Abstract
Background
In addition to extrasystoles of pulmonary vein (PV) origin, those arising from the superior vena cava (SVC) can precipitate atrial fibrillation (AF). The present study evaluates the electrophysiological properties of canine SVC sleeve preparations and the effect of ranolazine on late phase 3 early and delayed afterdepolarization (EAD and DAD)-induced triggered activity in SVC sleeves, and compares SVC and PV sleeve electrophysiologic properties.
Methods and Results
Action potentials (APs) were recorded from superfused SVC and PV sleeves using microelectrode techniques. Acetylcholine (1 μM), isoproterenol (1 μM), high calcium ([Ca2+]o=5.4 mM), or a combination were used to induce EADs, DADs and triggered activity. A marked diversity of action potential characteristics was observed in the SVC sleeve, including action potentials with short and long APs, with and without phase 4 depolarization. Rapid pacing induced hyperpolarization, accentuating the slope of phase 4 depolarization. Phase 4 depolarization and rapid pacing-induced hyperpolarization were reduced or eliminated following atropine (10 μM) or ranolazine (10 μM). APs displaying phase 4 depolarization (n=19) had longer APDs, smaller amplitude and Vmax, and a more positive take-off potential than APs lacking phase 4 depolarization (n=15). Ranolazine (5–10 μM) eliminated late phase 3 EADs and DAD-induced triggered activity as well as isoproterenol-induced automaticity elicited in SVC sleeves. Compared to PV, SVC sleeves display phase 4 depolarization, smaller Vmax and longer APs.
Conclusions
Autonomic influences promote spontaneous automaticity and triggered activity in SVC sleeves, thus generating extrasystolic activity capable of initiating atrial arrhythmias. Ranolazine can effectively suppress these triggers.
Keywords: atrial fibrillation, antiarrhythmic drugs, sodium channel blocker, electrophysiology, pharmacology
Introduction
Pulmonary veins (PV) have been shown to be a major site of ectopic foci capable of initiating atrial fibrillation (AF).1–3 In addition to PV, extrasystoles arising from a number of non-PV sites have been shown to be capable of initiating AF as well as other atrial arrhythmias.4–6 Among them, the superior vena cava (SVC), left atrial posterior free wall, crista terminalis, coronary sinus ostium, ligament of Marshall, and the interatrial septum.1 Of these sites, SVC is thought to be the most common source of ectopy, harboring 26–30% of non-PV foci.6–8 Like in PV sleeves, atrial myocardial tissue has been demonstrated to extend into the SVC sleeves for approximately 2–5 cm.5, 6 Extrasystolic activity arising from non-PV atrial structures, such as SVC, may compromise the success of PV isolation for the treatment of AF.7 In addition to PV isolation, SVC isolation improves the outcome of AF ablation in patients with paroxysmal AF.8
Previous studies have shown that ranolazine exerts antiarrhythmic effects in canine PV sleeves by suppressing late phase 3 early and delayed afterdepolarization (EAD and DAD)-induced triggered activity generated by parasympathetic and/or sympathetic stimulation.9–15 The effect of ranolazine on arrhythmias induced in SVC is unknown.
The present study was designed to evaluate the electrophysiological properties of canine SVC sleeve preparations, and establish the effectiveness of ranolazine to suppress arrhythmias induced in SVC sleeve preparations. Electrophysiological properties of SVC sleeve preparations were compared with those of PV sleeve preparations.
Methods
The experiments of this investigation conform to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publications No 85-23, Revised 1996) and was approved by the ACUC of the Masonic Medical Research Laboratory.
Adult mongrel dogs weighing 20–35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, IV). The chest was opened via a left-thoracotomy and the heart excised and placed in a cold cardioplegic solution ([K+]0 = 8 mM, 4°C).
Superfused superior vena cava and pulmonary vein sleeve preparations
SVC and PV sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from the right and left canine atria, respectively. The thickness of the preparations was approximately 2 mm. The preparations were placed in a small tissue bath and superfused with Tyrode’s solution of the following composition (mM): 129 NaCl, 4 KCl, 0.9 NaH2PO4, 20 NaHCO3, 1.8 CaCl2, 0.5 MgSO4, 5.5 glucose, buffered with 95% O2/5% CO2 (35 ± 0.5°C). SVC and PV sleeve preparations were stimulated at a basic cycle length (BCL) of 1000 ms during the equilibration period (1h) using electrical stimulation (1–3 ms duration, 2.5 times diastolic threshold intensity) delivered through silver bipolar electrodes insulated except at the tips. Transmembrane potentials were recorded using glass microelectrodes filled with 3 M KCl (10–20 Ω DC resistance) connected to a high input-impedance amplification system (World Precision Instruments, model KS-700, New Haven, CT). The following parameters were measured: action potential duration at 85% and 50% repolarization (APD85, APD50), action potential amplitude (APA), maximum rate of rise of action potential upstroke (Vmax), resting membrane potential (RMP), and take-off potential (TOP). Transmembrane action potentials (AP) were recorded at a sampling rate of 41 kHz.
