Abstract
Autism (AUT) is a complex neurodevelopmental disorder that, together with Asperger Syndrome and Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS), comprises the expanded classification of autistic spectrum disorder (ASD). The heterogeneity of ASD underlies the need to identify biomarkers or clinical features that can be employed to identify meaningful subtypes of ASD, define specific etiologies, and inform intervention and treatment options. Previous studies have shown that disordered porphyrin metabolism, manifested principally as significantly elevated urinary concentrations of pentacarboxyl- (penta) and copro- porphyrins, is commonly observed among some children with ASD. Here, we extend these observations by specifically evaluating penta and copro porphyrins as biological indicators of ASD among 76 male children comprising 30 with validated AUT, 14 with PDD-NOS and 32 neurotypical (NT) controls. ASD children (AUT and PDD-NOS) had higher mean urinary penta (p < 0.006) and copro (p < 0.006) concentrations compared to same-aged NT children, each characterized by a number of extreme values. Using Receiver Operating Characteristic (ROC) curve analysis, we evaluated the sensitivity and specificity of penta, copro and their combined Z-scores in ASD detection. The penta sensitivity was 30% for AUT and 36% for PDD-NOS, with 94% specificity. The copro sensitivity was 33% and 14%, respectively, with 94% specificity. The combined Z-score measure had 33% and 21% sensitivity for AUT and PDD-NOS, respectively, with 100% specificity. These findings demonstrate that porphyrin measures are strong predictors of both AUT and PDD-NOS and support the potential clinical utility of urinary porphyrin measures for identifying a subgroup of ASD subjects in whom disordered porphyrin metabolism may be a salient characteristic.
Keywords: autistic spectrum disorder, porphyrins, heme, biomarker, children
INTRODUCTION
Autism (AUT) (OMIM 209850) is a complex neurodevelopmental disorder that, along with Asperger Syndrome and Pervasive Developmental Disorders-Not Otherwise Specified (PDD-NOS), comprises the expanded classification of autistic spectrum disorder (ASD). The complexity of ASD and other pervasive developmental disorders, the multi-factorial nature of these disorders, and the difficulty in diagnosing and distinguishing between these at early ages have led many health care providers to view autism as a spectrum disorder. In this report we refer to autism as AUT and to PDD-NOS as a separate diagnosis, with the understanding that the lines between these may be blurred at times and that the latter is frequently included within the collective designation of ASD.
ASD afflicts as many as one in 110 children in the United States (Rice, 2009). The rising rate of diagnosis of ASD may be associated with heightened awareness, increased incidence or other factors (Fombonne, 2009; Ratajczak, 2011). What is clear is that early detection leads to improved efficacy of treatment and a better quality of life for many ASD patients. This concern has spurred the search for early biological markers of ASD. Genetic factors are likely to play a principal role in the etiology of ASD. However, at this time, only 10-15% of validated cases have known genetic causes, and fewer than 3% of cases are associated with any specific genetic factor (Levy et al., 2009). Thus, several non-genetic biological measures have been offered as biomarkers of ASD. A small study of placental tissue specimens from 13 ASD cases and 61 controls found abnormalities in 38.5% of cases and 13.1% of controls (Anderson et al., 2007), suggesting the potential utility of trophoblastic anomaly assessments in the early detection of ASD. In addition, the utility of functional MRI in detecting ASD has been recently reported (Perkins et al., 2010). Despite these observations, relatively few biological markers of ASD risk have been identified and brought into clinical use.
Porphyrins are formed as intermediates in the biosynthesis of heme, a process that ensues in essentially all eukaryotic tissues (Figure 1). In humans and other mammals, porphyrins with 8, 7, 6, 5 and 4 carboxyl groups are commonly formed in excess of those required for heme biosynthesis and are excreted in the urine in well-established concentration ranges (Ford et al., 1981; Woods, 1995; Woods et al., 2009). Changes in the urinary concentrations of specific porphyrins have been observed in association with mercury, lead and other toxicant exposures and have been proposed as biomarkers of such exposures in humans (Marks, 1985; Bowers et al., 1992; Woods, 1995). Over the past several years, there have been a number of reports of aberrant porphyrin excretion among children with ASD from Korea (Youn et al., 2010), Australia (Austin & Shandley, 2008), France (Nataf et al., 2006), and the United States (Geier et al., 2009; Kern et al., 2010). In most cases the authors attributed these findings to mercury or other toxicant exposures.
