Abstract
Background
We analyzed birth outcomes among infants of treatment-naïve, HIV-infected women from a series of mother-to-child transmission of HIV studies in Blantyre, Malawi.
Methods
Data from six prospective studies at one research site were analyzed. Mean birth weight (BW) and gestational age (GA), and frequency of low birth weight (LBW; <2500 g) and preterm (PT) birth (GA<37 weeks) were estimated. We assessed risk factors for LBW and PT birth using mixed-effects logistic regression. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) from earlier studies (1989-94) and later studies (2000-07) are presented separately.
Results
The analysis included 8874 HIV-exposed infants. Mean BW and GA ranged from 2793 to 3079 g, and 37.8 to 39.0 weeks. Greater maternal age was consistently (during both the early and late periods) associated with lower odds of LBW and PT birth; AOR (95% CI) for both outcomes in the early and late periods, respectively, were 0.98 (0.96-1.00) and 0.97 (0.95-0.99). Female infant gender was consistently associated with higher odds of PT birth during both periods and with higher odds of LBW during the later period. During the early period, higher maternal education was associated with lower odds of LBW (AOR 0.67 (0.48-0.95)) and PT birth (AOR 0.70 (0.51-0.95)) and later birth year was associated with lower odds of PT birth (AOR 0.35 (0.19-0.70)).
Conclusions
BW and GA remained stable within each time period. This analysis provides important baseline information for monitoring HIV treatment effects on birth outcomes. Modifiable factors affecting BW and GA should continue to be explored.
Keywords: antiretroviral therapy, pregnancy outcomes, HIV, Malawi, trends
The use of antiretroviral (ARV) drugs for HIV-1 treatment and prevention of mother-to-child transmission (MTCT) of HIV in low- and middle-income countries has expanded dramatically since 2003.1 Some African countries with high HIV prevalence and suboptimal coverage of prevention of MTCT programs are now considering universal ARV treatment (ART) for HIV-infected pregnant women.2
Globally, there are conflicting findings on the impact of combination ART on low birth weight (LBW) and preterm (PT) birth. Studies in Europe and the United States showed a relatively high rate of PT births among infants of HIV-infected mothers on highly active antiretroviral therapy (HAART)3-5 and some indication of very LBW among babies born to protease inhibitor users.6 Others studies from Europe, the Caribbean, Latin America and the United States, reported no association with birth weight (BW), gestational age (GA), or risk of stillbirths,7,8 or an increased risk of PT and LBW birth without ART.9 More broadly, the extent to which HIV contributes to adverse birth outcomes compared to established non-HIV-related risk factors, such as demographic, environmental and behavioral factors10 has been speculated but not well defined.11 A study from Rwanda, prior to ART, looking at intrauterine growth indicated that maternal HIV was significantly associated with LBW but not with PT birth12 while another report from the same study suggested infant HIV status is a more valuable predictor of BW.13
Existing information on maternal ART and adverse birth outcomes has been derived from observational studies conducted mainly in developed countries. In most African countries, information on BW is generated from small studies or national estimates which are not stratified by maternal HIV status. Similarly, there are no national data on GA. One published study in Botswana showed lower BW in HIV-uninfected infants exposed to HAART in-utero compared to those exposed to zidovudine alone; these lower birth weights were corrected in the first 6 months of life.14 The same trial concluded protease inhibitors were associated with increased PT delivery but not with excess hospitalization or mortality.15
Trend data on mean BW and GA among infants of ART-naïve, HIV-infected women could be instrumental in providing baseline estimates when monitoring birth outcomes associated with ART exposure. These data could also assist in establishing local and regional surveillance databases to study the effects of ART on these outcomes, as coverage and access continue to expand. In Malawi, several MTCT observational and interventional studies were conducted at a single site in Blantyre over the past 20 years.16 This provides a unique opportunity to document BW and GA over time in cohorts of HIV-infected women and their HIV-exposed children. These studies predated use of ART during pregnancy for prevention of MTCT of HIV in Malawi. In this paper, we examine trends in mean BW and GA, and the proportion of LBW and PT births in cohorts of infants born to HIV-infected, ART-naïve women screened and enrolled in multiple MTCT studies in Blantyre, Malawi. We also assess risk factors associated with these pregnancy outcomes.
MATERIALS AND METHODS
Setting and design of the studies
Blantyre District, including Blantyre City, has approximately one million inhabitants in the Southern Region of Malawi. HIV prevalence among women of reproductive age in the District’s health care settings is approximately 21%17. UNAIDS estimates that nationally, in 2009, 11% of individuals aged 15-49 years were living with HIV.1 The Johns Hopkins University Research Project in collaboration with Malawian investigators has conducted MTCT of HIV research in Blantyre from 1989 to the present.
