Abstract
The profile of HIV-associated neurocognitive disorders (HAND) has classically been characterized as “subcortical”, but questions have arisen as to whether aging with HIV in the antiretroviral therapy era has subtlety shifted the expression of HAND into a more “cortical” disorder (e.g., decay of semantic memory stores). We evaluated this hypothesis by examining semantic fluency and its component processes (i.e., clustering and switching) in 257 individuals across four groups stratified by age (<40 and ≥ 50 years) and HIV serostatus. Jonckheere-Terpstra tests revealed significant monotonic trends for the combined effects of HIV and aging on overall semantic (and letter) fluency and switching, but not cluster size, with greatest deficits evident in the older adults with HIV infection. Within the older HIV-infected cohort, poorer switching was uniquely associated with self-reported declines in instrumental activities of daily living and deficits in learning and executive functions, but not semantic memory. Results suggest that HIV infection and aging may confer adverse additive effects on the executive components of semantic fluency (i.e., switching), rather than a degradation of semantic memory stores (i.e., cluster size), which is a profile that is most consistent with combined frontostriatal neuropathological burden of these two conditions.
Keywords: Immunologic disorders, geropsychology, verbal fluency, executive functions, cognitive neuropsychology
With advances in the management of HIV infection and corresponding decreases in mortality rates, the prevalence of older individuals living with HIV/AIDS has steadily increased over the past 10 years (Centers for Disease Control and Prevention, 2009). Both HIV infection and aging are independently associated with neural abnormalities (e.g., frontostriatal pathology; Chang et al., 2005; Drachman, 2006; Jernigan et al., 1991; Langford et al., 2005), neurocognitive impairment (e.g., Reger Welsh, Razen, Martin & Boone, 2002; Salthouse, 1996) and corresponding functional declines (Cahn-Weiner et al., 2000; Heaton et al., 2004). As such, there is growing concern that the combined effects of these two risk factors amplifies their independent adverse effects on brain structure and function, which may have important implications for disease management, health outcomes, and quality of life.
Although findings across the HIV aging literature are not entirely consistent (e.g., Valcour, Paul, Neuhaus & Shikuma, 2011), a number of studies now show that older HIV-infected adults are at greater risk for adverse consequences on central nervous system (CNS) structure and function than their younger HIV-infected or older seronegative counterparts (e.g., Ernst & Chang, 2004; Green et al., 2005). For example, older age has been associated with increased HIV-associated neuropathological burden (e.g., Achim et al., 2009), and smaller brain volumes (e.g., Jernigan et al., 2005) in HIV-infected adults. Older individuals with HIV infection may also be at greater risk for HIV-Associated Neurocognitive Disorders (HAND; Becker, Lopez, Dew & Aizenstein, 2004; Valcour et al., 2004a). In fact, Valcour et al., (2004a) reported a three-fold increased risk of HIV-associated Dementia (HAD) in older (i.e., over 50 years of age) relative to younger HIV-infected individuals, even when taking into account other demographic factors, HIV disease severity, psychiatric comorbidities, and cART use. Even in the absence of dementia, many studies have found evidence for greater cognitive impairment in older HIV-infected individuals, particularly within the domains of executive functions, information processing speed, and episodic memory (e.g., Sacktor et al., 2007; Woods et al., 2010). Older HIV-infected individuals may also experience less favorable functional outcomes (e.g., poorer medication and financial management; Thames et al., 2011), particularly in the setting of neurocognitive impairment (e.g., Barclay et al., 2007).
One current controversy in the neuroAIDS of older adults posits that there has been a subtle shift toward neocortical (e.g., temporal and parietal lobe) neuropathology in the era of cART (e.g., Kieburtz et al, 1996). While this theory remains controversial, there is some evidence of pathology in HIV-infected individuals that may be similar to that observed in traditional “cortical” dementias (e.g., Alzheimer’s disease). For example, recent neuropathological studies have reported abnormal levels of beta amyloid (Green et al., 2005) and increased intraneuronal accumulation of amyloid plaques (Achim et al., 2009) in the brains of older HIV-infected adults. In addition, decreased CSF beta amyloid and increased tau concentrations have been found in HIV-infected individuals with HAND (Brew et al., 2005; cf. Ances et al., 2011). Neuroimaging studies have also revealed cortical thinning in parietal regions (e.g., Thompson et al, 2005) and smaller temporal lobe volumes (Jernigan et al., 2005) in HIV-infected individuals. Genetic studies have also found evidence for an association between Apolipoprotein E epsilon 4 (ApoEε4), a risk factor for Alzheimer’s disease (AD), and HAND among older adults with HIV (e.g., Valcour et al., 2004b). Despite these intriguing findings, controversy remains because many of these pathological and biomarker findings are non-specific to AD (e.g., ApoEε4 and tau), may reflect different mechanistic processes (e.g., absence of neurofibrillary tangles), and use conflicting terminology.
Nonetheless, based on this emergent theme of findings, some authors have argued that the profile of neurocognitive impairment in older HIV-infected adults may now be increasingly more consistent with that seen in “cortical” dementias (e.g., rapid forgetting, visuoperceptual deficits, and a degradation of semantic memory), rather than the classic “subcortical” pattern (e.g., executive dysfunction, bradyphrenia) that has historically been observed in younger and middle-aged HIV-infected adults (e.g., Kieburtz et al., 1996; Ragin et al., 2005). Evidence for this hypothesis arises from studies that have found HIV-associated deficits on tests of spatial cognitive (e.g., visuoperceptual) abilities, which are thought to be particularly reliant on the posterior parietal lobe (e.g., mental rotation; Olesen, Schendan, Amick, & Chronin-Golumb, 2007; Sharma, Amick, Shendan & Chronin-Golomb, 2003), and on tests of verbal and visual episodic memory (Sacktor et al., 2007). However, the interpretation of the visuoperceptual findings is controversial in light of data showing that HIV-associated deficits in spatial cognition may be driven by working memory and executive dysfunction (e.g., Weber et al., 2010). Moreover, the pattern of memory impairment observed in older HIV-infected adults (e.g., impaired retrieval in light of intact recognition memory) is more suggestive of executive dysfunction consistent with frontostriatal pathology (e.g., Gongvatana et al., 2007) than the prototypical shallow encoding, rapid forgetting, and intrusions observed in cortical dementias. Also arguing against the cortical hypothesis, Scott et al. (2011) observed no age by HIV interactions on a relatively comprehensive neurocognitive battery sensitive to posterior neocortical injury, including measures of consolidation, semantic knowledge, and visuoconstruction. As such, the available cognitive evidence is mixed regarding whether the neuropsychological profile of HAND in older HIV-infected adults includes impairments observed in cortical dementias in the era of cART.