Induction of arrhythmias
In 22 SVC sleeve preparations, we evaluated the effect of exposure of SVC to ACh (1 μM), isoproterenol (0.5–1 μM) and high calcium (5.4 mM) or their combination, conditions known to promote arrhythmias. This arrhythmia model has been previously shown to generate late phase 3 EADs and/or DAD-induced triggered activity in 100 % PV sleeve preparations.12–14, 16
Drugs
Ranolazine (Gilead, Foster City, CA) was dissolved in distilled water to form a stock solution of 10 mM and used at a final concentration of 5 to 10 μM. Isoproterenol (Sigma-Aldrich, St. Louis, MO) was dissolved in distilled water to form a stock solution of 1 mM and used at a final concentration of 1 μM. Acetylcholine (ACh) was dissolved in distilled water to form a stock solution of 10 mM and used at a concentration of 1 μM. Atropine (Sigma-Aldrich, St. Louis, MO) was dissolved in distilled water to form a stock solution of 10 mM and used at a final concentration of 10 μM. Ouabain (Sigma-Aldrich, St. Louis, MO) was dissolved in distilled water to form a stock solution of 1 mM and used at a final concentration of 1 μM.
Statistics
Statistical analysis was performed using one way repeated measures analysis of variance (ANOVA) followed by Bonferoni’s test. Mean values were considered to be different at p < 0.05. All data are reported as mean ± SD.
Results
We tested the viability and stability of superfused SVC and PV sleeve preparations by exposing them to normal Tyrode’s solution and continuously recording the electrical activity for a period of 120 min. No significant changes in action potential morphology were observed over a 120 min period.
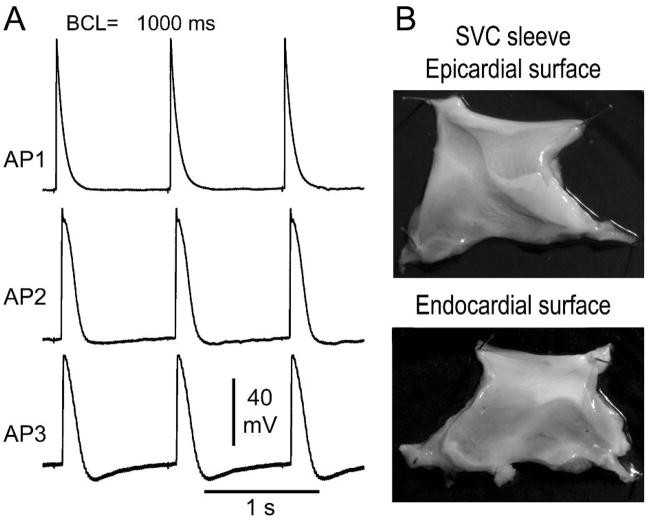
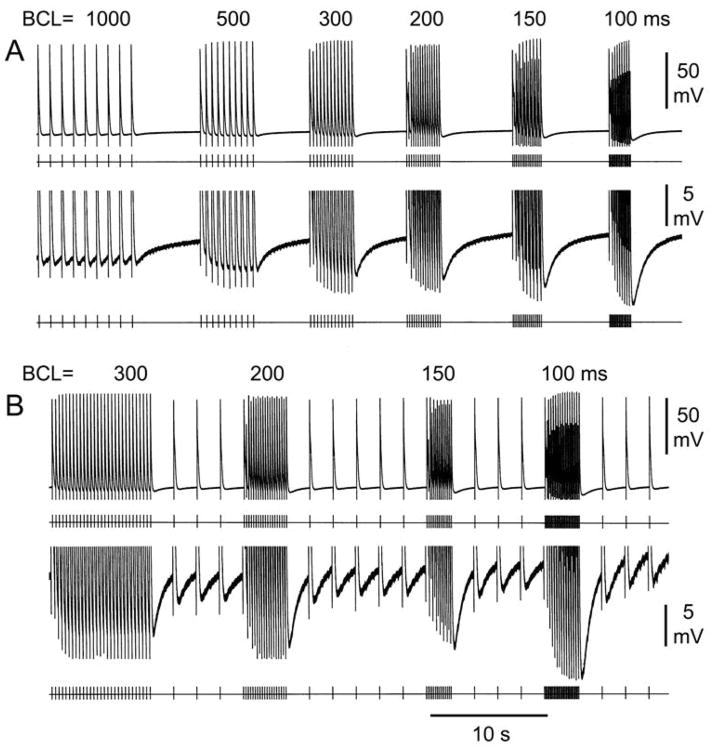
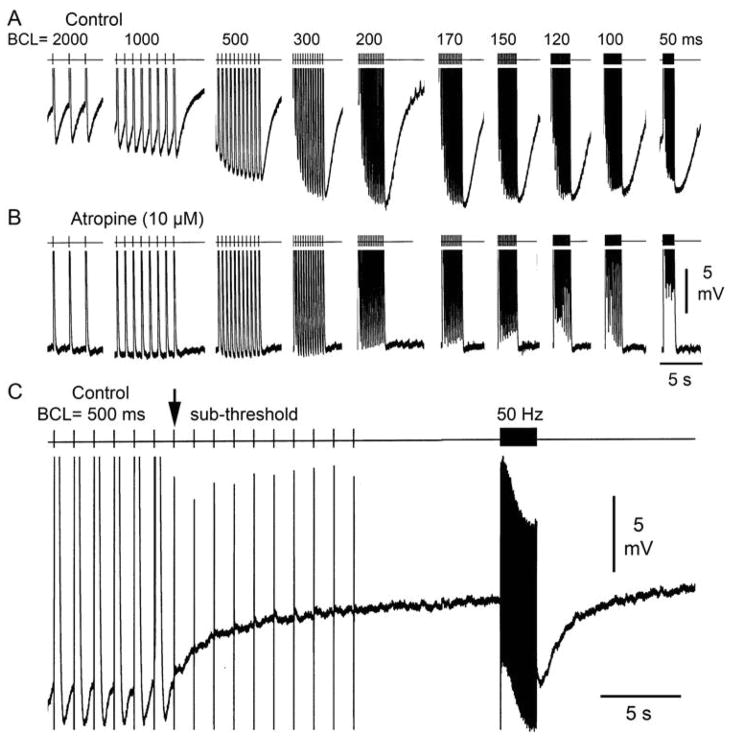
