Of 213,328 infants admitted to the NICU during the study period, 730 (0.3%) had a least 1 episode of candidiasis. The mean gestational age, birth weight, and day of life at the start of antifungal therapy were 27 weeks (5th, 95th percentile: 23, 37), 1035 g (515, 2730), and 23 days (6, 59), respectively (see Table, Supplemental Digital Content 1). There were no significant differences in the mean gestational age at birth between the cohorts of infants that received amphotericin B deoxycholate, amphotericin B lipid products, fluconazole, or combination therapy: 27 weeks (23, 37), 27 weeks (23, 38), 26 weeks (23, 32), and 28 weeks (23, 39), respectively (p=0.14). There were also no significant differences in the mean birth weight between the cohorts of infants that received amphotericin B deoxycholate, amphotericin B lipid products, fluconazole, or combination therapy: 1034 g (515, 2730), 1077 g (538, 3351), 948 g (503, 2075), and 1240 g (569, 2980), respectively (p=0.24). There were no significant differences in the mean day of life that antifungal therapy was started between the cohorts of infants that received amphotericin B deoxycholate, amphotericin B lipid products, fluconazole, or combination therapy: 24 days (6, 60), 23 days (5, 60), 22 days (7, 47), and 23 days (8, 70), respectively (p=0.88). Compared with whites, Hispanics were more likely to receive amphotericin B lipid products (p=0.001) and less likely to receive fluconazole (p=0.006).
Only 124 (17%) infants had a serum creatinine reported within 1 day of starting antifungal therapy. Mean serum creatinine was higher in infants receiving fluconazole (n=17) than in infants receiving amphotericin B deoxycholate (n=83)—1.0 mg/dL (0.4, 3.6) vs. 0.7 mg/dL (0.2, 1.5), respectively (p=0.02)—but not in infants receiving amphotericin B lipid products (n=21) (0.8 mg/dL [0.3, 1.5]; p=0.21). There was no significant difference in mean serum creatinine between infants treated with amphotericin B deoxycholate compared with amphotericin B lipid products (p=0.32).
Treatment Outcomes by Infection Site and Antifungal Agent
The mean duration of all episodes of candidiasis was 5 days (1, 17) (Table 1). The mean durations of candidiasis for those infants with isolated bloodstream (n= 474), urine (n= 152), central nervous system (CNS) infections (n= 5), and multiple sites of infection (n=99) were 5 days (1, 16), 2 days (1, 10), 2 days (1, 6), and 10 days (1, 25), respectively. Duration of candidiasis among infants with isolated bloodstream infections was higher compared to infants with isolated CNS or urine infections—5 days (1, 16) and 2 days (1, 10), respectively (p<0.001)—but lower compared to infants with multiple sites of infections (5 days [1, 16] and 10 days [1, 25], respectively; p<0.001).
On multivariable regression, there was no difference in the duration of candidiasis for infants treated with amphotericin B deoxycholate, amphotericin B lipid products, fluconazole, or combination therapy (p=0.47) (Table 2).
The overall mortality for infants in our study was 19% (138/730) (Table 2). Approximately half of all deaths (53%, 73/138) occurred within 14 days of the first positive Candida culture, and close to one-third of all deaths (34%, 47/138) occurred within 7 days of the first positive Candida culture. There were no significant differences in unadjusted mortality between infants with multiple sites of infection, isolated bloodstream infections, and isolated urine or CNS infections: 25% (25/99), 19% (88/474), and 16% (25/157), respectively (p=0.17). There were only 5 cases of isolated CNS infections in our cohort, none of which resulted in death.