Data from six HIV MTCT studies were included in this analysis: the International Collaboration of AIDS Research (ICAR, 1989-93), Preparation of AIDS Vaccine Evaluation (PAVE, 1992-94), Perinatal Infection Project (PIP, 1994-95), Nevirapine/Zidovudine (NVP/ZDV) trial (NVAZ, 2000-04), HIV Prevention Trials Network (HPTN, 2001-03), and Post-Exposure Prophylaxis of Infants (PEPI, 2004-07). All studies included prospective cohorts of HIV-infected women enrolled during pregnancy or at delivery, and followed with their children postnatally at regular intervals. All women were recruited from the antenatal clinics at the main hospital in Blantyre (Queen Elizabeth Central Hospital) and from five health centers throughout the City representing the hospital’s catchment area. ICAR18 and PAVE18 were observational cohort studies to determine rates and risk factors for HIV MTCT. PIP19, NVAZ20,21, HPTN22 and PEPI23,24 were phase 3 clinical trials to decrease the rate of HIV MTCT using ARV and non-ARV interventions. In NVAZ, HPTN and PEPI, women received single-dose oral NVP (200 mg) at the time of delivery and their infants received single-dose oral NVP immediately after birth (2 mg/kg). No participant received ART during pregnancy. No infants were enrolled from 1996 to 1999. Therefore, two distinct periods are considered: 1989-94 (IACR, PAVE and PIP studies) and 2000-07 (NVAZ, HPTN and PEPI studies). All studies were approved by appropriate institutional reviews boards in the United States (Johns Hopkins Bloomberg School of Public Health) and in Malawi (Ministry of Health and College of Medicine in Malawi).
Study procedures
Women were counseled, consented and tested for HIV using conventional HIV antibody tests at the time of each study (either ELISA with Western blot or rapid tests). HIV-infected women were enrolled after learning about study procedures and providing written informed consent. There were important exclusions of relevance to this analysis. In the NVAZ protocol (2000-04), infants who were PT or admitted to the neonatal care unit immediately following delivery were excluded (unless the clinician determined it was safe for the infant to receive oral ARV prophylaxis). In the HPTN and PEPI protocols (2001-2007) infants with severe clinical conditions were excluded, some of whom may have been born with low BW and/or low GA. The data from these three studies were grouped in the analysis for their similar study designs and populations. The results are presented with and without observations from the NVAZ study cohort, given its explicit exclusion of PT infants per the approved protocol. Demographic, medical, obstetric and behavioral information was collected on structured case report forms. Physical examinations of the mother and infant were conducted at birth and subsequent visits. BW and GA measurements were performed by trained research nurses as soon as possible following delivery, and always within 24 hours of an infant’s birth. GA was typically assessed using the Ballard score25; when this information was not available, GA was estimated based on date of last menstrual period and date of delivery. Ultrasounds were not used to confirm GA.
Data analyses
Data from the six studies were analyzed to determine mean BW in grams (g), mean GA in weeks and standard errors (SE) for each study and across all studies. The frequency of LBW (BW<2500 g), PT birth (GA<37 weeks) and LBW among full-term infants (BW<2500 g and GA ≥37 weeks) was also estimated. Mean BW and GA were plotted by birth year separately for the two periods, 1989-94 and 2000-07, to acknowledge differences in design and exclusion criteria. Including or excluding infants from multiple births did not impact the results and no exclusion for multiple births was applied.
Multiple logistic and linear regression models were used to assess the association of socio-demographic factors with LBW and PT birth separately for the two study periods. Selection of variables for inclusion in the models was based on epidemiological and biological considerations as well as availability of the same variables across all studies. We used generalized linear mixed models for dichotomous and continuous outcomes (a multilevel approach) to account for variation at the individual-level and potential clustering of outcomes at the study cohort level. Factors associated with BW and GA in the linear and logistic regression models were similar; therefore, we present only the results of the mixed effects logistic regression models. Birth year, which was the same as year of enrollment and highly correlated with study cohort, was included as a covariate in the logistic regression models to account for potential differences by calendar time. Infants with missing BW were excluded from the analysis. Body mass index, socio-economic status and mode of delivery were included in the univariable but not in the multivariable analyses because these variables were not collected in PIP (body mass index, socioeconomic status) and PAVE (mode of delivery) studies. We defined high socioeconomic status as living in a household with both electricity and piped water. P values <0.05 were considered statistically significant. SAS version 9.2 (SAS Institute, Carey, North Carolina) was used for all statistical analyses.