Figure 1.

Heme biosynthetic pathway. The figure summarizes the principal components of the heme biosynthetic pathway (left hand panel) and the corresponding constituents excreted into the urine (right hand panel).
To better define aberrant porphyrin excretion among ASD children, we conducted a study of male children aged 2-12 years with validated AUT or PDD-NOS diagnoses and age-matched neurotypical (NT) controls (Woods et al., 2010). The findings demonstrated significantly elevated concentrations of hexa-carboxyl porphyrin (OR=1.65[1.07-2.55]), penta-carboxyl porphyrin (OR=2.36[1.37-4.07]) and coproporphyrin (OR=2.03[1.15-3.57]) among children with ASD when compared with those of age-matched neurotypical (NT) controls. These findings suggested that porphyrin metabolism may be disordered or impaired among some ASD children and, moreover, that porphyrin measures might have potential clinical utility as a biomarker for ASD identification and/or classification.
Here, we pursued further analysis of the urinary concentrations of pentacarboxyl- and copro- porphyrins, referred to hereafter as “penta” and “copro”, as predictive biomarkers of risk for AUT and PDD-NOS. Specifically, we employed graphical and statistical analyses to evaluate the distribution of urinary concentrations of these porphyrins by diagnosis, and to determine the predictive (sensitivity and specificity) values of these measures for ASD detection.
METHODS
The study population
Subjects in this study were derived from a cohort of 118 male children, aged 2-12 years, with diagnosis of AUT, PDD-NOS or NT who participated in a recently completed investigation of altered urinary porphyrin excretion in relation to mercury exposure in children with AUT (Woods et al., 2010). All subjects were recruited from among families residing within the Pacific Northwest metropolitan areas of Seattle and Spokane, WA and Portland, OR and whose diagnoses had been performed by established autism diagnostic and treatment centers including the University of Washington Autism Center, the Seattle Children’s Autism Center, the Oregon Health and Sciences University and the Kaiser Permanente Developmental Assessment Clinic. Subjects were selected from among children for whom behavioral intervention was the principal therapeutic approach and who, therefore, had not received treatment with potentially porphyrinogenic drugs such as mood stabilizers or antidepressants, e.g. valproic acid (depakote, convulex) or risperidine (risperdal). We also assessed prior use of chelating agents, which might also alter porphyrin excretion, and excluded from the analysis those reporting a prior history of chelation therapy (n=42). Thus, the final study cohort of 76 eligible children included 30 diagnosed with AUT, 14 as having PDD-NOS, and 32 NT controls.
Diagnostic procedures
The diagnosis of AUT or PDD-NOS was made using a multidisciplinary approach that combined a clinical evaluation using the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association, 2000) criteria, along with a psychological evaluation using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000), supplemented by other established diagnostic procedures such as the Autism Diagnostic Interview-Revised (ADI-R) (Rutter et al., 2003) or the Childhood Autism Rating Scale (CARS) (Schopler et al., 1988). More than 90% of subjects were diagnosed since 2003, supporting continuity and homogeneity in the methods and procedures employed in the diagnoses.
Human subjects considerations
The study protocol was approved by the institutional review board at the University of Washington. All parents/caregivers gave written consent for themselves and their children prior to enrollment in the study.
Urinary porphyrin and creatinine measurements
Urinary porphyrin concentrations were measured as previously described (Bowers et al., 1992; Woods et al., 1991) and were expressed as nanomoles per gram of creatinine (nmol/gCr). This well-established procedure employs high performance liquid chromatography and spectrofluorometric detection to separate and quantify total concentrations of individual porphyrins. Urinary creatinine concentrations were measured using a standard colorimetric procedure (Sigma, St. Louis, MO, USA). For statistical analyses, urinary porphyrin concentrations were first creatinine-adjusted (nanomoles per gram) and then transformed using the natural logarithm.