Figure 1A illustrates a representative example of diversity of AP morphologies recorded form a SVC sleeve preparation. At a BCL of 1000 ms, AP1 shows a narrow AP and no phase 4 depolarization, whereas AP2 and AP3 display a wider AP, decreased amplitude as well as phase 4 depolarization, which is more accentuated in AP3. Similar heterogeneity of action potential characteristics was observed in all SVC sleeve preparations (n=9). Figure 1B illustrates an SVC sleeve preparation, showing how the muscular extension originating from the right atrium covers the endocardial (Endo) surface of the SVC. AP recordings were always obtained from the Endo surface of the SVC preparation. Figure 2 shows the effect of rapid pacing in 2 SVC sleeve preparations displaying phase 4 depolarization. Rapid pacing led to a marked hyperpolarization of maximum diastolic potential and accentuation of phase 4 diastolic depolarization. Similar behavior was observed in 9 experiments. APs hyperpolarized 1.4±0.6 mV at a BCL of 2000 ms; 2.8±1.1 mV at BCL 1000 ms; 3.7±1.7 mV at BCL 500 ms; 4.4±1.9 mV at BCL 300ms; 4.7±1.7 mV at BCL 200 ms; and 5.6±2.7 mV at BCL of 100 ms.
Figure 1.
A. Electrical heterogeneity in superior vena cava (SVC) sleeve preparation. A: Action potentials (APs) recorded in an SVC sleeve preparation at a basic cycle length (BCL) of 1000 ms. AP1 shows a relatively short action potential duration (APD) and no phase 4 depolarization, whereas APs 2 and 3 display a longer APD, smaller amplitude and phase 4 depolarization, more accentuated in AP3 than AP2. B: Picture of endocardial (Endo) and epicardial surface of a SVC sleeve. AP recordings were obtained from Endo surface.
Figure 2.
Effect of pacing at various cycle lengths on phase 4 depolarization in 2 superior vena cava (SVC) sleeve preparations. A: The upper panel displays action potentials (APs) elicited at basic cycle lengths (BCLs) of 1000 to 100 ms followed by pauses. The lower panel is an expanded view of the same sequence of APs. B: The upper panel displays APs elicited at BCLs of 300 to 100 ms followed by pacing at BCL of 2000 ms. The lower panel is an expanded view of the same sequence of APs. In both SVC preparations, faster pacing induces marked hyperpolarization and a steeper phase 4.
