Abstract
Huntington’s disease (HD) is associated with profound autonomic dysfunction including dysregulation of cardiovascular control often preceding cognitive or motor symptoms. Brain-derived neurotrophic factor (BDNF) levels are decreased in HD brain, and restoring BDNF levels prevents neuronal loss and extends lifespan. We reasoned that heart rate changes in HD may be associated with altered BDNF signalling in cardiovascular control nuclei in the brainstem. Here we show that heart rate is elevated in HD (N171-82Q) mice at presymptomatic and early disease stages, and heart rate responses to restraint stress are attenuated. BDNF and TrkB mRNA and protein levels were significantly decreased in brainstem cardiovascular nuclei in HD mice. Central administration of BDNF restored the heart rate to control levels. Our findings establish a link between diminished BDNF expression in brainstem cardiovascular nuclei and abnormal heart rates in HD mice, and suggest a novel therapeutic target for correcting cardiovascular dysfunction in HD.
Keywords: Huntington’s disease, brainstem, BDNF
1. Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder characterized by progressive striatal and cortical atrophy and motor, cognitive and psychiatric symptoms (Cardoso, 2009). HD patients also exhibit profound autonomic dysfunction which often precedes cognitive or motor symptoms (Aziz, et al., 2010). Further, cardiovascular disease is among the primary causes of death in HD patients, despite their relatively young age at death (Sorensen and Fenger, 1992).
BDNF levels are decreased in the cortex and striatum of HD patients (Zuccato, et al., 2008). Restoring cortical BDNF levels in huntingtin mutant mice suppresses HD pathology and extends lifespan (Duan, et al., 2003, Gharami, et al., 2008). BDNF is highly expressed in brainstem regions important in heart rate control (Clark, et al., 2011, Kawamoto, et al., 1996, Wang and Zhou, 2002), but whether a reduction in BDNF signaling in brainstem neurons contributes to cardiovascular dysfunction in HD is unknown. We hypothesized that heart rate dysregulation in HD is linked to deficient BDNF levels in brainstem cardiovascular control areas. Here we show that central heart rate regulation is disrupted in a mouse model of HD, an abnormality correlated with diminished levels of BDNF and its receptor TrkB in brainstem cardiovascular nuclei.
2. Methods
2.1 Mouse and human tissues
Male B6C3-Tg(HD82Gln)81Dbo/J (N171-82Q(Schilling, et al., 1999) mice and wild-type (WT) littermate controls (Jackson Laboratories; Bar Harbor, ME) were housed under a 12-hour light/dark cycle. Medulla samples from HD and control cases (Brain Resource Center; Johns Hopkins University) are summarized in Supplementary Table 1. All procedures were approved by the Institutional Animal Care and Use Committee of the National Institute on Aging.
2.2 Telemetry and BDNF infusion
Telemetry transmitters (TA10ETA-F20; Data Sciences International, St Paul, MN) were implanted at 8 weeks to continuously monitor mice in home cages and during restraint stress (at 14 weeks) as described (Wan, et al., 2003). In a separate set of animals, after transmitter implantation, mice were anaesthetized with isoflurane (1–3%) and a cannula (Brain infusion kit 3; Alzet, DURECT Corp., Cupertino, CA,) was implanted in the lateral ventricle (AP −0.25 mm, L 1.0 mm, Depth 2.5 mm) to allow intracerebroventricular (ICV) administration of BDNF. Preliminary studies determined the minimal effective dose of BDNF at 1.2 μg/24h. CSF or BDNF (Cell Sciences, Canton, MA) was delivered using a subcutaneous Alzet micro-osmotic pump (model 1002) attached to the cannula. Data were analyzed on day 7 of BDNF infusion.