Troyer’s clustering and switching model of verbal fluency provides a potentially useful framework within which to further explore this controversial hypothesis (Troyer, Moscovitch, & Winocur, 1997). Category fluency tasks require rapid, self-initiated search and retrieval from semantic stores to orally generate as many words as possible within 60 seconds that belong to a specific semantic category (e.g., animals). This influential conceptual model of verbal fluency deficits suggests that optimal performance depends on two related, but separable factors; viz., clustering and switching (Gruenewald & Lockhead, 1980; Troyer et al., 1997). In brief, clustering refers to the generation of words within specific semantic subcategories and is viewed as an automatic retrieval process associated with the integrity of semantic memory and the medial temporal lobes (e.g., Tröster et al., 1998). Switching describes the ability to disengage from one lexico-semantic cluster in order to search for, engage, and retrieve words from another relevant cluster (Troyer et al., 1997) and is considered to be a more controlled executive ability, likely associated with frontal systems (e.g., Ho et al., 2002). Research on verbal fluency components within neurodegenerative conditions has supported this neural dissociation between clustering and switching (e.g., Tröster et al., 1998; Troyer et al., 1998). For example, while individuals with both Parkinson’s disease (PD) and AD produce fewer total words on category fluency tasks, their clustering and switching profiles diverge. PD patients tend to switch less often than healthy adults, but produce normal mean cluster sizes, whereas individuals with AD show impairment in switching and lower mean cluster sizes (Troyer et al., 1998).
Semantic fluency declines are evident in both older healthy adults and HIV-infected individuals, and the available data suggest that these declines reflect dyscontrol of switching rather than an inability to generate sufficient cluster sizes (Iudicello et al., 2008; Troyer et al., 1997). Specifically, Troyer et al., (1997) found that older healthy adults switched between lexico-semantic categories less frequently relative to their younger counterparts, but generated similar cluster sizes. Similarly, HIV infection is associated with impairments in letter (e.g., Woods et al., 2004) and category switching (e.g. Iudicello et al., 2008), whereas clustering remains largely unaffected. In addition, HIV-associated category fluency deficits may be exacerbated when the switching demands became more explicit (i.e., using an alternating fluency task; Iudicello et al., 2008). This verbal fluency profile observed in both healthy aging and HIV infection is most consistent with the pattern of clustering and switching observed in conditions with prominent frontostriatal pathology (e.g., Tröster et al., 1998; Troyer et al., 1998).
While there is evidence for semantic fluency impairment in older HIV-infected adults relative to younger seropositive individuals (Hardy et al., 1999), no studies have examined the combined effects of HIV and aging on the component processes (i.e., clustering and switching) of category fluency. Viewed through the lens of the cortical hypothesis, one would expect that older HIV-infected adults might evidence impairment in switching in addition to smaller cluster sizes, reflecting both executive dysfunction and the degradation of the semantic stores, respectively. Alternatively, the traditional frontostriatal viewpoint of HAND would predict that the combined effects of aging and HIV infection would confer additive adverse effects on switching, but not clustering. With these competing predictions in mind, the current study sought to explore the cortical hypothesis controversy by applying Troyer’s model of clustering and switching in order to elucidate the verbal fluency profile of older adults living with HIV infection, including its neurocognitive and everyday functioning correlates.
Method
Participants
This study included 257 baseline participants enrolled in NIH-funded studies conducted within the San Diego HIV Neurobehavioral Research Program. Each participant was classified with respect to HIV serostatus (i.e., HIV+ and HIV−) and age (i.e., older ≥ 50 and younger < 40). This classification yielded 63 Older HIV+; 51 Older HIV−; 50 Younger HIV+; and 93 Younger HIV− participants. HIV infection was indicated by enzyme linked immunosorbent assays (ELISA) and a Western Blot confirmatory test.
Individuals were excluded if they met Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (DSM-IV; American Psychiatric Association, 1994) criteria for substance dependence within six months of evaluation or if they tested positive for illicit drugs (except marijuana) on a urine toxicology screen on the day of testing. Additional exclusion criteria included a history of severe psychiatric (e.g., schizophrenia) or neurological (e.g., active CNS opportunistic infections, cerebrovascular accidents, seizure disorders, closed head injuries) illness, or an estimated verbal IQ score less than 70 on the Wide Range Achievement Test, Revision 3 (WRAT-3 Revised; Wilkinson, 1993). Demographic characteristics for the four groups are presented in Table 1. The four groups did not differ in terms of years of education, estimated verbal IQ, ethnicity, or sex (all ps > 0.10).
Table 1.