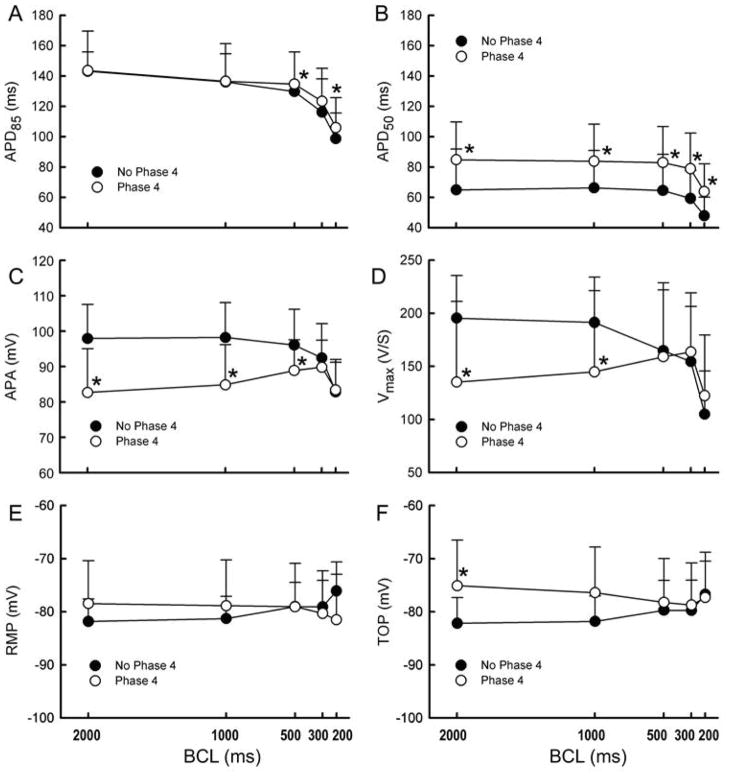
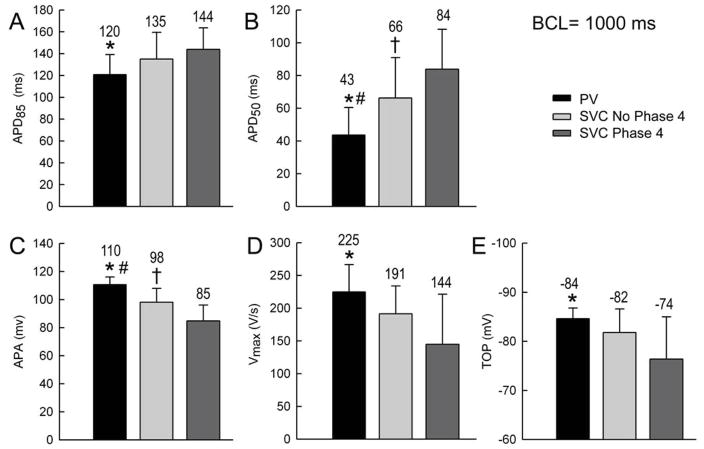
Composite data of the electrophysiological characteristics of SVC sleeve preparations with and without phase 4 depolarization are shown in Figure 3. APs with phase 4 depolarization (n=19) displayed significantly longer APD85 and APD50, smaller APA and Vmax, and exhibited a more positive TOP at slow rates, compared to APs lacking phase 4 depolarization (n=15) (p=0.04, p=0.03, p=0.001, p=0.01, p=0.02, respectively at a BCL of 500 for APD85 and BCL of 2000 ms for all others.)
Figure 3.
Composite data of electrophysiological parameters of superior vena cava (SVC) sleeve preparations with and without phase 4 depolarization. In SVC with phase 4, action potential duration measured at 85% and 50% repolarization (APD85 and APD50, A and B) were significantly increased, action potential amplitude (APA) and maximum upstroke velocity of action potential upstroke (Vmax) (C and D) were significantly decreased. Vmax shows a progressive decrease with acceleration in SVC cells with no phase 4 depolarization but a biphasic relationship for cells with phase 4. No differences were observed in resting membrane potential (RMP, E), but a significant increase in take-off potential (TOP, F) was observed at slow rates in SVC cells with phase 4. P<0.05, * SVC with phase 4 vs. SVC with no phase 4.