Statistical procedures
Variations of porphyrin concentrations by age among children younger than 8 years, whether NT or diagnosed with AUT or PDD-NOS, have not been well described. Therefore, we conducted both graphical and statistical analyses to fully evaluate the distributions of urinary porphyrin concentrations within this cohort. All analyses were conducted using the statistical package PASW statistics 18 (IBM® SPSS®, Chicago, IL).
Initial graphical analyses included scatter plots by diagnostic status (AUT, PDD-NOS and NT) against age for urinary concentrations penta and copro, as well as for a combined score. Due to the large absolute differences in urinary concentrations of penta and copro, their Z-scores were calculated and added together to create a combined metric. These graphical plots allowed us to visually identify subjects with values above the 95% confidence interval (CI) controlling for age. They also facilitated identification of cutoff concentrations for potential biomarkers – values maximizing the number of cases identified (true positives) while simultaneously minimizing the number of controls (false positives) included. Receiver operating characteristic (ROC) curves were used to provide a graphical display of the various combinations for sensitivity and specificity of these biomarkers.
RESULTS
A description of the study population by diagnostic status is provided in Table 1. The study population was comprised of male children ranging in age from 2.5 to 12.4 years of age. There were no statistical differences in the means for age and creatinine concentrations between any of the groups. Penta (p < 0.02) and copro (p < 0.004) concentrations were significantly higher among AUT children compared to same-age NT controls, but not between PDD-NOS and controls. We found no association of altered porphyrin concentrations with any measure of lead, mercury or other xenobiotic exposure (not shown).
Table 1.
Characteristics of Study Population (all Males)
| Case Status | Measure | Age | Creatinine (g/l) | Porphyrins (nmol/gCr)
|
|
|---|---|---|---|---|---|
| Penta | Copro | ||||
|
Control (NT) (N=32) |
Mean | 7.18 | 1.06 | 1.21 | 56.44 |
| Range | 2.3-11.7 | 0.23-2.48 | 0.22-3.10 | 10.1-126.4 | |
| Std. Dev. | (3.02) | (0.46) | (0.65) | (32.42) | |
|
Autistic (N=30) |
Mean (p-value)* | 6.08 (.09) | 0.96 (.36) | 1.95 (.02) | 89.01 (.004) |
| Range | 3.2-11.3 | 0.44-1.80 | 0.50-7.43 | 18.5-199.6 | |
| Std. Dev. | (1.96) | (0.39) | (1.49) | (49.48) | |
|
PDD-NOS (N=14) |
Mean (p-value)* | 6.59 (.51) | 0.96 (.48) | 1.86 (.13) | 66.36 (.32) |
| Range | 2.5-12.4 | 0.44-1.92 | 0.39-5.91 | 16.1-121.5 | |
| Std. Dev. | (2.64) | (0.43) | (1.47) | (29.29) | |
|
Total (N=44) |
Mean | 6.64 | 1.00 | 1.62 | 71.12 |
| Range | 2.3-12.4 | 0.23-2.48 | 0.22-7.43 | 10.1-199.6 | |
| Std. Dev. | (2.59) | (0.43) | (1.24) | (41.88) | |
equality of means compared to controls (t-test, equal variance not assumed)
Figure 2 graphically shows the distribution of urinary porphyrin concentrations by age for 2a) penta and 2b) copro. The graphs clearly distinguish between AUT, PDD-NOS cases and NT controls. In addition, we show (green lines) the simple regression of porphyrin concentrations with age and associated 95% CIs calculated only among NT controls in order to estimate normal distributions among children in this age range. For both graphs, it is clear that AUT and PDD-NOS cases dominate the highest porphyrin concentrations. Thus, we selected two cutoff concentrations (shown by the horizontal dotted lines) for each porphyrin. The higher cutoff excludes all NT controls, providing 100% specificity (32/32) (no false positives), whereas the lower cutoff allows for two false positive NT controls, providing 94% specificity (30/32).
Figure 2.