RESULTS
Six MTCT studies conducted in Blantyre, Malawi contributed data from 8753 HIV-infected mothers (range: 235-3352 per study) and 8874 HIV-exposed infants to this analysis (3042 infants from the ICAR, PAVE and PIP studies; 5832 infants from the NVAZ, HPTN and PEPI studies). Table 1 describes key socio-demographic and clinical characteristics of the mothers and children. Differences across studies were small but statistically significant, with the exception of infant gender, which was distributed similarly in all studies. Notably, the rate of cesarean sections (elective and non-elective combined) in the PIP study (12.3%) was substantially higher than in other studies (1.0-5.3%).
Table 1.
Distribution of select characteristics among HIV-infected mothers and their HIV-exposed infants, 1989–2007, Blantyre, Malawi
| Characteristics | ICARM (1989-92) |
PAVE (1992-4) |
PIP (1994-95) |
HPTN (2001-03) |
NVAZ (2000-4) |
PEPI (2004-7) |
P valuea |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Mothers | 644 (100.0) | 235 (100.0) | 2103 (100.0) | 414 (100.0) | 2019 (100.0) | 3352 (100.0) | |
| Age – mean ± SE | 24.1 ± 0.2 | 23.6 ± 0.3 | 24.1 ± 0.1 | 25.4 ± 0.3 | 24.9 ± 0.1 | 26.2 ± 0.1 | <0.001 |
| Parity – mean ± SE | 3.1 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.04 | 3.0 ± 0.1 | 3.0 ± 0.03 | 3.3 ± 0.03 | <0.001 |
| Marital status | <0.001 | ||||||
| Married | 597 (92.7) | 219 (93.2) | 1925 (91.6) | 311 (75.1) | 1823 (90.4) | 3072 (91.7) | |
| Other | 47 (7.3) | 16 (6.8) | 177 (8.4) | 103 (24.9) | 193 (9.6) | 280 (8.3) | |
| Education | <0.001 | ||||||
| Grade 1-8 | 460 (71.5) | 154 (65.5) | 1279 (60.8) | 243 (58.7) | 1260 (62.5) | 1781 (53.1) | |
| Form 1-4 | 121 (18.8) | 55 (23.4) | 522 (24.8) | 144 (34.8) | 468 (23.2) | 1211 (36.1) | |
| None or other | 27 (9.6) | 26 (11.1) | 302 (14.4) | 27 (6.5) | 289 (14.3) | 360 (10.7) | |
| Husband’s education | <0.001 | ||||||
| Grade 1-8 | 247 (41.2) | 93 (46.7) | 807 (45.4) | 139 (35.0) | 846 (42.4) | 1099 (33.5) | |
| Form 1-4 | 345 (57.5) | 102 (51.3) | 923 (51.9) | 253 (63.7) | 877 (43.9) | 2093 (63.7) | |
| None or other | 8 (1.3) | 4 (2.0) | 47 (2.6) | 5 (1.3) | 274 (13.7) | 93 (2.8) | |
| SES | <0.001 | ||||||
| Highb | 122 (18.9) | 43 (18.3) | -- | 104 (25.1) | 298 (14.8) | 510 (15.2) | |
| Low | 522 (81.1) | 192 (81.7) | -- | 310 (74.9) | 1720 (85.2) | 2842 (84.8) | |
| BMI – mean ± SE | 22.0 ± 0.1 | 22.9 ± 0.2 | -- | 22.2 ± 0.1 | 22.7 ± 0.1 | 23.8 ± 0.1 | <0.001 |
| Infants | 656 (100.0) | 244 (100.0) | 2142 (100.0) | 417 (100.0) | 2020 (100.0) | 3395 (100.0) | |
| Gender | 0.88 | ||||||
| Female | 332 (50.9) | 113 (46.3) | 1075 (50.2) | 209 (50.2) | 1020 (50.5) | 1694 (49.9) | |
| Male | 320 (49.1) | 131 (53.7) | 1066 (49.8) | 207 (49.8) | 1000 (22.6) | 1701 (50.1) | |
| Mode of delivery | <0.001 | ||||||
| Cesarean section | 20 (3.1) | -- | 263 (12.3) | 22 (5.3) | 20 (1.0) | 92 (2.7) | |
| Vaginal birth | 629 (96.9) | -- | 1797 (83.9) | 394 (94.7) | 1984 (98.2) | 3274 (96.5) | |
| Other | 0 (0.0) | -- | 81 (3.8) | 0 (0.0) | 16 (0.8) | 28 (0.8) | |
BMI=body mass index; HIVNET=HIV Network; HPTN=HIV Prevention Trials Network; GA=gestational age; ICAR=International Collaboration for AIDS Research; LBW=low birth weight; NVAZ=Nevirapine and Zidovudine; PAVE=Preparation for AIDS Vaccine Evaluation; PIP= Perinatal Infection Project; PEPI=Post-exposure Prophylaxis of Infants; SE=standard error; SES=socioeconomic status;
P values were calculated using the Kruskal Wallis test for continuous data, and the Chi-square test for categorical data
Defined as living in a household with both electricity and piped water
The mean BW and GA of HIV-exposed infants across the six cohorts was 2953 g (SE ±5.3) and 38.5 weeks (SE±0.03), respectively (Table 2). The differences in mean BW and GA across studies was <300 g for BW and 1.2 weeks for GA. Table 2 also shows the overall proportions of LBW, PT and LBW among full-term infants which were 12.9%, 7.6% and 5.2%, respectively. During the earlier period (1989-94; ICAR, PAVE and PIP studies), proportions for LBW (range 19.9-21.5%), PT (13.9-14.9%) and LBW (5.9-7.0%) for full term infants were similar across time (p>0.05); during the later period (2000-07; NVAZ, HPTN and PEPI), proportions for the three outcomes (ranges 5.1-17.8%; 1.3-12.9%; and 3.7-5.4%, respectively) were variable across time (p<0.01).