Demographic, psychiatric, medical, and HIV-disease characteristics of the study groups.
| HIV−
|
HIV+
|
p | Groupa | |||
|---|---|---|---|---|---|---|
| Younger (n = 93) | Older (n = 51) | Younger (n = 50) | Older (n = 63) | |||
| Demographic Characteristics | ||||||
| Age (years) | 32.2 (5.2) | 56.8 (4.9) | 32.8 (4.0) | 58.2 (6.2) | <0.001 | Y− = Y+ < O− = O+ |
| Education (years) | 14.0 (2s.2) | 13.4 (2.3) | 14.2 (1.9) | 13.9 (2.6) | 0.390 | ----- |
| Ethnicity (% Caucasian) | 66.7% | 78.4% | 62.0% | 74.6% | 0.218 | ----- |
| Sex (% male) | 68.8% | 74.5% | 78.0% | 82.5% | 0.249 | ----- |
| Estimated Verbal IQb | 103.4 (10.1) | 100.1 (12.0) | 103.2 (8.7) | 101.5 (11.7) | 0.282 | ----- |
| Psychiatric Characteristics | ||||||
| Total Mood Disturbance (POMS)c | 41.0 (22.8, 73.3) | 40.0 (17.0, 81.0) | 54.0 (30.0, 79.0) | 62.0 (46.8, 91.5) | 0.006 | Y−, O− < O+ |
| POMS Depression/Dejection | 4.0 (1.0, 14.0) | 4.0 (1.0, 18.0) | 6.0 (2.0, 16.0) | 9.0 (4.0, 17.0) | 0.160 | ----- |
| Medical Characteristics | ||||||
| Hepatitis C Virus (% infected) | 5.1% | 37.5% | 7.3% | 25.0% | <0.001 | Y− = Y+ < O− = O+ |
| Vascular Risk (%) | 10% | 41% | 18% | 43% | <0.001 | Y− = Y+ < O− = O+ |
| HIV Disease Characteristics | ||||||
| Duration of Infection (years) | ----- | ----- | 4.6 (4.5) | 10.7 (6.5) | <0.001 | Y+ < O+ |
| Proportion with AIDS (%) | ----- | ----- | 32.0% | 71.0% | <0.001 | Y+ < O+ |
| Proportion on ARVs (%) | ----- | ----- | 37.5% | 76.7% | <0.001 | Y+ < O+ |
| Nadir CD4c (cells/μl) | ----- | ----- | 282.0 (170.3, 463.5) | 153.0 (50.0, 227.0) | <0.001 | Y+ > O+ |
| Current CD4c (cells/μl) | ----- | ----- | 432.0 (273.5, 623.5) | 419.0 (271.0, 596.8) | 0.564 | ----- |
| Plasma HIV RNAc (log10) | ----- | ----- | 3.9 (2.6, 4.7) | 2.6 (1.7, 2.9) | <0.001 | Y+ > O+ |
Note. Means (SD) unless otherwise noted. HIV = Human Immunodeficiency Virus; Y = younger (< 40 years); O = older (≥ 50 years). POMS = Profile of Mood States; AIDS = Acquired Immune Deficiency Syndrome; ARVs = antiretroviral therapies; CD4 = Cluster of Differentiation 4; CSF = Cerebrospinal Fluid;
p < 0.05;
Based on the Reading subtest from the Wide Range Achievement Test, Revision 3;
Median (interquartile range).
Table 1 also displays the medical characteristics for the study participants. The older groups had a significantly greater proportion of individuals infected with HCV and with cardiovascular risk factors relative to the younger groups (ps < 0.05). As might be expected, the Older HIV+ group had a longer duration of disease, lower nadir CD4 counts, and a greater proportion of individuals diagnosed with acquired immune deficiency syndrome (AIDS) as compared with the Younger HIV+ group (ps < 0.001). Nevertheless, a larger proportion of the Older HIV+ group was taking antiretroviral medications (p < 0.001), and had lower plasma (p < 0.001) and CSF (p = 0.017) HIV viral loads relative to their younger counterparts.
Procedure
Each participant provided written, informed consent and was administered a standard measure of category (i.e., animal) and letter (i.e., FAS) fluency (Benton, Hamsher & Sivan, 1994) alongside a comprehensive neuropsychological and medical evaluation. Fluency performance was determined by the total number of correct words generated, and scored for clustering and switching according to the rules and criteria established by Troyer et al. (Troyer, 2000; Troyer et al., 1997). Briefly, mean cluster size was indexed by the average number of successfully generated words belonging to the same semantic subcategory (e.g., animals used as pets, such as cat and dog). Switching was determined by the total number of times an individual disengaged from one semantic cluster and switched to another.
The remaining neuropsychological test battery included an estimate of premorbid verbal IQ (reading subtest of the WRAT-3; Wilkinson, 1993), and measures from designed to assess neurocognitive domains relevant to HAND in accordance with Frascati research criteria (Antinori et al., 2007) as listed below:
(1) Speed of Information Processing: Trail Making Test Part A (Army Individual Test Battery, 1944; Heaton et al., 2004), Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Symbol and Symbol Search (Heaton, Taylor, & Manly, 2001; The Psychological Corporation, 1997), Stroop Color Naming (Golden, 1978); (2) Attention/Working Memory: Paced Auditory Serial Addition Test (PASAT; Heaton et al., 2004; Gronwall, 1977; Gronwall & Sampson, 1974), WAIS-III Letter Number Sequencing (Heaton et al., 2001; The Psychological Corporation, 1997); (3) Executive Functions: Halstead Category Test (Heaton et al., 1991; Reitan & Wolfson, 1993), Wisconsin Card Sorting Test-64 Card Version (WCST-64; Kongs, Thompson, Iverson, & Heaton, 2000) Perseverative Responses, Trail Making Test Part B (TMT-B; Army Individual Test Battery, 1944; Heaton et al., 2004), Stroop Interference (Golden, 1978); (4) Learning: Hopkins Verbal Learning Test-Revised (HVLT-R; Benedict, Schretlen, Groninger, & Brandt, 1998) Total Trial 1–3 Recall, Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997) Total Trial 1–3 Recall; (5) Memory: HVLT-R Percent Retained (Benedict et al., 1998), BVMT-R Percent Retained (Benedict, 1997); (6) Motor: Grooved Pegboard Dominant and Non-dominant hand (Heaton et al., 2004; Klove, 1963).