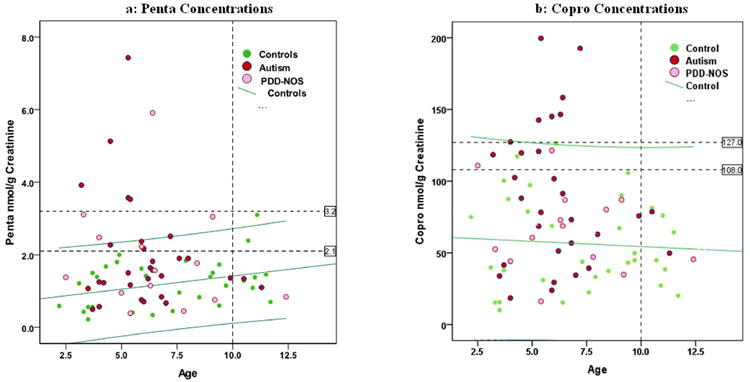
Distribution of porphyrin concentrations by age with regression-predicted levels and 95% CI for Controls. Figure 2a shows graphical distribution of urinary pentacarboxyl porphyrin concentration by age among AUT, PDD-NOS and NT subjects. Figure 2b shows graphical distribution of urinary coproporphyrin concentration by age among AUT, PDD-NOS and NT subjects. Green lines in each figure represent regression of the porphyrin concentrations with age and associated 95% CI, and show that the highest concentrations of either are associated with AUT and PDD-NOS cases.
Results for penta are shown in Figure 2a. The higher cutoff (3.2 nmol/gCr) identifies 5 AUT and 1 PDD-NOS cases, whereas the lower cutoff (2.1 nmol/gCr) identifies 9 AUT and 5 PDD-NOS cases. Notably, all identified cases are under 10 years of age (the oldest being 7.2 and 9.1 for AUT and PDD-NOS, respectively), whereas both the NT controls identified by this lower cutoff are over 10 (10.7 and 11.1). There are very few cases in our study over the age of 10, and it is possible that the specificity of this cutoff might be artificially reduced by the age range of this study. The upper 95% CI for the controls (age adjusted) falls midway between these two cutoff levels, and identifies 6 AUT and 4 PDD-NOS cases, along with one NT control.
Results for copro are shown in Figure 2b. Here the higher concentration cutoff (127 nmol/gCr) identifies 7 AUT and no PDD-NOS cases, whereas the lower cutoff (108 nmol/grCr) identifies 10 AUT and 2 PDD-NOS cases. The upper 95% CI closely parallels the higher cutoff concentration for this porphyrin, and identifies 6 AUT cases and one NT control.
We used the sum of Z-scores (measured in standard deviations or SD) for penta and copro concentrations to create a combined metric for these two porphyrins (Figure 3). The combined Z-score has a range of -2.32 to 6.52 with a mean of 0.055 (SD=1.69). As can be seen in Figure 3a, a cutoff of 1.13 SD identifies 10 AUT and 3 PDD-NOS cases with no NT controls.
Figure 3.
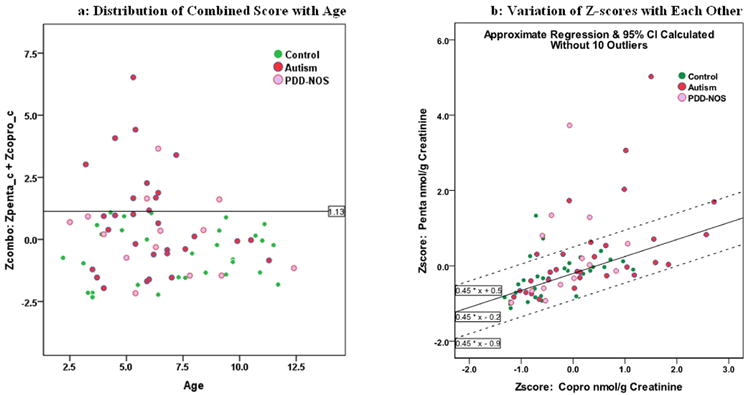
Distribution of Z-scores. Figure 3a shows the graphical distribution of the combined Z-scores by age among AUT, PDD-NOS and NT subjects. Figure 3b shows the individual Z-scores plotted against each other and their regression slope and 95% CI after eliminating 10 ‘outlier’ cases.