Table 2.
Birth weight and gestational age by study among infants born to HIV-infected mothers, 1989–2007, Blantyre, Malawi
| Study | Birth weight (g) | Low birth weight (<2500 g at birth) |
Gestational age (weeks) | Preterm birth (<37 weeks GA) |
LBW among full-term infants (<2500 g and ≥37 weeks gestational age at birth) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SE | P-valuea | n | % | P-valuea | N | Mean ± SE | P-valuea | n | % | P-valuea | n | % | P-valuea | |
| ICAR | 656 | 2795.5 ±23.8 | 0.93 | 141 | 21.5 | 0.64 | 648 | 38.1 ±0.1 | <0.001 | 97 | 14.9 | 0.81 | 38 | 5.9 | 0.79 |
| PAVE | 244 | 2793.0 ±34.6 | 52 | 21.3 | 236 | 38.1 ±0.3 | 34 | 14.0 | 17 | 7.0 | |||||
| PIP | 2142 | 2825.8 ±11.1 | 427 | 19.9 | 2140 | 37.8 ±0.05 | 298 | 13.9 | 127 | 5.9 | |||||
| HPTN | 417 | 2881.3 ±28.9 | <0.001 | 74 | 17.8 | <0.001 | 405 | 38.3 ±0.1 | <0.001 | 53 | 12.9 | <0.001 | 15 | 3.7 | 0.01 |
| NVAZ | 2020 | 3078.7 ±9.1 | 103 | 5.1b | 2020 | 39.0 ±0.1 | 27 | 1.3b | 76 | 3.8 | |||||
| PEPI | 3395 | 3010.4 ±7.8 | 349 | 10.3 | 3395 | 38.6 ±0.03 | 166 | 4.9 | 183 | 5.4 | |||||
| Total | 8874 | 2953.4 ±5.3 | 1146 | 12.9 | 8844 | 38.5 ±0.03 | 675 | 7.6 | 456 | 5.2 | |||||
HIVNET=HIV Network; HPTN=HIV Prevention Trials Network; GA=gestational age; ICAR=International Collaboration for AIDS Research; LBW=low birth weight; NVAZ=Nevirapine and Zidovudine; PAVE=Preparation for AIDS Vaccine Evaluation; PIP= Perinatal Infection Project; PEPI=Post-exposure Prophylaxis of Infants; SE=standard error
P values compare means and proportions across the three studies within each time period (earlier and later studies). Kruskal Wallis test used for means. Chi-square test used for proportions.
Preterm infants and infants with very low birth weight excluded from study per protocol.
Figure 1 show trends in mean BW by birth year. Although the number of infants born each year was variable (range: 93-2142 infants), the mean BW for each year was not substantially different within each of the two study periods. From 1989-94, mean BW ranged from 2772 g (SE ±26.7) to 2936 g (SE ±42.3); and from 2925 g (SE ±43.6) to 3092 g (SE ±15.2) in 2000-07 (Figure 1). The variability in GA during the two study periods was also relatively minimal ranging from 37.8 (SE ±0.1) to 39.2 (SE ±0.2) weeks (Figure 2, supplemental digital conent).
Figure 1. Mean birth weight of infants born to treatment-naïve HIV-infected mothers, 1989–1994 and 2000–2007.