2.4 RNA and protein measurements
RNA and proteins were measured from isolated cardiovascular nuclei from pons (parabrachial nucleus, locus coeruleus, and A5 region), dorsal medulla (nucleus of the solitary tract and dorsal motor nucleus of the vagus) and ventral medulla (nucleus ambiguus and ventrolateral region). RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and DNaseI digested prior to cDNA synthesis. cDNA was synthesized using SuperScript III First-strand kit (Invitrogen). Real-time PCR with SYBR green detection was performed using a PTC-200 thermocycler (MJ Research) with a Chromo4 fluorescence detector (BioRad). Primers: BDNF - Forward 5′GCGCCCATGAAAGAAGTAAA3′, Reverse 5′TCGTCAGACCTC TCGAACCT 3′; TrkB Forward 5′TTCAGCTGCTGTTGCTGCTTCT3′, Reverse 5′AACCGCTAAACCGGCACGAATATC3′. Protein levels were quantified by immunoaffinity capillary electrophoresis as described (Arumugam, et al., 2010). Raw BDNF and TrkB protein levels are listed in Supplementary table 2. For phosphorylated TrkB, proteins from microdissected nuclei were resolved on a 4–12% bis-tris polyacrylamide gel, transferred to a PVDF membrane and incubated with an antibody against phosphorylated TrkB (Abcam; ab52191) or full-length TrkB (Santa Cruz; sc-8316).
2.5 Statistical analysis
Data were analyzed by two-way ANOVA with Bonferroni’s posthoc test and Student’s t test for individual comparisons between groups. Significance was set at p< 0.05.
3. Results
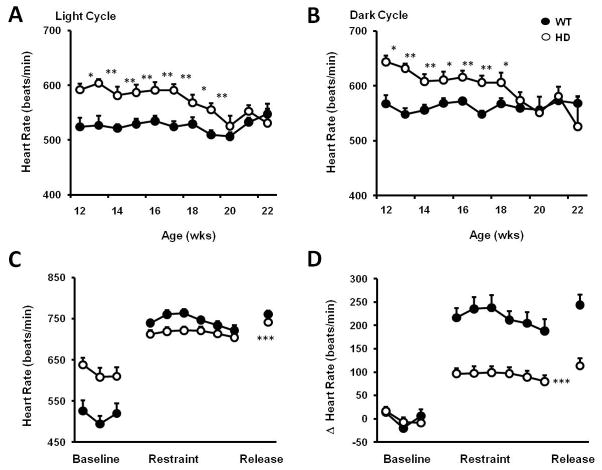
Similar to HD patients (Andrich, et al., 2002), HD mice had significantly elevated heart rates compared to wild type (WT) controls (Fig. 1A,B). Elevated heart rates in HD mice were not correlated with lower body weights (Supplementary Fig. 1A,B), or higher activity levels (Supplementary Fig. 1B,C). HD patients exhibit attenuated heart rate responses to stress (Kobal, et al., 2009). To test whether HD mice also exhibit dysregulation of heart rate control during stress, we exposed mice to restraint stress. During restraint, heart rate was elevated in WT and HD mice (Fig. 1C). However heart rate increases were attenuated in HD mice. Further, the change in heart rate in HD mice during restraint was diminished (Fig. 1D).
Figure 1. HD mice exhibit dysregulation of heart rate.
(A and B) Heart rates of HD mice (n=11) are significantly higher than WT mice (n=12) at presymptomatic and early symptomatic ages during the light (A) and dark (B) periods. (C) HD mice have attenuated heart rates during a 60 minute restraint stress and release. Data points represent 10-minute bins. (D) The change in heart rate in HD mice is significantly lower than WT mice, both during restraint and release. Data are represented as mean +/− s.e.m. *p<0.05, **p<0.01, ***p<0.001
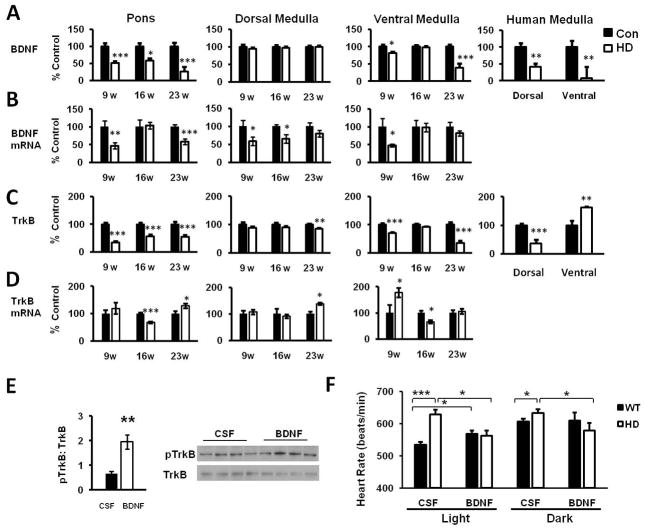
BDNF levels are reduced in HD cortex and striatum (Zuccato, et al., 2001). However, whether BDNF is also diminished in HD brainstem is unknown. We measured BDNF in brainstem nuclei responsible for cardiovascular control. BDNF protein was decreased at pre- (9 weeks), early (16 weeks) and late (23 weeks) symptomatic stages in pontine nuclei and ventral medulla of HD mice, but unchanged in dorsal nuclei (Fig. 2A). Further, BDNF was also diminished in the medulla of human HD patients. Consistent with this, BDNF mRNA was diminished in brainstem cardiovascular nuclei of HD mice (Fig. 2B). Similar to BDNF, TrkB was reduced in HD mouse pons and ventral and dorsal medulla across disease stages (Fig. 2C). TrkB was decreased in dorsal medulla of human HD patients, but increased in ventral medulla. TrkB mRNA was also diminished in brainstem cardiovascular nuclei of HD mice (Fig. 2D).