Another interesting pattern is seen in Figure 3b, where we graphed the two Z-scores against each other for the entire cohort. In order to better define the dominant underlying relationship (slope) between the two Z-scores, we twice calculated a simple regression with 95% CI and eliminated subjects outside of these limits, resulting in the elimination of 10 subjects. The third calculation, with 10 subjects eliminated, is shown superimposed on Figure 3b (note that the two subjects just above the upper CI in Figure 3b were not among the 10 eliminated). This final slope shows that penta increases approximately 0.45 SD for each change of one SD for copro, and appears to fairly represent the relationship between these porphyrins for the majority of subjects. This observation is consistent with the sequential formation of each porphyrin along the heme biosynthetic pathway (Figure 1).
It is also clear from Figure 3b that the 10 subjects eliminated from the calculation vary considerably from the calculated slope, and that these subjects are dominated by AUT and PDD-NOS cases. Among the 10 outliers were 4 AUT and 4 ASD cases and only 2 NT controls. Variation from the dominant ratio between these two porphyrins may be another indicator of case status.
Finally, ROC curves shown in Figure 4 demonstrate the combinations of sensitivity (true positives) and [1 minus specificity] (false positives) for the three porphyrin measures described above. Figure 4a demonstrates that, while the combined Z-scores and copro appear to be the best predictors for AUT cases in terms of overall sensitivity, all three measures are within the same range. However, for PDD-NOS cases (Figure 4b), copro falls behind the other two measures. Selected sensitivity and specificity calculations for the three porphyrin measures in detecting AUT and PDD-NOS cases individually and combined are presented in Table 2. The combined Z-score measure provides one of the best combinations of sensitivity and specificity, identifying 33% of ASD and 21% of PDD-NOS cases as true positives before identifying the first NT control (false positive) within the cohort of all 76 study subjects.
Figure 4.
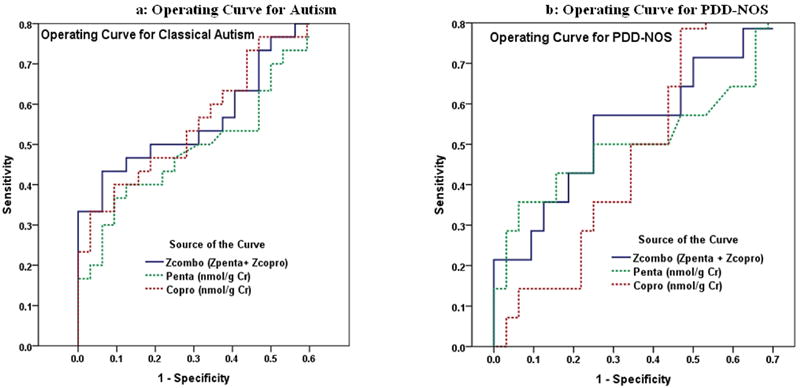
Receiver Operating Characteristic (ROC) Curves for 3 Porphyrin Measures Predicting Autism and PDD-NOS. Individual lines depict the proportion of AUT (Figure 4a) or of PDD-NOS (Figure 4b) identified as true positives (Sensitivity) in relation to the proportion identified as false positives (1- Specificity), from among all 76 study subjects (30 AUT + 14 PDD-NPS + 32 NT).
Table 2.