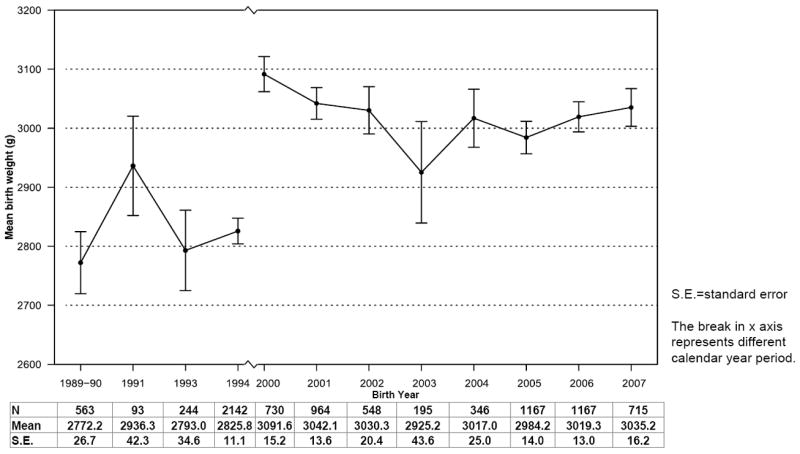
charts the mean birth weight and 95% confidence intervals (CI) among infants enrolled in six prospective studies in Blantyre, Malawi by year of birth, with sample sizes, point estimates and standard errors (SE) provided below the graph. Figure 2, available in the supplemental digital content, charts a similar distribution for mean weeks of gestation by birth year in the two study periods.
Table 3 shows the adjusted odds ratios (AOR) and 95% confidence intervals (CI) from mixed effects logistic regression for the LBW and PT birth outcomes in the early period (1989-94) and Table 4 shows results for the later period (2000-07). In the early period, maternal education was protective against LBW (AOR 0.67, CI: 0.48-0.95) and PT birth (AOR 0.70, 0.51-0.95). Maternal age was also protective against LBW (AOR 0.98, CI: 0.96-1.00) and PT birth (AOR 0.98, CI: 0.96-1.00). Birth year was associated with lower PT risk (AOR 0.35, CI: 0.19-0.70). Female gender was associated with a higher risk of PT compared to male gender (AOR 1.24, CI: 1.04-1.48). In the later period (Table 4), maternal age protected against LBW (AOR 0.97, CI: 0.95-0.99) and PT birth (AOR 0.98, CI: 0.96-1.00), while female infant gender was associated with higher odds for LBW (AOR 1.48, CI: 1.22-1.79) and PT birth (AOR 1.23, CI: 1.05-1.44). Sensitivity analyses excluding observations from NVAZ revealed virtually no differences for the LBW outcome, as shown in Table 4. There was some attenuation in the effect of maternal age and infant gender on PT birth without NVAZ data, although the direction of the effect remained the same for both covariates.
Table 3.
Multivariable mixed effects logistic regression for the association between select risk factors and low birth weighta/pretermb birth for the study period 1989–94, Blantyre, Malawi
| Risk factorsc | ICAR, PAVE, PIP (1989–94)
|
|||||
|---|---|---|---|---|---|---|
| A*: Low birth weight
|
B*: Preterm birth
|
|||||
| AOR | 95% CI | P value | AOR | 95% CI | P value | |
| Birth year (per 1 calendar year) | 0.95 | 0.84, 1.07 | 0.38 | 0.35 | 0.19, 0.70 | 0.001 |
| Maternal age (per 1 year) | 0.98 | 0.96, 1.00 | 0.02 | 0.98 | 0.96, 1.00 | 0.03 |
| Maternal education | ||||||
| None (ref) | ||||||
| Grade 1–8 | 0.84 | 0.63, 1.12 | 0.24 | 0.91 | 0.69, 1.19 | 0.49 |
| Form 1 & above | 0.67 | 0.48, 0.95 | 0.02 | 0.70 | 0.51, 0.95 | 0.02 |
| Marital status | ||||||
| Other (ref) | ||||||
| Married | 1.63 | 0.76, 3.49 | 0.21 | 1.13 | 0.60, 2.13 | 0.71 |
| Infant gender | ||||||
| Male (ref) | ||||||
| Female | 1.11 | 0.91, 1.34 | 0.30 | 1.24 | 1.04, 1.48 | 0.02 |
AOR=adjusted odds ratio; CI=confidence interval; ref=reference category
<2500 g at birth;
<37 weeks gestational age;
Parity and husband’s education were not included in these models due to strong collinearity with maternal age and maternal education, respectively.
Model A: ICC = 0.01 and Model B: ICC = 0.62
Table 4.