Vmax showed a progressive decrease with acceleration in APs with no phase 4 but a biphasic relationship in AP with phase 4 (Figure 3D). This was a constant feature in all SVC sleeve preparations studied.
Development of late phase 3 EADs and DADs in SVC sleeves. Effect of ranolazine
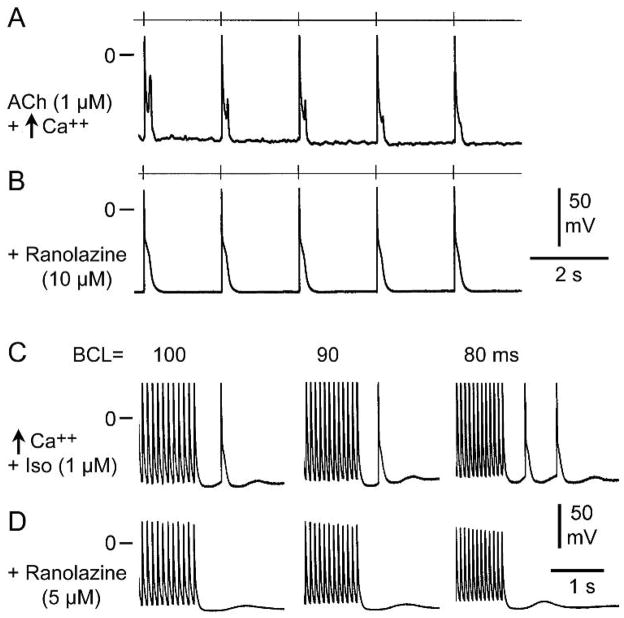
Previous studies have shown that phase 3 EADs- and DAD-induced triggered activity are observed in PV sleeves following exposure to ACh, isoproterenol, high calcium, or their combination, and that ranolazine, in concentrations within the therapeutic range (2–10 μM), is capable of suppressing triggered activity and reducing or suppressing EADs and DADs.9, 10, 12, 15 We performed a series of experiments in order to establish whether similar behavior is observed in SVC sleeve preparations. Figure 4A illustrates the development of ACh and high calcium-induced late phase 3 EADs in a SVC sleeve preparation. Late phase 3 EADs are observed at slow rates in beats immediately following rapid pacing (BCL = 200 ms). Ranolazine (10 μM) completely eliminated the late phase 3 EAD activity (Figure 4B). Figure 4C illustrates the development of DAD-induced triggered responses in a SVC preparation following exposure to isoproterenol and high calcium. Ranolazine (5μM) eliminated the triggered beats, but DADs persisted (figure 4D).
Figure 4.
Ranolazine eliminates late phase 3 early afterdepolarizations (EADs) and delayed afterdepolarizations (DAD)-induced triggered activity in superior vena cava sleeve preparations. A: ACh (1 μM) and high calcium (5.4 mM)- induce prominent late phase 3 EADs at basic cycle length (BCL) of 2000 ms following a train of 20 beats at BCL 200 ms (not shown). B: Addition of ranolazine (10μM) eliminates late phase 3 EADs. C. Isoproterenol (Iso, 1 μM) and high calcium (5.4 mM) induce a triggered response followed by a DAD following pacing at BCLs of 100 and 90 ms and 2 triggered beats followed by a DAD at BCL of 80 ms. D. Addition of ranolazine (5 μM) eliminates the triggered beats but DADs persisted.
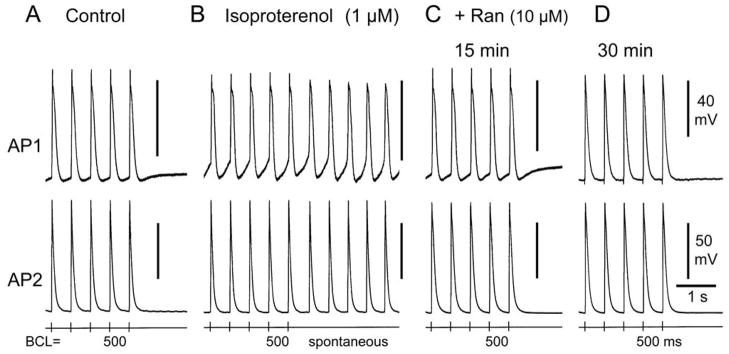
Late phase 3 EADs and/or DADs and triggered activity and/or increased automaticity could be induced in all SVC preparations exposed to ACh, isoproterenol, high calcium, alone, or in combination (n=22). Isoproterenol, alone or combined with high calcium often caused an increase in automaticity. Figure 5 illustrates an example of isoproterenol-induced increase in automaticity. Spontaneous activity due to an increase in phase 4 depolarization was observed following pacing at BCL of 500 ms. Ranolazine (10μM, 30 min of exposure) suppressed the spontaneous activity as well as phase 4 depolarization. In SVC sleeves, ranolazine (5–10 μM) eliminated late phase 3 EADs, DAD-induced triggered activity and decreased automaticity in 10 of 11 preparations.