Selected Sensitivity & Specificity for AUT, PDD-NOS & Combined Cases for 3 Porphyrin Measure
| Test Criteria | Control | Autism | PDD-NOS | Combined | Case/Control |
|---|---|---|---|---|---|
| Penta Criteria: | Urinary Penta > 2.1 nmol/gCr | ||||
| True Positive (Sensitivity) | 9 (30%) | 5 (36%) | 14 (32%) | Cases | |
| False Negative (1-Sensitivity) | 21 (70%) | 9 (64%) | 30 (68%) | ||
| True Negative (Specificity) | 30 (94%) | Controls | |||
| False Positive (1-Specificity) | 2 (6%) | ||||
| Total | 32 | 30 | 14 | 44 | |
| Copro Criteria: | Copro > 108 nmol/gCr | ||||
| True Positive (Sensitivity) | 10 (33%) | 2 (14%) | 7 (16%) | Cases | |
| False Negative (1-Sensitivity) | 20 (67%) | 12 (86%) | 37 (84%) | ||
| True Negative (Specificity) | 30 (94%) | Controls | |||
| False Positive (1-Specificity) | 2 (6%) | ||||
| Total | 32 | 30 | 14 | 44 | |
| Z-Score Combined Criteria: | Z-Penta + Z-Copro > 1.13 | ||||
| True Positive (Sensitivity) | 10 (33%) | 3 (21%) | 13 (30%) | Cases | |
| False Negative (1-Sensitivity) | 20 (67%) | 11 (79%) | 31 (70%) | ||
| True Negative (Specificity) | 32 (100%) | Controls | |||
| False Positive (1-Specificity) | 0 | ||||
| Total | 32 | 30 | 14 | 44 | |
DISCUSSION
ASD is generally accepted to be a multi-factorial biological disorder that is characterized by a broad spectrum of behavioral and clinical symptoms. This heterogeneity remains a major challenge in the diagnosis of ASD as well as to the selection of intervention and treatment options. Identification of biological markers that can be employed to identify categorical ASD subgroups is critical to reducing this heterogeneity, defining etiologies, and informing response to treatment. The present analyses support the potential clinical utility of urinary porphyrin measurements as a specific biomarker for identifying a subgroup of ASD subjects in whom disordered porphyrin metabolism may be a salient characteristic. Whereas the present study reports a sensitivity of porphyrin measures of approximately 30% among all ASD children, the sensitivity of these measures might, in fact, be much higher, possibly approaching 100%, for a specific subset of ASD subjects in whom disordered porphyrin metabolism may be associated with a specific neurological phenotype. In this respect, this non-intrusive, rapid, relatively inexpensive, and readily available clinical test has very favorable predictive characteristics compared with more complex genetic and placental abnormality screens described in recent literature.
Support for the use of penta and copro as biomarkers of ASD is provided from a study conducted among Korean children (Youn et al., 2010). Although the authors focused on porphyrins as a biomarker of oxidative stress, they did report on the number of outliers for each specific porphyrin concentration among ASD children and controls. Among the ASD children, outliers for penta and copro were in the range of 30%, whereas there were no outliers among typically developing controls. These findings are comparable to those observed in the present study, and support the underlying view that measures based on elevated urinary concentrations of these specific porphyrins might serve as a biomarker to distinguish some children with ASD.
The mechanistic association of elevated urinary porphyrin concentrations with an autistic neurobehavioral phenotype that defines a specific ASD category remains to be delineated. However, heme is known to play a critical role as a signaling molecule in glutaminergic neuronal receptor processing and synapse development (Sengupta et al., 2005; Chernova et al., 2006), as well as in the regulation of serotonin (5-hydroxytryptamine) synthesis and signaling in the central nervous system (Litman & Correia, 1983; 1985). Disorders of both systems have been implicated as etiological factors in autism (Anderson et al, 2009; Bill & Geschwind, 2009). In terms of glutaminergic signaling, heme has been identified as a regulatory factor in the formation and heteromeric assembly of N-methyl-D-aspartate (NMDA) receptor subunits NR1 and NR2 (Chernova et al., 2007, 2011), both of which are constituents of putative convergent biological pathways comprising the core ASD phenotype (Bill & Geschwind, 2009; Wall et al., 2009). In the serotonin system, heme regulates the activity of tryptophan 2,3-dioxygenase (TDO2), the rate-limiting enzyme in the catabolism of tryptophan, the immediate precursor of serotonin. Defects in serotonin metabolism as well as abnormalities in circulating and brain serotonin levels have been reported in a wide variety of human behavioral and psychiatric disorders including autism (Chugani et al., 1999; Cook and Leventhal, 1996; Leboyer et al., 1999). Notably, hyperserotonemia has been consistently reported among approximately one third of autistic children (Cook and Leventhal, 1996; Owley et al., 2003), comparable to the proportion of ASD children identified as having significantly elevated porphyrin measures in the present study. These observations provide a scientific rationale for a mechanistic association wherein disruption of heme biosynthesis, as manifested by aberrant porphyrin excretion, might underlie a pathology associated with a specific autistic neurologic phenotype. Additional studies are needed to further identify the associations of elevated porphyrin concentrations and the mechanistic link(s) defining a specific behavioral subset of ASD.