Multivariable mixed effects logistic regression for the association between select risk factors and low birth weighta/pretermb birth for the study period 2000–07, Blantyre, Malawi
| Risk factorsc | NVAZ, HPTN, PEPI (2000–07)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low birth weight
|
Preterm birth
|
|||||||||||
| A*: With NVAZ | B*: Without NVAZ | C*: With NVAZ | D*: Without NVAZ | |||||||||
| AOR | 95% CI | P value | AOR | 95% CI | P value | AOR | 95% CI | P value | AOR | 95% CI | P value | |
| Birth year (per 1 calendar year) | 1.01 | 0.91, 1.12 | 0.86 | 0.96 | 0.86, 1.07 | 0.44 | 1.02 | 0.94, 1.12 | 0.60 | 1.08 | 0.98, 1.20 | 0.13 |
| Maternal age (per 1 year) | 0.97 | 0.95, 0.99 | 0.01 | 0.97 | 0.95, 1.00 | 0.02 | 0.98 | 0.96, 1.00 | 0.03 | 0.98 | 0.96, 1.00 | 0.10 |
| Maternal education | ||||||||||||
| None (ref) | ||||||||||||
| Grade 1–8 | 0.98 | 0.71, 1.34 | 0.88 | 0.97 | 0.67, 1.41 | 0.89 | 1.08 | 0.83, 141 | 0.56 | 1.08 | 0.78, 1.49 | 0.66 |
| Form 1 & above | 1.14 | 0.82, 1.59 | 0.44 | 1.07 | 0.73, 1.57 | 0.73 | 1.09 | 0.83, 1.45 | 0.54 | 1.12 | 0.80, 1.57 | 0.50 |
| Marital status | ||||||||||||
| Other (ref) | ||||||||||||
| Married | 0.94 | 0.67, 1.33 | 0.73 | 0.97 | 0.65, 1.46 | 0.90 | 1.29 | 0.94, 1.77 | 0.12 | 1.48 | 0.99, 2.20 | 0.05 |
| Infant gender | ||||||||||||
| Male (ref) | ||||||||||||
| Female | 1.48 | 1.22, 1.79 | <0.001 | 1.56 | 1.26, 1.95 | <0.001 | 1.23 | 1.05, 1.44 | 0.01 | 1.15 | 0.95, 1.38 | 0.15 |
AOR=adjusted odds ratio; CI=confidence interval; ref=reference category
<2500 g at birth;
<37 weeks gestational age;
Parity and husband’s education were not included in these models due to strong collinearity with maternal age and maternal education, respectively.
Model A: ICC = 0.12, Model B: ICC = 0.03, Model C: ICC = 0.09, Model D: ICC = 0.11
DISCUSSION
Data from six prospective studies conducted in Blantyre, Malawi were analyzed to assess trends in mean BW and GA, and frequency of LBW and PT birth during two periods which approximately represent non-ARV (1989-94) and ARV (2000-07) eras of prevention of HIV MTCT. Mothers of these HIV-exposed infants did not receive ARV drugs or HAART for their own health during pregnancy.
Our findings show that mean BW and GA remained relatively stable with no notable differences within each time period. The frequency of LBW (range 19.9-21.5%) and PT (range 13.9-14.9%) also did not vary substantially during the earlier period (1989-94) when study exclusion criteria for infants were minimal. The proportion of LBW infants in these studies was higher than national estimate of 16% for Malawi in 2000, and World Health Organization estimates for the Southern Africa Region in 2000 of 14.6%, possibly because all mothers in these studies were HIV-infected.26 Estimates for the proportion of PT births were lower than the overall estimate for the Southern Africa Region of 17.5% but similar to other estimates from Africa (range 8.7-14.3%).27 The studies in the later period (2000-07) were all major clinical trials and required exclusion of newborns with severe illness, which is closely associated with LBW and PT birth, for safety reasons. Therefore, the proportions of LBW or PT infants were variable during this later period, and most likely underestimate true incidence of these outcomes. Because of the substantial number of observations (n=2020, 23%) contributed by the NVAZ study to the overall analysis, we opted to include this study and present the results with and without the NVAZ data. The frequency of LBW among full term infants showed limited variability over time (3.7-7.0%).
BW is a major predictor of infant morbidity and mortality. The duration of gestation and fetal growth in-utero are important determinants of BW and are influenced by maternal, infant and environmental factors. Maternal HIV infection is an additional factor associated with LBW and PT birth.28-32 This could result from complications to the mother herself, intrauterine infection of the fetus, or both. In this analysis, several factors were independently associated with LBW and PT birth. An increase in maternal age was consistently protective; e.g., an increase in age of 1 year was associated with a 2-3% decrease in odds of LBW or PT birth in all models, irrespective of the time of the study. Increase in maternal education was also protective against both LWB and PT birth during the earlier period (1989-94). Maternal education, as a socio-economic indicator, may influence maternal nutrition or access to care. Consistent with other studies,26 female infant gender in Blantyre, Malawi was associated with higher odds of being LBW or PT. The risk factors examined in this analysis were not comprehensive and although other relevant covariates were available from individual studies, such as maternal syphilis status, comparability of data across studies precluded inclusion of additional variables.