Figure 2. Central BDNF administration restores HD heart rate.
(A) BDNF protein is significantly diminished in cardiovascular nuclei of the pons and ventral medulla of HD mice (n=6 for each group) and HD patients (Control n=3, HD n=4). (B) BDNF mRNA is significantly diminished in cardiovascular nuclei in the pons, dorsal and ventral medulla of HD mice (n=5 for each group). (C) TrkB receptor levels are significantly decreased in pons, and dorsal and ventral medulla in HD mice (n=6 for each group). While TrkB is reduced in dorsal medulla from HD patients, it is increased in ventral regions of the medulla (Control n=3, HD n=4) (D) TrkB mRNA is significantly decreased in brainstem cardiovascular nuclei of HD mice (n=5 for each group). (E) ICV BDNF administration significantly increased TrkB phosphorylation in brainstem cardiovascular control nuclei. (F) BDNF was delivered by ICV cannulae for 7 days and heart rates were quantified during light and dark cycles. BDNF significantly increased heart rate in WT (n=6) mice. Heart rates in HD mice (n=5) were restored to WT levels upon BDNF administration. Data are mean +/− s.e.m. *p<0.05, **p<0.01, ***p<0.001
Because BDNF and TrkB were diminished in HD brainstem, we tested whether administration of BDNF could restore heart rate in HD mice. WT mice receiving ICV BDNF (1.2 μg/24h) had significantly elevated levels of phosphorylated TrkB compared ICV CSF, confirming increased BDNF signaling in the brainstem (Fig. 2E). ICV administration of BDNF increased WT heart rates (Fig. 2F). In contrast, heart rates in HD mice were decreased and restored to WT levels (Fig. 2F).
4. Discussion
Our data show that: 1) HD mice exhibit elevated heart rates and dysregulation of heart rate during stress; 2) BDNF levels are diminished in brainstem regions responsible for cardiovascular regulation in HD mice and human HD tissue; and 3) central administration of BDNF to HD mice restores heart rate to control levels. The literature exploring heart rate control in HD patients is limited. In the few existing studies examining central cardiovascular control, HD patients exhibit early sympathetic dominance and parasympathetic withdrawal at late disease stages associated with dizziness when standing up and tachycardia during pre- and early symptomatic stages of HD (Bar, et al., 2008). In addition to tachycardia, these patients experience impaired modulation of cardiovascular tone and attenuated heart rate responses to stress (Kobal, et al., 2009). Our data are consistent with these studies, as HD mice have elevated heart rates at pre- and early symptomatic stages of the disease and depressed heart rate responses to stress, suggesting that central cardiovascular modulation is interrupted prior to the appearance of motor symptoms.