On multivariable regression, mortality was higher in the cohort of infants treated with amphotericin B lipid products compared with the cohort treated with amphotericin B deoxycholate (29% vs. 18%; odds ratio [OR] 1.96 [95% confidence interval {CI}: 1.16, 3.33]) and compared with the cohort treated with fluconazole (29% vs. 16%; OR 2.39 [1.18, 4.83]) (see Table, Supplemental Digital Content 2). The mortality rates for fluconazole and combination therapy did not differ significantly compared with amphotericin B deoxycholate. Therapeutic failure (the combined outcome of death, prolonged duration of candidiasis, or additional therapy) was also higher in infants treated with amphotericin B lipid products compared to those treated with amphotericin B deoxycholate (47% vs. 38%; OR 1.62 [1.00, 2.64]) (see Table, Supplemental Digital Content 2). Therapeutic failure was higher but did not reach statistical significance when comparing the cohort treated with amphotericin B lipid products to the cohort treated with fluconazole (47% vs. 40%; OR 1.34 [0.73, 2.46]). Therapy failure rates for fluconazole and combination therapy did not differ significantly compared with amphotericin B deoxycholate.
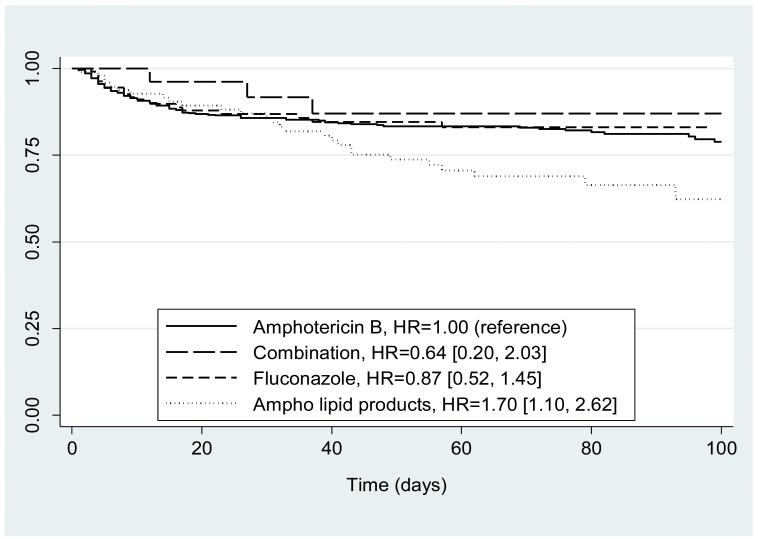
Cox regression showed similar evidence of higher mortality in the cohort treated with amphotericin B lipid products than in the cohort treated with amphotericin B deoxycholate. Using amphotericin B deoxycholate as the baseline (hazard ratio [HR] =1.00), HRs for amphotericin B lipid product, fluconazole, and combination were 1.70 (1.10, 2.62), 0.87 (0.52, 1.45), and 0.64 (0.20, 2.03), respectively (Figure 1).
Figure 1.
Kaplan-Meier curve for mortality by antifungal therapy. Hazard ratios (HRs) from Cox regression controlling for: gestational age groups (<26, 26–29, 30–33, >33 weeks), day of life at onset of infection groups (0–7, 8–30, >30 days), delay in therapy (days), and site of infection groups (any blood, any urine, and any CSF positive cultures).
DISCUSSION
In the present study, only 0.3% of all infants (730/213,328) admitted to the NICU were diagnosed with culture-proven invasive Candida infections. Current management guidelines for neonatal candidiasis recommend removing any source of infection and antifungal treatment with amphotericin B deoxycholate, and suggest treatment with fluconazole or amphotericin B lipid products as alternatives.18 Amphotericin B deoxycholate is the most commonly used antifungal for treating invasive Candida infections in infants,4,19 although fluconazole, amphotericin B lipid products, and echinocandins such as caspofungin are increasingly used.19
Current treatment recommendations for infants come from non-comparative, open-label studies of invasive Candida infections in infants, which demonstrate efficacy with amphotericin B deoxycholate,7 amphotericin B lipid products,10-13,20 and fluconazole.8,9 However, comparative outcomes data in infants are lacking and limited to small cohort studies or underpowered trials.5,14,15,17 In this large retrospective cohort study, we observed that monotherapy with amphotericin B lipid products was associated with higher mortality compared to monotherapy with amphotericin B deoxycholate or fluconazole, and higher rates of antifungal therapy failure compared to amphotericin B deoxycholate.