Consistent with our observation in the univariable analysis that mean BW and frequency of LBW and PT were stable during the two study periods, birth year was not significantly (p>0.1) associated with either LBW or PT birth after adjusting for other factors. The exception was for PT birth in the early period (1989-94); an increase of one year was associated with significantly lower risk of PT birth in the multivariable analysis. Birth year may be a proxy for a factor we did not include in the analysis.
This analysis of 8874 HIV-exposed infants provides important baseline information for monitoring impact of ARV use during pregnancy and its potential effects on pregnancy outcomes among women who deliver in the health facilities. In Malawi, an ambitious program of life-long HAART for all HIV-infected pregnant women regardless of CD4 cell count has been introduced to prevent HIV MTCT for current and future pregnancies and to improve maternal health.2,33 Monitoring of this wider use of ARVs should include both safety and effectiveness. A major strength of this analysis is the availability of data from a single research site to assess trends across several years that encompassed the earlier and later periods of the HIV epidemic in Malawi. All studies had prospective designs and followed comparable protocols for recruitment and study procedures. Exclusion of sick infants in the later studies limits generalizability of these findings, which should not be taken to represent all infants born in Malawi during the study period. Nonetheless, the differences in mean BW compared with earlier years were not substantial. We were not able to include births from 1995 to 1999 in this analysis. Expectations are high that ART will substantially improve health outcomes in sub-Saharan Africa as has been achieved in developed countries.34,35 Treatment for mothers should be equally accompanied by ongoing support for the infant from the time of birth. BW and GA are established indicators to monitor progress and reverse high levels of morbidity and mortality.
Supplementary Material
Acknowledgments
Funding sources: The ICAR and PAVE studies were supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, grants PO1-AI-26499, R21-AI-33874-01, and NO1-CP-33005. The PIP study was supported by the National Cancer Institute, National Institutes of Health. The NVAZ study was funded by the Fogarty International Center, National Institutes of Health (AIDS FIRCA Award #5R03TW01199 and Supplement), and the Doris Duke Charitable Foundation, New York. The HPTN study was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, subcontracts N01-AI-35173-117 and U01-AI-48005. The PEPI study was supported by a cooperative agreement from the U.S. Centers for Disease Control and Prevention and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health, 5-U50-PS022061-05, award U50-CC0222061.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.UNAIDS. UNAIDS report on the global AIDS epidemic 2010. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2010. [Google Scholar]
- 2.Zolfo M, De Weggheleire A, Schouten E, Lynen L. Time for “test and treat” in prevention of mother-to-child transmission programs in low- and middle-income countries. J Acquir Immune Defic Syndr. 2010;55(3):287–9. doi: 10.1097/QAI.0b013e3181eef3da. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzi P, Spicher VM, Laubereau B, Hirschel B, Kind C, Rudin C, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS. 1998;12(18):F241–7. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cotter AM, Garcia AG, Duthely ML, Luke B, O’Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193(9):1195–201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 5.Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989-2004. Pediatrics. 2007;119(4):e900–6. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 6.Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346(24):1863–70. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 7.Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS. 2006;20(18):2345–53. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 8.Patel K, Shapiro DE, Brogly SB, Livingston EG, Stek AM, Bardeguez AD, et al. Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J Infect Dis. 2010;201(7):1035–44. doi: 10.1086/651232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Is zidovudine therapy in pregnant HIV-infected women associated with gestational age and birthweight? The European Collaborative Study. AIDS. 1999;13(1):119–24. doi: 10.1097/00002030-199901140-00016. [DOI] [PubMed] [Google Scholar]
- 10.U S CDC Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings - June 27–28, 2006. Atlanta, Georgia: Centers for Disease Control and Prevention, and National Center on Birth Defects and Developmental Disabilities; [Google Scholar]