Raw scores from the measures listed above were converted to demographically (e.g., age, education) corrected T-scores, which were used to derive individual and global deficit scores (i.e., GDS; Carey et al., 2004). Consistent with prior research, a GDS cut-off score of 0.5 was used to classify individuals as neuropsychologically impaired (NP Impaired; which corresponds approximately to a T-score < 40) or NP Unimpaired. HIV-infected individuals were classified as having HAND if they met recent Frascati research (see Antinori et al., 2007) criteria for one of three conditions: asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), and HIV-associated Dementia (HAD). HAND diagnoses required that participants demonstrate global NP impairment that was not better explained by comorbid conditions. Within the 27 older HIV-infected individuals with HAND (i.e., 43% of the older HIV-infected cohort), approximately 67% (n = 18) were classified as ANI, 30% (n = 8) as having MND, and 3% (n = 1) with HAD. These proportions of HAND subdiagnoses are generally consistent with current epidemiological estimates (see Heaton et al., 2010).
Current mood was assessed using the Profile of Mood States (POMS; McNair, Loor & Droppleman, 1981), which is a 65-item, self-report measure of current (i.e. the week prior to evaluation) affective distress. Participants were asked to rate various adjectives (e.g., “unhappy”) on a five-point Likert-type scale ranging from 0 (i.e., “not at all”) to 4 (i.e., “extremely”), which were then used to derive a total mood score (i.e., POMS Total Mood Disturbance) that was used for all analyses. The Older HIV+ cohort endorsed significantly greater overall affective distress relative to the Older HIV− (p = 0.031) and Younger HIV− (p = 0.004) individuals, but did not differ from the Younger HIV+ group (p = 0.206). No significant omnibus difference was observed between the groups specifically on the Depression/Dejection subscale (p = 0.160).
Everyday functioning was measured using a modified version of the Lawton and Brody (1969) Activities of Daily Living Scale, which requires participants to self-rate their current and best levels of functioning with regard to 10 instrumental activities of daily living (i.e., IADLs) including Financial Management, Home Repair, Medication Management, Laundry, Transportation, Grocery Shopping, Shopping, Housekeeping (Cleaning), Cooking, and Telephone Use. Participants were classified as IADL dependent if they reported decline (i.e., current rating of functioning is lower than their best level of functioning) in two or more IADLs (consistent with Heaton et al., 2004). Analyses regarding the predictors of IADL dependence were conducted in the Older HIV+ group only. Using this system, within the older HIV-infected cohort, 39% (n=24) were classified as “IADL Dependent”, whereas 61% (n=37) were considered “IADL Independent”. The IADL Dependent and Independent samples were generally comparable with respect to rates of overall neuropsychological impairment (p = 0.142), and most demographic (e.g., age, ethnicity, gender), medical (i.e., hepatitis C virus, hypertension, diabetes mellitus, and hypercholesterolemia) and HIV disease characteristics (e.g., AIDS status, Nadir CD4 lymphocyte count, current CD4 count, plasma and CSF viral loads) that may influence an individual’s everyday functioning abilities (all ps > 0.10). However, the IADL Dependent group endorsed significantly higher levels of current affective distress (i.e., POMS Total Mood Disturbance; p < 0.001) and slightly higher education levels (p = 0.087) and longer estimated durations of HIV infection (p = 0.082).
Data Analyses
Prior to analyses, the data were reviewed for outliers (i.e., > 3.5 SD from the overall mean) and normality using the Shapiro-Wilk W Test. Given the non-normal distributions of some of the fluency variables (e.g., mean cluster size), non-parametric statistics were used wherever possible for consistency. The critical alpha was set to 0.05 for all analyses. A series of Jonckheere-Terpstra (i.e., J–T) tests for ordered monotonic trends (Jonckheere, 1954; Terpstra, 1952) were conducted to test the hypotheses that median verbal fluency scores would decrease in a stepwise additive fashion as the number of neurocognitive risk factors increased. Thus, it was predicted that the Younger HIV− group would have the highest median scores, followed by those with only one risk factor (i.e., Younger HIV+ or Older HIV−), with the Older HIV+ demonstrating the poorest performance. Linear regression analyses using the four-level aging and HIV grouping variable as a predictor of overall category fluency performance was then conducted to explore the confounding effects of the demographic, medical, and psychiatric factors that differed between the study groups. Despite the non-normal distributions of some of the primary variables of interest, this regression approach was deemed appropriate given the large sample sizes and largely normal residuals. Follow-up pairwise comparisons using Wilcoxon Rank-Sum tests were then conducted and were restricted to comparisons involving the older HIV+ group only to reduce Type I error risk. Effect sizes for group comparisons were conducted using Hedge’s g.
Raw scores for the individual cognitive tests were converted to population-based z-scores (i.e., individual score minus the group mean, divided by the group standard deviation) using raw score distributions from the clinical sample (i.e., older HIV-infected sample) for standardization and comparison purposes. The use of the clinical sample’s raw score distributions is based off of a standard published approach that we have used in previous studies (e.g., Zogg et al., 2011). The individual z-scores were then averaged to create cognitive domain z-scores, which were used to explore the cognitive correlates of switching within the Older HIV-infected group. Spearman’s rho correlation coefficients and linear regression analyses were conducted to examine the associations between category fluency switching performance and the HIV disease variables and cognitive domains in the Older HIV-infected group. Logistic regression procedures were used to explore the relationship between switching performance and self-reported IADL dependence in the older, HIV-infected sample, alongside global NP impairment, as well as possible confounding psychiatric and HIV disease characteristics noted above that differed between the IADL dependent and independent groups.