We observed significantly higher mortality in infants treated with amphotericin B lipid products compared to fluconazole: 29% vs. 16%, respectively; adjusted mortality OR of amphotericin B lipid products vs. fluconazole: 2.39 (1.18, 4.83). We observed a higher incidence of therapeutic failure associated with amphotericin B lipid products that did not reach statistical significance: 47% vs. 40% therapeutic failure, respectively; adjusted therapeutic failure OR of amphotericin B lipid products vs. fluconazole: 1.34 (0.73, 2.46). We also observed significantly higher mortality in infants treated with amphotericin B lipid products compared to amphotericin B deoxycholate: 29% vs. 18% mortality, respectively; adjusted mortality OR of amphotericin B lipid products vs. amphotericin B deoxycholate: 1.96 (1.16, 3.33). Furthermore, we estimated significantly higher therapeutic failure associated with amphotericin B lipid products compared to amphotericin B deoxycholate: 47% vs. 38% therapeutic failure, respectively; adjusted therapeutic failure OR of amphotericin B lipid products vs. amphotericin B deoxycholate: 1.62 (1.00, 2.64). These findings differ from a small, single-center prospective cohort study in infants with proven candidemia that found similar mortality rates in infants treated with different amphotericin formulations.14 This prior study of 56 infants determined antifungal use by serum creatinine levels because of concern for amphotericin B deoxycholate-associated nephrotoxicity. Infants with serum creatinine <1.2 mg/dL received amphotericin B deoxycholate, and infants with serum creatinine >1.2 mg/dL received either liposomal amphotericin B or amphotericin B colloidal dispersion. Even though infants treated with amphotericin B lipid products were smaller and younger than infants treated with amphotericin B deoxycholate, mortality was similar between study groups.14 Our results also differ from previous large randomized trials in adults and children that demonstrate similar efficacy for amphotericin B lipid products compared with amphotericin B deoxycholate.21-24
We observed no difference in outcomes between amphotericin B deoxycholate and fluconazole: 18% vs. 16% mortality, respectively; adjusted mortality OR of fluconazole vs. amphotericin B deoxycholate: 0.82 (0.46, 1.47); 38% vs. 40% therapeutic failure, respectively; adjusted therapeutic failure OR of fluconazole vs. amphotericin B deoxycholate: 1.21 (0.76, 1.93). In a small, underpowered randomized trial of antifungal therapy in young infants (n=23), investigators also found no difference in mortality between infants treated with amphotericin B deoxycholate and fluconazole (46% and 33%, respectively; p=0.56).5
Worse outcomes with amphotericin B lipid products may be explained by the different tissue distributions of amphotericin B lipid products compared with amphotericin B deoxycholate and fluconazole. Invasive Candida infections in infants have a propensity to affect the CNS and urinary tract.25 Several adult studies show fluconazole has excellent penetration into the CNS and urinary tract, achieving CSF concentrations similar to serum levels and urine concentrations 10–20 times higher than serum levels.26,27 There are few pharmacokinetic data on amphotericin B formulations in infants and children, so tissue distribution is largely extrapolated from preclinical and adult studies. Preclinical studies comparing kidney tissue concentrations of amphotericin B formulations show amphotericin B lipid products achieve lower concentrations in the kidneys compared with equivalent doses of amphotericin B deoxycholate.28-30 In adult studies, liposomal amphotericin B achieves less kidney penetration than amphotericin B deoxycholate.31 Preclinical studies of amphotericin B formulation concentrations in brain tissue show liposomal amphotericin B achieves relatively higher brain tissue concentrations than amphotericin B deoxycholate and other amphotericin B lipid products,32 In infants, amphotericin B deoxycholate appears to have improved CNS penetration compared with adults.7 A recent population pharmacokinetics study of amphotericin B lipid complex in infants with invasive candidiasis found less drug exposure in urine than amphotericin B deoxycholate and low or no detectable concentrations in the CSF.33
Overall unadjusted mortality did not significantly vary by infection site: bloodstream-only infections (19%), urine-only infections (16%), CNS-only infections (0%), and multiple site infections (25%) (p=0.17). Our observation differs from a previous multi-center cohort study of invasive Candida infections in ELBW infants, which found higher mortality associated with multiple site infections (57%) compared with urine (26%) and bloodstream (28%) infections.34 We included infants with isolated positive urine cultures as having invasive candidiasis, but it is possible that these infants may have only been colonized with Candida. We performed our analysis excluding infants with urine–only-positive cultures and observed similar adjusted mortality and therapeutic failure ORs.