Finally, we acknowledge several limitations of the present study. First is the relatively small number of ASD subjects among whom we sought to distinguish differences in porphyrin excretion and, hence, the possibly tentative nature of the proposed porphyrin measures as predictive biomarkers for ASD. Despite this limitation, the findings suggest a potentially important new approach toward categorizing ASD that may serve to improve identification and inform treatment of affected children. A related limitation is the restriction of the present analyses to a cohort of ASD children and NT controls, without the inclusion of a second control population comprised of developmentally delayed children without ASD. This omission precludes evaluation of disordered porphyrin measures in other neurodevelopmental disorders that do not have ASD as a clinical feature. Further studies in a larger cohort that includes both ASD and other control groups will aim to define aberrant porphyrin excretion among ASD children in terms of quantitative differences in communication, social and repetitive behavior characteristics that comprise a circumscribed ASD core phenotype and to identify genetic and mechanistic factors that may underlie this association.
Acknowledgments
Partial funding for this research was provided by Center Grant P30ES07033 to the University of Washington from the National Institute of Environmental Health Sciences, National Institutes of Health. Additional funding was provided by the Autism Research Institute and by the Wallace Research Foundation.
References
- American Psychiatric Association. Text Revision. 4. Washington, DC: American Psychiatric Press; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anderson BM, Schnetz-Boutaud NC, Bartlett J, Wotawa AM, Wright HH, Abramson RK, et al. Examination of association of genes in the serotonin system to autism. Neurogenetics. 2009;10:209–216. doi: 10.1007/s10048-009-0171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM, Jacobs-Stannard A, Chawarsha K, Volkmar FR, Kliman HJ. Placental trophoblast inclusions in autism spectrum disorder. Biological Psychiatry. 2007;61:487–491. doi: 10.1016/j.biopsych.2006.03.068. [DOI] [PubMed] [Google Scholar]
- Austin DW, Shandley K. An investigation of porphyrinuria in Australian children with autism. Journal of Toxicology and Environmental Health. 2008;71:1349–1351. doi: 10.1080/15287390802271723. [DOI] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Current Opinion in Genetic Development. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MA, Aicher LD, Davis HA, Woods JS. Quantitative determination of porphyrins in rat and human urine and evaluation of urinary porphyrin profiles during mercury and lead exposures. Journal of Laboratory and Clinical Medicine. 1992;120:272–281. [PubMed] [Google Scholar]
- Chernova T, Nicotera P, Smith AG. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Molecular Pharmacology. 2006;69:697–705. doi: 10.1124/mol.105.016675. [DOI] [PubMed] [Google Scholar]
- Chenova T, Steinert JR, Guerin CJ, Nicotera P, Forsythe ID, Smith AG. Neurite degeneration induced by heme deficiency mediated via inhibition of NMDA receptor-dependent extracellular signal-regulated kinase1/2 activation. Journal of Neuroscience. 2007;27:8475–8485. doi: 10.1523/JNEUROSCI.0792-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova T, Steinhert JR, Richards P, Mistry R, Challiss RAJ, Jukes-Jones R, et al. Early failure of N-methyl-D-aspartate receptors and deficient spine formation induced by reduction of regulatory heme in neurons. Molecular Pharmacology. 2011 doi: 10.1124/mol.110.069831. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Musik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Annals of Neurology. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Leventhal BL. The serotonin system in autism. Current Opinion in Pediatrics. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Research. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Ford RE, Ou C-N, Ellefson RD. Liquid-chromatographic analysis for urinary porphyrins. Clinical Chemistry. 1981;27:397–401. [PubMed] [Google Scholar]