Results
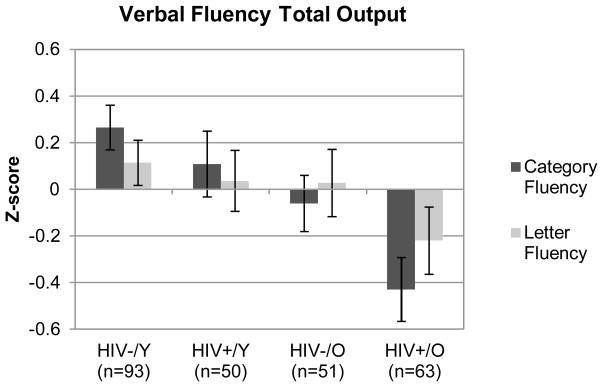
Figure 1 shows the additive effects of HIV and aging on overall Category Fluency performance, as indicated by a significant monotonic trend across the four study groups (J–T statistic = 3.03; p = 0.001). Specifically, the Older HIV+ group produced significantly fewer correct words overall relative to the Older HIV− (p = 0.038, g = −0.37), Younger HIV+ (p = 0.017, g = −0.51), and Younger HIV− (p < 0.001, g = −0.70) groups. Follow-up regression analyses revealed that the aging/HIV group effect remained a significant predictor of overall category fluency performance (adjusted R2 = 0.09; p = 0.004), even while accounting for the characteristics that differed between the groups (e.g., current mood symptoms). There were no significant effects of HCV infection on fluency performance in the older groups (ps > 0.10), which was evaluated separately given the low prevalence of HCV in the younger cohorts.
Figure 1.
Z-scores displaying the effects of HIV infection and aging on category and letter fluency overall performance. Error bars in this figure represent standard errors. Y = younger (< 40 years); O = older (≥ 50 years).
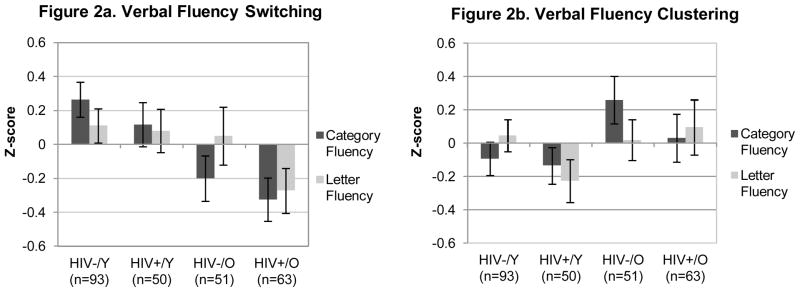
Figure 2 shows a similar, significant additive effect for the switching component of animal fluency (J–T statistic = 2.16; p = 0.015). Follow-up comparisons indicated that the Older HIV+ cohort switched less often relative to the Younger HIV+ (p = 0.025, g = −0.51) and Younger HIV− (p < 0.001, g = −0.70) groups, although they performed comparably to the Older HIV− adults (p = 0.914, g = −0.13). A closer examination of this null finding revealed that the Older HIV-infected individuals with HAND (n = 27) demonstrated significantly worse switching relative to the both unimpaired older HIV+ participants (n = 36; p = 0.004, g = −0.68) and older HIV-seronegatives (p = 0.016, g = −0.48). A follow-up multiple regression showed that the four-level age/HIV grouping variable remained a significant predictor of switching (adjusted R2 = 0.10; p < 0.001) even when mood symptoms and vascular risk factors were included in the model. Moreover, neither POMS Total Mood Disturbance nor the Depression/Dejection subscale significantly correlated with switching performance in the Older HIV+ group (both ps > 0.10). In contrast, no significant effects of age or HIV were found for the category fluency clustering variable (J–T statistic = −1.37; p = 0.915; See Figure 2).
Figure 2.
Z-scores displaying the effects of HIV infection and aging on category and letter fluency component processes (i.e., switching and clustering). Error bars in these figures represent standard errors. Y = younger (< 40 years); O = older (≥ 50 years).
Figure 1 also displays the significant additive effects of HIV and aging on overall letter fluency performance (J–T statistic = 1.65; p = 0.049) in the expected direction. Follow-up pairwise comparisons indicated that the Older HIV+ group produced fewer overall words on the letter fluency test relative to the Younger HIV− group (p = 0.032, g = −0.33). No other significant group differences in overall letter fluency output were observed (all ps > 0.10). A similarly modest monotonic trend was found for letter fluency switching (J–T statistic = 1.56; p = 0.060; See Figure 2). Follow-up analyses indicated that the Older HIV+ group switched significantly less than the Younger HIV− group (p = 0.024, g = −0.39), with more modest, trend-level effects relative to the Older HIV− (p = 0.095, g = −0.30) and Younger HIV+ (p = 0.060, g = −0.39) groups. Similar to the category fluency results, there were no orderly between-group differences on letter fluency mean cluster size (J–T statistic = 0.372; p = 0.355; See Figure 2).
Category switching performance correlated with attention/working memory (Spearman’s ρ = 0.349; p = 0.006), learning (ρ = 0.486, p < 0.001), speed of information processing (ρ = 0.345, p = 0.006), motor skills (ρ = 0.304, p =0.018) and memory (ρ= 0.328, p = 0.010), along with executive functions at a trend level (ρ = 0.224; p = 0.082). In contrast, switching performance was not significantly associated with semantic memory as measured by the WRAT-3 (Wilkinson, 1993; Spearman’s ρ = 0.182; p = 0.226). Regression analyses revealed executive functions (p = 0.011) and learning (p = 0.024), as independent predictors of animal switching performance [overall model F(6, 50) = 4.47; p = 0.001; adjusted R2 = 0.27].