Figure 5.
Ranolazine eliminates isoproterenol-induced increase in automaticity in a superior vena cava sleeve preparation. Shown are the last 5 beats of a train of 20 beats at basic cycle length (BCL) of 500 ms followed by a pause. A. Control action potentials (AP). AP1 but not AP2 displays phase 4 depolarization. B. Isoproterenol (1 μM) induced increase in phase 4 depolarization and spontaneous activity following pacing. C. Addition of ranolazine (Ran) (10 μM, 15 min of exposure) decreases phase 4 depolarization and eliminates spontaneous activity. D. Ranolazine (30 min of exposure) suppresses phase 4 depolarization.
Effects ouabain, atropine, and ranolazine on SVC with phase 4 depolarization
In order to determine the mechanism of phase 4 depolarization and pacing-induced hyperpolarization and increase in phase 4 depolarization, we evaluated the effects of ouabain, a Na+/K+-pump inhibitor, atropine, a muscarinic acetylcholine receptor blocker, and ranolazine, a late sodium channel blocker, on SVC sleeve preparations exhibiting phase 4 depolarization. Ouabain (1 μM) exerted no effect on phase 4 or pacing-induced hyperpolarization in any of the SVC preparations studied (n=4). Atropine markedly reduced or eliminated phase 4 depolarization as well as rapid pacing-induced hyperpolarization (Figure 6A and B). Similar results were observed in 4 of 4 SVC preparations. Subthreshold pulses (50–100 Hz) delivered to SVC preparations induced a 6 mV hyperpolarization and development of phase 4 depolarization (Figure 6C), suggesting stimulation of parasympathetic nerves innervating the SVC sleeve as the basis for the hyperpolarization and enhanced phase 4 depolarization accompanying rapid pacing. Similar effects of subthreshold stimulation were observed in 3 SVC sleeve preparations.
Figure 6.
Effect of atropine (10 μM) and subthresold pulses on phase 4 depolarization in superior vena cava sleeve preparations. A: Control action potentials (APs) recorded at basic cycle length (BCL) of 2000 to 50 ms displaying phase 4 depolarization and marked hyperpolarization following fast pacing. B: Atropine eliminates phase 4 depolarization as well as fast pacing-induced hyperpolarization. C. Following pacing at BCL of 500 ms, subthresold pulses introduced at a frequency of 50 Hz induce marked hyperpolarization.
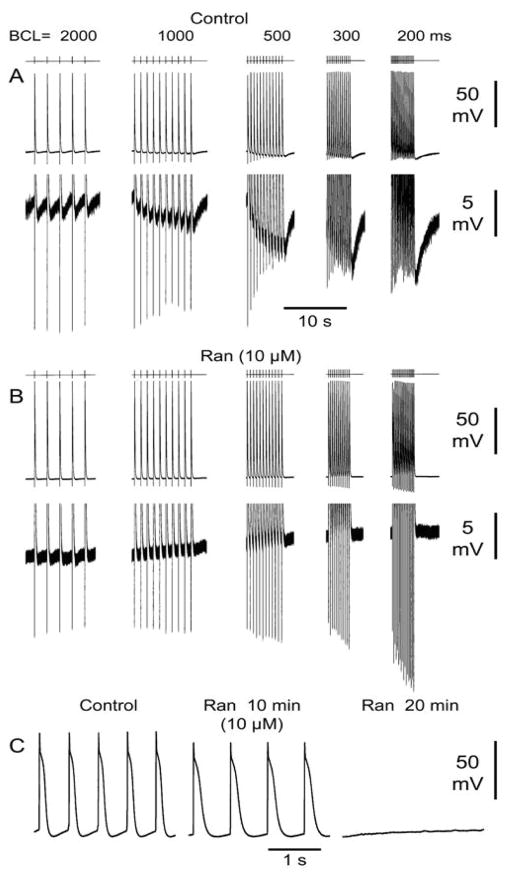
The effects of ranolazine on phase 4 depolarization were evaluated in 4 experiments. Figure 7(A and B) shows an example of the effect of ranolazine on an SVC preparation exhibiting phase 4 depolarization. Ranolazine (10μM) eliminated spontaneous phase 4 depolarization as well as rapid pacing-induced hyperpolarization and enhanced phase 4 depolarization. Ranolazine (5–10μM) reduced or eliminated phase 4 depolarization and rapid pacing-induced hyperpolarization in 4 of 4 experiments. Isolated spontaneous ectopic beats were often observed in SVC preparations, however spontaneous beating was only observed in 1 of 24 SVC sleeve preparations. In this preparation (Figure 7C) ranolazine (10 μM) reduced phase 4 depolarization from a CL of 550 to of 700 ms after 10 min of exposure and subsequently totally suppressed automaticity.