To our knowledge, this is the largest study comparing outcomes of antifungal therapies in infants. Strengths of this study include population diversity from academic and community institutions; recorded results of every blood, urine, and CSF culture obtained for each patient during hospitalization; and ability to use multivariate analysis controlling for gestational age, day of life at onset of infection, delay in therapy, and site of infection. Our results are generalizable to young infants admitted to the NICU who develop invasive Candida infections.
There are several limitations to this study that need to be considered. This is a retrospective study and lacked clinical data regarding underlying illnesses; risk factors for invasive candidiasis such as presence and removal of central venous catheter35; use of broad-spectrum antibiotics36 and total parenteral nutrition; source of blood culture (central or peripheral); Candida species; and cause of death. Our definition of duration of candidiasis is limited by the fact that follow-up cultures were obtained per standard of care instead of daily. An important limitation in any non-experimental outcomes study is confounding by indication. Because amphotericin B deoxycholate is often the preferred first-line therapy and amphotericin B lipid products are more expensive and have a narrower indication, there may be a tendency to use amphotericin B lipid products in sicker infants. This may overestimate the increase in mortality associated with amphotericin B lipid products. A recent study of antifungal utilization in infants with invasive candidiasis during part of our study period showed the ratio of amphotericin B lipid product use to amphotericin B deoxycholate use is increasing.19 It is unclear what is motivating the change in prescribing practices, but this may suggest less heterogeneity in use of amphotericin B formulations. Furthermore, amphotericin B deoxycholate and amphotericin B lipid product treatment groups did not differ in mean gestational age at birth, birth weight, day of life at onset of infection, or serum creatinine—important prognostic indicators in infants. We were unable to determine whether more critically ill infants with higher serum creatinine were selected for amphotericin B lipid products, as only 124 infants (17%) had serum creatinine reported within 1 day of beginning treatment, and only 21 were treated with amphotericin B lipid products. Due to concern for amphotericin B deoxycholate-associated nephrotoxicity, sicker infants with higher serum creatinine may have been selected for amphotericin B lipid products. We also lacked information on whether fluconazole administration was oral or parenteral. Infants who received oral fluconazole may have been healthier at baseline compared with infants receiving a parenteral antifungal agent. This would overestimate the increase in mortality associated with amphotericin B lipid products compared with fluconazole.
We lacked information to distinguish between the 3 amphotericin B lipid products; therefore, our study did not assess differences between types of amphotericin B lipid products. We grouped lipid products as 1 treatment category, although there may be important differences between the lipid formulations, such as CNS penetration. Aggregate efficacy estimates from open-label studies in adult and child populations treated for invasive fungal infections show similar response rates between the 3 amphotericin B lipid products.24 Additionally, a previous multi-center prospective cohort study compared amphotericin B formulations in 118 infants with proven invasive candidiasis and found no difference in mortality rates between liposomal amphotericin B and amphotericin B colloidal dispersion.15
Another limitation of these data was the lack of dosing information for each antifungal. Inappropriate dosing may account for some of the observed differences in outcomes. While dosing recommendations for amphotericin B deoxycholate and amphotericin B lipid products have not changed since our study period, recent studies have evaluated the pharmacokinetics of fluconazole for treatment of invasive candidiasis in young infants.37,38 The studies found that a higher than previously recommended maintenance dose is needed to achieve therapeutic exposure (12 mg/kg vs. 6 mg/kg).37 As these fluconazole studies were published after our study period, our data suggest that amphotericin B lipid products were associated with worse outcomes compared to low doses of fluconazole.