Finally, amongst the older HIV-infected group, poorer switching performance was a significant independent predictor of IADL dependence (p = 0.041), even when accounting for global neuropsychological impairment (i.e., NP Impaired/Unimpaired), as well as HIV disease characteristics (i.e., duration of infection), current affective distress (i.e., POMS Total Mood Disturbance), and years of education (overall model χ2 = 34.6; p < 0.001). The unit odds ratio associated with switching in the older HIV-infected group was 1.5 (95% confidence interval =1.11 – 2.19).
Discussion
This current study investigated the “cortical” hypothesis of aging and neuroAIDS by adopting Troyer’s conceptual model to examining the clustering and switching aspects of category fluency in well-characterized samples of older and younger adults with and without HIV infection (Troyer et al., 1997). Overall, our findings argue against a posterior neocortical pattern of neuropsychological impairment associated with the combined effects of aging and HIV infection. While we found significant adverse additive effects of HIV infection and aging on overall category fluency performance, this effect was exclusively driven by the switching (i.e., executive) components of the task, rather than an inability to generate sufficient cluster sizes. Specifically, a significant stepwise decline in performance was observed for category fluency switching, with performance levels highest in the Younger HIV seronegative group and lowest in the Older HIV+ group. In contrast, no significant stepwise deleterious effects were observed for mean cluster size. It is unlikely that these findings are better explained by potentially confounding HIV disease and treatment characteristics or co-morbid medical conditions (e.g., vascular disease, HCV infection). While the older HIV+ group endorsed significantly more overall affective distress on the POMS relative to the older and younger HIV− samples, though their ratings did not differ significantly from their younger HIV+ counterparts. However, there were not any significant correlations between current affective distress and switching within the older HIV-infected cohort and HIV/age group status remained a significant predictor of switching when the POMS was included in a multiple regression model. Thus, in our cohort, it is unlikely that switching declines in our older HIV-infected cohort could be attributed to current mood symptoms, which is consistent with most literature that has failed to find additive effects of depression and HIV infection on cognition (e.g., Cysique et al., 2007). Collectively, these findings provide support for the additional burden of HIV infection and aging on the controlled executive (i.e., switching) components of fluency and underlying frontostriatal systems, rather than a more widespread pattern of neocortical (e.g., temporal and parietal) dysfunction and corresponding degradation of semantic memory stores.
Although there was no serostatus effect on category fluency switching in the older groups, post-hoc analyses revealed that older HIV-infected individuals with HAND switched significantly less often than older persons without HAND (g = −0.68) and the seronegatives (g = −0.48). Interestingly, there was no effect of HAND on clustering among the older cohort (ps > 0.10). A similar pattern was observed for letter fluency, whereby the older HAND group demonstrated significantly lower switching (ps <.05), but not clustering (ps > 0.10), relative to older HIV-infected individuals without HAND (g = −0.64) and older seronegative adults (g = −0.60), who did not differ from each other (g = 0.00). Collectively, this evidence suggests that a subset of older HIV+ individuals (i.e., those with HAND) may experience impairment in switching, which is consistent with prior research showing an increased risk of cognitive impairment in older HIV-infected adults (e.g., Valcour et al., 2004). These data are of possible clinical relevance given research to suggest that older HIV-infected adults, and particularly those with cognitive impairment (e.g., Barclay et al., 2007), are at greater risk for functional declines and highlights the need for early identification and remediation of cognitive impairment in this growing subpopulation.
Although inferential, this verbal fluency evidence is most consistent with the sequelae of pathological and structural brain abnormalities within the frontostriatal regions in older HIV-infected individuals (e.g., Ernst & Chang, 2004; Jernigan et al., 2005; Wiley et al., 1998), rather than an increased burden of age and HIV on posterior neocortical structures. In other words, the pattern of impaired switching in the setting of normal clustering observed in older adults with HIV infection is not consistent with that observed in conditions characterized by more widespread cortical dysfunction. For example, both clustering and switching are impaired in Alzheimer’s disease (i.e., AD; Troyer, et al., 1998), which associated with prominent neocortical pathology (e.g., Davies, Mann, Sumpter, & Yates, 1987). These findings are consistent with the prior data showing intact semantic memory (e.g., naming objects and famous faces) in older HIV-infected adults (Scott et al., 2011). Indeed, the profile of older HIV-infected adults mirrors the pattern of clustering and switching performance that has been independently observed in aging (e.g., Troyer et al., 1997) and HIV infection (e.g., Iudicello et al., 2008; Miliken et al, 2004; Woods et al., 2005), as well as in other conditions affecting the integrity of the frontostriatal systems (e.g., PD; Troyer, et al., 1998; Tröster et al., 1998).
Findings regarding the underlying cognitive mechanisms of switching impairment in older HIV-infected adults also argue against the cortical hypothesis. While switching was associated with a majority of the cognitive domains assessed (e.g., attention/working memory, executive functions, motor skills, episodic memory, and information processing speed), only executive functions (e.g., mental flexibility, inability to inhibit inappropriate responses, attention shifting) and learning emerged as the strongest predictors. These findings are consistent with previous research demonstrating modest associations between HIV-associated category switching deficits and executive dysfunction (e.g., Iudicello et al., 2008). The association between switching impairment and learning in the older HIV-infected group is somewhat difficult to interpret given prior research suggesting an association between HIV-associated episodic verbal learning and memory deficits and medial temporal (i.e., hippocampal) dysfunction (e.g., Maki et al., 2009). However, HIV-associated episodic memory impairment is largely driven by dysfunction in the executive processes involved in encoding (e.g., use of effective organizational strategies) and retrieval (e.g., Gongvatana et al., 2007; Woods et al., 2005), which have been associated with frontal systems (e.g., Baldo & Shimamura, 2002). In contrast, there is no strong evidence to suggest that HAND is associated with high rates of semantically-related intrusions or rapid forgetting (e.g., Woods et al., 2005; cf. Scott et al., 2006). As mentioned above, no significant associations were found between switching impairment and a measure of semantic memory in the older HIV-infected group (e.g., WRAT-3 Reading). Collectively, this evidence suggests that the category fluency impairment in this older HIV-infected sample may be primarily driven by a deficiency in the executive processes involved in switching.