Figure 7.
Effect of ranolazine (10 μM) on rapid pacing induced hyperpolarization and associated phase 4 depolarization. A: Control action potentials (APs) recorded at basic cycle lengths (BCLs) of 2000 to 200 ms. The lower panel is an expanded view of the same sequence of APs. B: Ranolazine (Ran) (10μM) eliminates fast pacing-induced hyperpolarization and increased phase 4 depolarization. The lower panel is an expanded view of the same sequence of APs. C: Spontaneous beating in a SVC sleeve preparation at a spontaneous cycle length (SCL) of 550 ms. Addition of ranolazine (10μM, 10 min) reduces phase 4 depolarization leading to an increase in SCL to 700 ms at 10 min and subsequent total suppression of automaticity.
Comparison of SVC and PV sleeve preparations
In SVC sleeve preparations, muscular atrial extensions (sleeves) are observed on the endocardial side (luminal) of the SVC whereas in PV sleeve preparations atrial extensions penetrate on the epicardial side (abluminal) of the PV. Compared to PV, SVC sleeve preparations exhibit phase 4 depolarization, display smaller Vmax (p=0.001) and APA (p=0.001) and a longer APD50 (p=0.001) and APD85 (p=0.07) and exhibited a more negative TOP (p=0.01) (Figure 8). Isoproterenol-induced DADs and triggered activity were observed in both PV and SVC sleeves. However, isoproterenol-induced automaticity was observed more frequently in SVC preparations than in PV sleeve preparations.
Figure 8.
Comparison of distinct electrophysiological parameters in superior vena cava (SVC) vs. pulmonary vein (PV) sleeve preparations. Basic cycle length (BCL) = 1000 ms. In each panel, the black bar represents PV parameters, the light grey bar SVC parameters in cells with no phase 4, and the dark grey bar SVC parameters in cells with phase 4. Compared to SVC sleeve, in PV sleeve, action potential duration at 85% and 50% repolarization (APD85, APD50) were significantly decreased, action potential amplitude (APA) significantly increased, maximum upstroke velocity of action potential upstroke (Vmax) significantly increased and takeoff potential (TOP) significantly increased. P<0.05, * PV vs. SVC with phase 4, # PV vs. SVC with no phase 4, † SVC with no phase 4 vs. SVC with phase 4.
Discussion
The superior vena cava is the main non-PV site of origin of extrasystoles capable of triggering AF.6–8 Paroxysmal atrial tachycardia is known to originate from the SVC.17, 18 Similar to extensions of left atrial muscle into PV, right atrial muscle extends into SVC.5, 6 Chen et al18 previously described in canine SVC cardiomyocytes, the presence of spontaneously beating APs displaying phase 4 depolarization. The results of our study demonstrate a marked diversity of action potential characteristics in the canine SVC sleeve, including action potentials with short and long durations, with and without phase 4 depolarization. Like in PV sleeves, APs of cells in SVC sleeves develop late phase 3 EADs and DAD-induced triggered activity following exposure to ACh, isoproterenol, high calcium or their combination. In addition, SVC sleeves exhibit increased automaticity secondary to an increase in phase 4 depolarization following isoproterenol or isoproterenol combined with high calcium.