- Geier DA, Kern JK, Geier MR. A prospective blinded evaluation of urinary porphyrins verses the clinical severity of autism spectrum disorders. Journal of Toxicology and Environmental Health. 2009;72:1585–1591. doi: 10.1080/15287390903232475. [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Mehta JA, Grannemann BD, Geier MR. Toxicity biomarkers in autism spectrum disorder: a blinded study on urinary porphyrins. Pediatrics International. 2011;53:147–153. doi: 10.1111/j.1442-200X.2010.03196.x. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, et al. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biological Psychiatry. 1999;45:158–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. The Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman DA, Correia MA. L-tryptophan: a common denominator of biochemical and neurological events of acute hepatic porphyria? Science. 1983;222:1031–1033. doi: 10.1126/science.6648517. [DOI] [PubMed] [Google Scholar]
- Litman DA, Correia MA. Elevated brain tryptophan and enhanced 5-hydroxytryptamine turnover in acute hepatic heme deficiency: clinical implications. Journal of Pharmacology and Experimental Therapeutics. 1985;232:337–345. [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Marks GS. Exposure to toxic agents: the heme biosynthetic pathway and hemoproteins as indicator. Critical Review in Toxicology. 1985;15:151–179. doi: 10.3109/10408448509029323. [DOI] [PubMed] [Google Scholar]
- Nataf R, Skorupka C, Amet L, Lam A, Sorubgbett A, Lathe R. Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicology and Applied Pharmacology. 2006;214:99–108. doi: 10.1016/j.taap.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Owley T, Leventhal BL, Cook EH. Childhood Disorders: The autism spectrum disorders. In: Tasman A, Kay J, Lieberman JA, editors. Psychiatry. 2. Chichester: Wiley; 2003. pp. 757–770. [Google Scholar]
- Perkins T, Stokes M, McGillivray J, Bittar R. Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience. 2010;17:1239–1243. doi: 10.1016/j.jocn.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Ratajczak HV. Theoretical aspects of autism: causes – a review. Journal of Immunotoxicology. 2011;8:68–79. doi: 10.3109/1547691X.2010.545086. [DOI] [PubMed] [Google Scholar]
- Rice C. Prevalence of autistic spectrum disorders – Autism and developmental disabilities monitoring network, United States. MMRW Morbidity and Mortality Weekly Report. 2009;58(SS10):1–20. [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R: Autism Diagnostic Interview – Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sengupta A, Hon T, Zhang L. Heme deficiency suppresses the expression of key neuronal genes and causes neuronal cell death. Brain Research and Molecular Brain Research. 2005;137:23–30. doi: 10.1016/j.molbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. CARS: The Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- Wall DP, Esteban FJ, DeLuca TF, Huyck M, Monaghan T, Valez de Mendizabal N, et al. Comparative analysis of neurological disorders focuses genome-wide search for autism genes. Genomics. 2009;93:120–129. doi: 10.1016/j.ygeno.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Woods JS. Porphyrin metabolism as indicator of metal exposure and toxicity. In: Goyer RA, Cherian M, editors. Handbook of Experimental Pharmacology: Toxicology of Metals-Biochemical Aspects. Vol. 115. Berlin: Springer-Verlag; 1995. pp. 19–52. [Google Scholar]
- Woods JS, Armel SA, Fulton DI, Allen J, Wessels K, Simmonds PL, et al. Urinary porphyrin excretion in neurotypical and autistic children. Environmental Health Perspectives. 2010;118:1450–1457. doi: 10.1289/ehp.0901713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Bowers MA, Davis HA. Urinary porphyrin profiles as biomarkers of trace metal exposure and toxicity: studies on urinary porphyrin excretion patterns in rats during prolonged exposure to methyl mercury. Toxicology & Applied Pharmacology. 1991;110:464–476. doi: 10.1016/0041-008x(91)90047-i. [DOI] [PubMed] [Google Scholar]
- Woods JS, Martin MD, Leroux BG, DeRouen TA, Bernardo MF, Luis HS, et al. Urinary porphyrin excretion in normal children and adolescents. Clinica Chimica Acta. 2009;405:104–109. doi: 10.1016/j.cca.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn SI, Jin SH, Kim SH, Lim S. Porphyrinuria in Korean children with autism: correlation with oxidative stress. Journal of Toxicology and Environmental Health. 2010;73:701–710. doi: 10.1080/15287391003614000. [DOI] [PubMed] [Google Scholar]