- 11.Lambert JS, Watts DH, Mofenson L, Stiehm ER, Harris DR, Bethel J, et al. Risk factors for preterm birth, low birth weight, and intrauterine growth retardation in infants born to HIV-infected pregnant women receiving zidovudine. Pediatric AIDS Clinical Trials Group 185 Team. AIDS. 2000;14(10):1389–99. doi: 10.1097/00002030-200007070-00012. [DOI] [PubMed] [Google Scholar]
- 12.Bulterys M, Chao A, Munyemana S, Kurawige JB, Nawrocki P, Habimana P, et al. Maternal human immunodeficiency virus 1 infection and intrauterine growth: a prospective cohort study in Butare, Rwanda. Pediatr Infect Dis J. 1994;13(2):94–100. doi: 10.1097/00006454-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Weng S, Bulterys M, Chao A, Stidley CA, Dushimimana A, Mbarutso E, et al. Perinatal human immunodeficiency virus-1 transmission and intrauterine growth: a cohort study in Butare, Rwanda. Pediatrics. 1998;102(2):e24. doi: 10.1542/peds.102.2.e24. [DOI] [PubMed] [Google Scholar]
- 14.Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden-Peterson S, van Widenfelt E, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56(2):131–8. doi: 10.1097/QAI.0b013e3181ffa4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powis KM, Kitch D, Ogwu A, Hughes MD, Lockman S, Leidner J, et al. Increased Risk of Preterm Delivery Among HIV-Infected Women Randomized to Protease Versus Nucleoside Reverse Transcriptase Inhibitor-Based HAART During Pregnancy. J Infect Dis. 2011;204(4):506–14. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taha TE. Mother-to-child transmission of HIV-1 in sub-Saharan Africa: past, present and future challenges. Life Sci. 2011;88(21-22):917–21. doi: 10.1016/j.lfs.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Taha TE, Li Q, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr. 2011;57(4):319–25. doi: 10.1097/QAI.0b013e318217877a. [DOI] [PubMed] [Google Scholar]
- 18.Taha TE, Dallabetta GA, Hoover DR, Chiphangwi JD, Mtimavalye LA, Liomba GN, et al. Trends of HIV-1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi. AIDS. 1998;12(2):197–203. doi: 10.1097/00002030-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Biggar RJ, Miotti PG, Taha TE, Mtimavalye L, Broadhead R, Justesen A, et al. Perinatal intervention trial in Africa: effect of a birth canal cleansing intervention to prevent HIV transmission. Lancet. 1996;347(9016):1647–50. doi: 10.1016/s0140-6736(96)91486-5. [DOI] [PubMed] [Google Scholar]
- 20.Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;292(2):202–9. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]
- 21.Taha TE, Kumwenda NI, Gibbons A, Broadhead RL, Fiscus S, Lema V, et al. Short postexposure prophylaxis in newborn babies to reduce mother-to-child transmission of HIV-1: NVAZ randomised clinical trial. Lancet. 2003;362(9391):1171–7. doi: 10.1016/S0140-6736(03)14538-2. [DOI] [PubMed] [Google Scholar]
- 22.Taha TE, Brown ER, Hoffman IF, Fawzi W, Read JS, Sinkala M, et al. A phase III clinical trial of antibiotics to reduce chorioamnionitis-related perinatal HIV-1 transmission. AIDS. 2006;20(9):1313–21. doi: 10.1097/01.aids.0000232240.05545.08. [DOI] [PubMed] [Google Scholar]
- 23.Taha TE, Li Q, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, et al. Post-Exposure Prophylaxis of Breastfeeding HIV-Exposed Infants with Antiretroviral Drugs to Age 14 Weeks: Updated Efficacy Results of the PEPI-Malawi Trial. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e318217877a. In press. [DOI] [PubMed] [Google Scholar]
- 24.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359(2):119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 25.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 26.UNICEF. Low Birthweight: Country, Regional and Global Estimates. Geneva: United Nations Children’s Fund and World Health Organization; 2004. [Google Scholar]
- 27.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 29.Ickovics JR, Ethier KA, Koenig LJ, Wilson TE, Walter EB, Fernandez MI. Infant birth weight among women with or at high risk for HIV infection: the impact of clinical, behavioral, psychosocial, and demographic factors. Health Psychol. 2000;19(6):515–23. doi: 10.1037//0278-6133.19.6.515. [DOI] [PubMed] [Google Scholar]
- 30.Mwanyumba F, Claeys P, Gaillard P, Verhofstede C, Chohan V, Mandaliya K, et al. Correlation between maternal and infant HIV infection and low birth weight: a study in Mombasa, Kenya. J Obstet Gynaecol. 2001;21(1):27–31. doi: 10.1080/01443610020022078. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone FD, Raab GM, Hamilton BA. The effect of human immunodeficiency virus infection and drug use on birth characteristics. Obstet Gynecol. 1996;88(3):321–6. doi: 10.1016/0029-7844(96)00201-3. [DOI] [PubMed] [Google Scholar]
- 32.Braddick MR, Kreiss JK, Embree JB, Datta P, Ndinya-Achola JO, Pamba H, et al. Impact of maternal HIV infection on obstetrical and early neonatal outcome. AIDS. 1990;4(10):1001–5. doi: 10.1097/00002030-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378(9787):282–4. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 34.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53(1):124–30. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