Of note, it is possible that the relatively young age of our “older” HIV-infected sample (mean = 58.2 years old, standard deviation = 6.2), which is quite young in comparison to traditional cognitive aging studies, may have limited our ability to detect the more prominent and/or differential neurocognitive effects that may be observed in samples with substantial proportions of individuals over age 60. Thus, we cannot rule out the possibility that more widespread cognitive impairment (e.g., semantic memory deficits) may evolve as individuals age into their late 60s and 70s with HIV infection. Moreover, the older HIV-infected adults in this study were relatively healthy (only 30% had detectable viral loads, mean current CD4 count = 435 cells/μl), and only one individual was classified with HAD. Thus, our findings can be most comfortably interpreted for older individuals with ANI and MND. It is possible that older HIV-infected cohorts with more advanced disease or those with HAD may demonstrate cognitive impairment reflective of more widespread cortical neuropathology (e.g., Scott et al., 2006). In support of this notion, increased cortical damage has been observed in HIV-infected individuals with more advanced disease (e.g., Kruman, Nath & Mattson, 1998) and deficits in episodic learning and memory that are generally more consistent with underlying cortical pathology (e.g., recency list learning effects consistent with a primary encoding deficit) have been demonstrated in individuals with HAD (e.g., Scott et al., 2006). Nevertheless, the general pattern of switching versus clustering deficits persisted when analyses were restricted to only the older HIV-infected individuals with HAND, the majority of whom had ANI and MND.
A few additional limitations are worth noting. First, this study tested an additivity model, thus inferences regarding potential interactive or synergistic effects of aging and HIV infection may not be gleaned from these results. Second, our older HIV-infected sample was predominantly Caucasian (approximately 75%), and mostly male (approximately 83%), which may limit the generalizability of these findings. Third, due to the inclusion of participants from different protocols, a consistent measure of lifetime substance (i.e., alcohol and illicit drug) use was not available for this study. Lastly, some of the observed cognitive impairment observed in this older HIV-infected cohort may be attenuated by issues related to survival bias (e.g., an attrition of those who may have survived for long periods of time without substantial HIV-associated CNS damage). Future research is needed to carefully examine these potentially confounding factors (e.g., substance use) and their effects on the profile of HAND in older HIV-infected individuals. For example, studies could examine factors that may delay the clinical presentation of symptoms (e.g., cognitive reserve) that would otherwise reflect the more widespread neocortical damage that may be present in older HIV-infected adults.
Of possible clinical significance, results of this study suggested that switching impairment in older HIV-infected adults was a significant predictor of self-reported declines in instrumental activities of daily living (IADLs), even when accounting for potentially confounding variables that differed between IADL independent and dependent groups (i.e., education, duration of HIV infection, and affective distress). Odds ratio analyses revealed that older HIV-infected individuals were approximately 1.5 times more likely to be classified as IADL dependent per switching unit decrease. Moreover, including global neurocognitive impairment in the model did not alter the significance of switching as a predictor of IADL dependence (p = 0.04). This data is consistent with research suggesting a significant association between executive functions, and specifically, verbal fluency impairment, and declines in everyday functioning in both HIV infection (e.g., Heaton et al., 2004; Woods et al., 2006) and in normal aging (e.g., Cahn-Weiner, Boyle, & Malloy, 2002). Collectively, this evidence provides novel insight into the cognitive predictors (e.g., category fluency switching) of IADL dependence in older HIV-infected individuals and for the clinical utility of category fluency switching as a potential useful diagnostic tool in determining whether older individuals with HIV infection may be particularly vulnerable to declines in everyday functioning.
Acknowledgments
The San Diego HIV Neurobehavioral Research Program [HNRP] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S., Tanya Wolfson, M.A.