Our data suggest that diminished BDNF signaling in cardiovascular nuclei accounts for dysregulation of heart rate control. Both BDNF and TrkB mRNA and protein levels were depressed in cardiovascular regions on the brainstem, and heart rate was restored by ICV application of BDNF. A recent study revealed that humans with a BDNF polymorphism (Val66Met) resulting in decreased activity-dependent BDNF secretion, have exhibit sympathetic dominance (Yang, et al., 2010) and attenuated heart rate responses to stress (Alexander, et al., 2010). Taken together with our data, this suggests that reduced central BDNF expression in HD impairs heart rate stress responses and cardiovascular function. Relatively little is known regarding the effects of BDNF on central modulation of heart rate. Our data indicate that reduced BDNF levels in HD mice lead to elevated heart rate. Whereas chronic central BDNF infusion increased heart rate in WT mice, BDNF administration depressed heart rate in HD mice, restoring the heart rate to control levels. This may be due to the differences in TrkB expression or downstream signaling caused by mutant huntingtin. Low TrkB expression is independent of BDNF levels in models of HD; increasing BDNF protein expression in HD models does not restore TrkB levels (Gines, et al., 2006). Therefore, the opposing effects of central BDNF on heart rate in HD and WT mice may result from differences in TrkB receptor expression and/or signaling. Our findings establish a link between diminished BDNF expression in brainstem regions responsible for cardiovascular control and abnormal heart rates in HD mice, and suggest BDNF signaling as a novel therapeutic target for correcting cardiovascular dysfunction in HD.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging
Footnotes
6. Disclosure statement: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Alexander N, Osinsky R, Schmitz A, Mueller E, Kuepper Y, Hennig J. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Andrich J, Schmitz T, Saft C, Postert T, Kraus P, Epplen JT, Przuntek H, Agelink MW. Autonomic nervous system function in Huntington’s disease. Journal of neurology, neurosurgery, and psychiatry. 2002;72(6):726–31. doi: 10.1136/jnnp.72.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Annals of neurology. 2010;67(1):41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NA, Anguelova GV, Marinus J, van Dijk JG, Roos RA. Autonomic symptoms in patients and pre-manifest mutation carriers of Huntington’s disease. Eur J Neurol. 2010 doi: 10.1111/j.1468-1331.2010.02973.x. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Andrich J, Epplen JT, Fischer F, Cordes J, Koschke M, Agelink MW. Cardiovagal modulation upon postural change is altered in Huntington’s disease. Eur J Neurol. 2008;15(8):869–71. doi: 10.1111/j.1468-1331.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- Cardoso F. Huntington disease and other choreas. Neurologic clinics. 2009;27(3):719–36. vi. doi: 10.1016/j.ncl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci. 2011;31(34):12318–29. doi: 10.1523/JNEUROSCI.0746-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2911–6. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington’s disease phenotypes in mice. Journal of neurochemistry. 2008;105(2):369–79. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gines S, Bosch M, Marco S, Gavalda N, Diaz-Hernandez M, Lucas JJ, Canals JM, Alberch J. Reduced expression of the TrkB receptor in Huntington’s disease mouse models and in human brain. The European journal of neuroscience. 2006;23(3):649–58. doi: 10.1111/j.1460-9568.2006.04590.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto Y, Nakamura S, Nakano S, Oka N, Akiguchi I, Kimura J. Immunohistochemical localization of brain-derived neurotrophic factor in adult rat brain. Neuroscience. 1996;74(4):1209–26. doi: 10.1016/0306-4522(96)00245-x. [DOI] [PubMed] [Google Scholar]
- Kobal J, Melik Z, Cankar K, Bajrovic FF, Meglic B, Peterlin B, Zaletel M. Autonomic dysfunction in presymptomatic and early symptomatic Huntington’s disease. Acta neurologica Scandinavica. 2009 doi: 10.1111/j.1600-0404.2009.01251.x. [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Human molecular genetics. 1999;8(3):397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Sorensen SA, Fenger K. Causes of death in patients with Huntington’s disease and in unaffected first degree relatives. Journal of medical genetics. 1992;29(12):911–4. doi: 10.1136/jmg.29.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. The Journal of nutrition. 2003;133(6):1921–9. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou XF. Injection of brain-derived neurotrophic factor in the rostral ventrolateral medulla increases arterial blood pressure in anaesthetized rats. Neuroscience. 2002;112(4):967–75. doi: 10.1016/s0306-4522(02)00085-4. [DOI] [PubMed] [Google Scholar]
- Yang AC, Chen TJ, Tsai SJ, Hong CJ, Kuo CH, Yang CH, Kao KP. BDNF Val66Met polymorphism alters sympathovagal balance in healthy subjects. Am J Med Genet B Neuropsychiatr Genet. 2010 doi: 10.1002/ajmg.b.31069. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science (New York, NY. 2001;293(5529):493–8. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain pathology (Zurich, Switzerland) 2008;18(2):225–38. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.