In summary, this large multicenter cohort study provides new information on comparative outcomes of antifungal treatment in infants with invasive Candida infections. The results of this study indicate treatment with amphotericin B lipid products is associated with higher mortality compared to treatment with amphotericin B deoxycholate or fluconazole. Clinicians choosing amphotericin B lipid products should be cautioned about lack of penetration to the CNS and kidneys. There are no adequately powered randomized trials comparing the efficacy of different antifungal regimens for treatment of invasive candidiasis in infants. These findings may help inform the selection of antifungal therapies for future clinical trials in this patient population. Well-powered randomized controlled trials are needed to more fully investigate outcomes to provide neonatologists with a clear roadmap for the management of invasive candidiasis.
Supplementary Material
Acknowledgments
This work was supported by the United States government (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C to D.K.B. Jr.); the nonprofit organization Thrasher Research Foundation (to D.K.B. Jr. and M.C.W); the National Institute of Child Health and Human Development (1K23HD060040-01 and DHHS-1R18AE000028-01 to P.B.S; 1K23HD064814-01 to M.C.W.); and CTSA biostatistical services through the Duke Division of Pediatric Quantitative Sciences (NIH CTSA grant 1UL 1RR024128-01 to C.M., NIH-5UL-1RR024128-01 to D.K.B. Jr.).
Footnotes
Conflicts of interest and sources of funding: D.K.B. Jr. is a consultant for Pfizer, Cerexa, Biosynexus, and Johnson & Johnson and a principal investigator for Astellas Pharma, AstraZeneca, and UCB Pharma, the protocol chair of a randomized trial comparing micafungin to amphotericin B deoxycholate in young infants (registered on clinicaltrials.gov), and an advisor to a trial comparing fluconazole to an echinocandin that has not yet been approved by the EMA. P.B.S. is a consultant for Pfizer, Cubist Pharmaceuticals, Pangen Biosystems, Johnson & Johnson, and Astellas Pharma and a principal investigator for Cubist Pharmaceuticals. M.C.W. is a consultant and a principal investigator for Pfizer. C.M. is a consultant for Pfizer and a principal investigator for Astellas Pharma. The remaining authors have no conflicts to declare.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112(3 Pt 1):543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 4.Rowen JL, Tate JM. Management of neonatal candidiasis. Neonatal Candidiasis Study Group. Pediatr Infect Dis J. 1998;17:1007–1011. doi: 10.1097/00006454-199811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driessen M, Ellis JB, Cooper PA, et al. Fluconazole vs. amphotericin B for the treatment of neonatal fungal septicemia: a prospective randomized trial. Pediatr Infect Dis J. 1996;15:1107–1112. doi: 10.1097/00006454-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008;27:820–826. doi: 10.1097/INF.0b013e31817275e6. [DOI] [PubMed] [Google Scholar]
- 7.Baley JE, Meyers C, Kliegman RM, Jacobs MR, Blumer JL. Pharmacokinetics, outcome of treatment, and toxic effects of amphotericin B and 5-fluorocytosine in neonates. J Pediatr. 1990;116:791–797. doi: 10.1016/s0022-3476(05)82674-5. [DOI] [PubMed] [Google Scholar]
- 8.Wainer S, Cooper PA, Gouws H, Akierman A. Prospective study of fluconazole therapy in systemic neonatal fungal infection. Pediatr Infect Dis J. 1997;16:763–767. doi: 10.1097/00006454-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Huttova M, Hartmanova I, Kralinsky K, et al. Candida fungemia in neonates treated with fluconazole: report of forty cases, including eight with meningitis. Pediatr Infect Dis J. 1998;17:1012–1015. doi: 10.1097/00006454-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Scarcella A, Pasquariello MB, Giugliano B, Vendemmia M, de Lucia A. Liposomal amphotericin B treatment for neonatal fungal infections. Pediatr Infect Dis J. 1998;17:146–148. doi: 10.1097/00006454-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Juster-Reicher A, Leibovitz E, Linder N, et al. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection. 2000;28:223–226. doi: 10.1007/s150100070040. [DOI] [PubMed] [Google Scholar]