This research was supported by National Institutes of Health grants P30-MH62512, P01-DA12065, and T32-DA31098. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E The HNRC Group. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. Journal of Neuroimmune Pharmacology. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P, Clifford DB. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: A case-control study. Neurology. 2011;75:111–115. doi: 10.1212/WNL.0b013e3181e7b66e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd D, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army Individual Test Battery. Manual of directions and scoring. Washington, D.C: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 2002;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, Durvasula RS. Age-associated predictors of medication adherence in HIV positive adults: Health benefits, self-efficacy, and neurocognitive status. Health Psychology. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18:S11–S18. [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test - Revised. Odessa, Florida: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid B42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Mallow PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. The Clinical Neuropsychologist. 2000;14:187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD The HNRC Group. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. The Clinical Neuropsychologist. 2004;18:234–248. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. HIV/AIDS Surveillance Report, 2007. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention; 2009. [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. American Journal of Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L The HNRC Group. Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society. 2007;13:1–11. doi: 10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DMA, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. Journal of the Neurological Sciences. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Delis DC, Peavy G, Heaton R, Butters N, Salmon DP, Taylor M The HNRC Group. Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? Evidence using the California Verbal Learning Test. Assessment. 1995;2:151–165. [Google Scholar]
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): Norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Drachman DA. Aging of the brain, entropy, and Alzheimer’s disease. Neurology. 2006;67:1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L. Effects of aging on brain metabolism in antiretroviral-naïve HIV patients. AIDS. 2004;18:61–67. [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM. Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia. 1993;31:17–28. doi: 10.1016/0028-3932(93)90077-d. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test. Chicago: Stoelting; 1978. [Google Scholar]
- Gongvatana A, Woods SP, Taylor MJ, Vigil O, Grant I The HNRC Group. Semantic clustering inefficiency in HIV-associated dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:36–42. doi: 10.1176/jnp.2007.19.1.36. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–375. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gronwall DM, Sampson H. The psychological effects of concussion. Auckland, New Zealand: Auckland University Press; 1974. [Google Scholar]
- Gruenewald PJ, Lockhead GR. The free recall of category examples. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:225–240. [Google Scholar]
- Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore LH. Age differences and neurocognitive performance in HIV-infected individuals. New Zealand Journal of Psychology. 1999;28:94–101. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F the CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H The HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Odessa, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Heaton RK, Taylor MJ, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky DS, Heaton RK, Chelune G, Ivnik R, Bornstein RA, Prifitera A, Ledbetter M, editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Ho AK, Sahakian BJ, Robbins TW, Barker RA, Rosser AE, Hodges JR. Verbal fluency in Huntington’s disease: A longitudinal analysis of phonemic and semantic clustering and switching. Neuropsychologia. 2002;40:1277–1284. doi: 10.1016/s0028-3932(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Weber E, Dawson MS, Scott JC, Carey CL the HNRC Group. Cognitive mechanisms of switching in HIV-associated category fluency deficits. Journal of Clinical and Experimental Neuropsychology. 2008;30:797–804. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part 1: Localization of age-related changes. Biological Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Rivera-Mindt M, Marcotte T, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometricka. 1954;41:133–145. [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, Caine ED. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology. 1996;53:155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- Kløve H. Grooved Pegboard. Lafayette, IN: Lafayette Instruments; 1963. [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test - 64 Card Computerized Version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Experimental Neurology. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: A preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Loor M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Millikin CP, Trepanier LL, Rourke SB. Verbal fluency component analysis in adults with HIV/AIDS. Journal of Clinical and Experimental Neuropsychology. 2004;26:933–942. doi: 10.1080/13803390490510842. [DOI] [PubMed] [Google Scholar]
- Oleson PJ, Schendan HE, Amick MM, Chronin-Golumb A. HIV infection affects parietal-dependent spatial cognition: Evidence from mental rotation and hierarchical pattern perception. Behavioral Neuroscience. 2007;121:1163–1173. doi: 10.1037/0735-7044.121.6.1163. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients with human immunodeficiency virus. Journal of Neurovirology. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of Neurovirology. 2007;13:203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Weber E, Dawson MS, Bondi MW, Grant I The HNRC Group. Neurocognitive consequences of HIV infection in older adults: an evaluation of the “cortical” hypothesis. AIDS and Behavior. 2011;15:1187–1196. doi: 10.1007/s10461-010-9815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Patterson KA, Morgan EE, Heaton RK, Grant I, … & the HNRC Group. Recency effects in HIV-associated dementia are characterized by deficient encoding. Neuropsychologia. 2006;44:1336–1343. doi: 10.1016/j.neuropsychologia.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Terpstra TJ. The asymptotic normality and consistency of Kendall’s test against trend, when ties are present in one ranking. Indigationes Mathematicae. 1952;14:327–333. [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, Hinkin CH. Medication and finance management among HIV-infected adults: The impact of age and cognition. Journal of Clinical and Experimental Neuropsychology. 2011;33:200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Author; 1997. [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröster AI, Fields JA, Testa JA, Paul RH, Blanco CR, Hames KA, Beatty WW. Cortical and subcortical influences on clustering and switching in the performance of verbal fluency tasks. Neuropsychologia. 1998;4:295–304. doi: 10.1016/s0028-3932(97)00153-x. [DOI] [PubMed] [Google Scholar]
- Troyer AK. Normative data for clustering and switching on verbal fluency tasks. Journal of Clinical and Experimental Neuropsychology. 2000;22:370–378. doi: 10.1076/1380-3395(200006)22:3;1-V;FT370. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Leach L, Freedman M. Clustering and switching on verbal fluency tests in Alzheimer’s and Parkinson’s disease. Journal of the International Neuropsychological Society. 1998;4:137–143. doi: 10.1017/s1355617798001374. [DOI] [PubMed] [Google Scholar]
- Valcour V, Paul R, Neuhaus J, Shikuma C. The effects of age and HIV on neuropsychological performance. Journal of the International Neuropsychological Society. 2011;17:190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: The Hawaii Aging with HIV-1 Cohort. Neurology. 2004a;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, Sacktor N. Age, apolipoprotein E4, and the risk of HIV dementia: The Hawaii Aging with HIV Cohort. Journal of Neuroimmunology. 2004b;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Weber E, Woods SP, Cameron MV, Gibson SA, Grant I The HNRC Group. A brief report on mental rotation of hands in HIV infection: Neuropsychological evidence of dysfunction in fronto-striato-parietal networks. Journal of Neuropsychiatry and Clinical Neuroscience. 2010;22:115–122. doi: 10.1176/appi.neuropsych.22.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathology. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test Administration Manual. 3. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Woods SP, Conover E, Rippeth JD, Carey CL, Gonzalez R, Marcotte TD The HNRC Group. Qualitative aspects of verbal fluency in HIV-associated dementia: A deficit in rule-guided lexical-semantic search processes? Neuropsychologia. 2004;42:801–809. doi: 10.1016/j.neuropsychologia.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK The HNRC Group. Construct validity of Hopkins Verbal Learning Test Revised component process measures in an HIV-1 sample. Archives of Clinical Neuropsychology. 2005;20:1061–1071. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I The HNRC Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical & Experimental Neuropsychology. 2010;32:522–527. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Dawson M, Scott JC, Grant I The HNRC Group. Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. Journal of Clinical and Experimental Neuropsychology. 2006;28:1030–1042. doi: 10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Doyle K, Grant I The HNRP Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]