- 12.Adler-Shohet F, Waskin H, Lieberman JM. Amphotericin B lipid complex for neonatal invasive candidiasis. Arch Dis Child Fetal Neonatal Ed. 2001;84:F131–F133. doi: 10.1136/fn.84.2.F131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juster-Reicher A, Flidel-Rimon O, Amitay M, Even-Tov S, Shinwell E, Leibovitz E. High-dose liposomal amphotericin B in the therapy of systemic candidiasis in neonates. Eur J Clin Microbiol Infect Dis. 2003;22:603–607. doi: 10.1007/s10096-003-0993-4. [DOI] [PubMed] [Google Scholar]
- 14.Linder N, Klinger G, Shalit I, et al. Treatment of candidaemia in premature infants: comparison of three amphotericin B preparations. J Antimicrob Chemother. 2003;52:663–667. doi: 10.1093/jac/dkg419. [DOI] [PubMed] [Google Scholar]
- 15.Lopez Sastre JB, Coto Cotallo GD, Fernandez Colomer B. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol. 2003;20:153–163. doi: 10.1055/s-2003-40008. [DOI] [PubMed] [Google Scholar]
- 16.Odio CM, Araya R, Pinto LE, et al. Caspofungin therapy of neonates with invasive candidiasis. Pediatr Infect Dis J. 2004;23:1093–1097. [PubMed] [Google Scholar]
- 17.Jeon GW, Koo SH, Lee JH, et al. A comparison of AmBisome to amphotericin B for treatment of systemic candidiasis in very low birth weight infants. Yonsei Med J. 2007;48:619–626. doi: 10.3349/ymj.2007.48.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad PA, Coffin SE, Leckerman KH, Walsh TJ, Zaoutis TE. Pediatric antifungal utilization: new drugs, new trends. Pediatr Infect Dis J. 2008;27:1083–1088. doi: 10.1097/INF.0b013e31817eeee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weitkamp JH, Poets CF, Sievers R, et al. Candida infection in very low birth-weight infants: outcome and nephrotoxicity of treatment with liposomal amphotericin B (AmBisome) Infection. 1998;26:11–15. doi: 10.1007/BF02768745. [DOI] [PubMed] [Google Scholar]
- 21.White MH, Bowden RA, Sandler ES, et al. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clin Infect Dis. 1998;27:296–302. doi: 10.1086/514672. [DOI] [PubMed] [Google Scholar]
- 22.Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 23.Prentice HG, Hann IM, Herbrecht R, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol. 1997;98:711–718. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 24.Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: time for a new “gold standard”. Clin Infect Dis. 2003;37:415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112(3 Pt 1):634–640. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 26.Brammer KW, Farrow PR, Faulkner JK. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990;12(Suppl 3):S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- 27.Debruyne D. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin Pharmacokinet. 1997;33:52–77. doi: 10.2165/00003088-199733010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Clark JM, Whitney RR, Olsen SJ, et al. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob Agents Chemother. 1991;35:615–621. doi: 10.1128/aac.35.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fielding RM, Smith PC, Wang LH, Porter J, Guo LS. Comparative pharmacokinetics of amphotericin B after administration of a novel colloidal delivery system, ABCD, and a conventional formulation to rats. Antimicrob Agents Chemother. 1991;35:1208–1213. doi: 10.1128/aac.35.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991;28(Suppl B):49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- 31.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46:828–833. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000;182:274–282. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 33.Wurthwein G, Groll AH, Hempel G, Adler-Shohet FC, Lieberman JM, Walsh TJ. Population pharmacokinetics of amphotericin B lipid complex in neonates. Antimicrob Agents Chemother. 2005;49:5092–5098. doi: 10.1128/AAC.49.12.5092-5098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin DK, Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126:e865–e873. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 36.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK., Jr. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118:717–722. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 37.Wade KC, Benjamin DK, Jr, Kaufman DA, et al. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J. 2009;28:717–723. doi: 10.1097/INF.0b013e31819f1f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piper L, Smith PB, Hornik CP, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. 2011;30:375–378. doi: 10.1097/INF.0b013e318202cbb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.