Phase 4 depolarization in SVC
The presence of phase 4 depolarization was consistently observed in the SVC preparations. It should be noted that spontaneous beating was only rarely observed in SVC preparations (1 out of 24) and phase 4 depolarization was of relatively modest magnitude and only increased following stimulation of vagal efferents. These characteristics and behavior of phase 4 depolarization differ markedly from phase 4 depolarization typically observed in sinus node, AV node or Purkinje fiber pacemakers cells. Phase 4 depolarization was observed at slow rates in SVC sleeves, and was markedly enhanced following rapid-pacing induced hyperpolarization. Atropine and ranolazine but not ouabain were able to markedly decrease and/or eliminate phase 4 depolarization as well as hyperpolarization induced by fast pacing. The effect of atropine to suppress rapid–pacing induced hyperpolarization and the ability of subthreshold stimulation to induce this phenomena are consistent with concomitant stimulation of parasympathetic nerves coursing through the SVC. Stimulation of human parasympathetic efferent nerves that lie along the surface of the SVC induces negative chronotropic and dromotropic effects.19 Lu et al showed that superior vena cava-aorta ganglionated plexi can act as “the head stage” for the autonomic mechanism underlying rapid SVC firing that initiates AF.20 We did not systematically study the effect of the site of stimulation and are therefore unable to determine whether the presence of neuronal clusters could have influenced the effect of subthreshold stimulation. The development of phase 4 depolarization in SVC may also be linked to the Lakatta calcium clock hypothesis described for sinus node cells suggesting that spontaneous calcium releases may contribute to accentuation of phase 4 depolarization.21, 22 Interestingly Chen et al.18 showed that densities of ICa,L and IK are similar, but Ito densities differ between cardiomyocytes with and without phase 4 depolarization.
One of the mechanisms responsible for the initiation of AF is the increase in automaticity due to spontaneous firing and diastolic depolarizations.23, 24 It has been demonstrated that some AF episodes are initiated by rapid firing of non-PV triggers, such as the SVC, in particular in patients with obesity and sleep apnea.25
The effect of ranolazine to suppress phase 4 as well as fast pacing–induced hyperpolarization suggest a role of the late sodium current in the genesis of phase 4 depolarization. In fact, using the enhancer of late INa, anemone toxin II (ATX-II), Song et al. demonstrated that an increase of late INa contributes to the development of diastolic depolarization and spontaneous activity in guinea pig atrial myoctes26 and that ranolazine can suppress spontaneous diastolic depolarization and action potential firing in these myocytes. Similarly, in our experiments, ranolazine markedly reduced or eliminated phase 4 depolarization occurring either spontaneously or following fast pacing or isoproterenol. Moreover, preliminary experiments in SVC sleeves indicate that phase 4 depolarization increases following exposure to ATX-II (4 nM).(Sicouri et al, unpublished observation) The presence of Ca2+-activated K+ channels (SK channels)27 leading to the development of phase 4 depolarization in SVC is yet another hypothesis which remains to be evaluated.
Arrhythmias induced in SVC sleeves
The development of late phase 3 EADs and DAD-induced triggered activity in SVC sleeves following ACh, isoproterenol, high calcium or their combination are similar to observations in canine PV models reported by Patterson et al.,28, 29 Sicouri et al.,12–14, 16 and Burashnikov et al.,10 in coronary-perfused atrial preparations. Similar characteristics of late phase 3 EADs were observed in PV sleeve preparations.12, 14, 16 Late-phase 3 EADs and late-phase 3 EAD-induced triggered activity, originally described by Burashnikov and Antzelevitch30 in coronary-perfused atrial preparations, represent a relatively new concept of arrhythmogenesis. Abbreviated repolarization permits normal sarcoplasmic reticulum calcium release and associated sodium-calcium exchange inward current to induce a late phase 3 EAD, resulting in closely coupled triggered responses when it reaches threshold.9 Conditions permitting intracellular calcium loading (isoproterenol, high calcium, fast pacing rates) facilitate the development of late phase 3 EADs. Autonomic stimulation can also give rise to this phenomenon in canine PV sleeves and in some cases result in a run of triggered responses.12, 14, 16, 28, 29
Inhibition of peak and late INa have been shown to have important consequences on intracellular calcium homeostasis. Inhibition of peak and late INa can reduce calcium overload. Suppression of DADs by ranolazine, similarly to TTX and other Na channel blockers, is likely due to a decrease in ITi as a consequence of the decrease in intracellular diastolic calcium concentration (via NCX). Song Y et al31 showed that ranolazine (10 μM) and TTX (2 μM) abolish DADs by inhibiting ITi caused by Na-dependent calcium overload due to exposure to ATX-II. Inhibition of late INa has also been shown to lead to a decrease in spontaneous diastolic depolarizations due to inhibition of a slowly inactivating TTX-sensitive sodium current in atrial myocytes26 and in newborn rabbit SA node cells.32 The reduction of phase 4 depolarization by ranolazine in SVC cells could in part be mediated by this mechanism as well as by a reduction in the activity of the calcium clock.33
In addition to the development of EADs and DADs, isoproterenol frequently induced an increase in automaticity. This was a mechanism of arrhythmia induced by isoproterenol or isoproterenol + high calcium in the SVC sleeves. The increase in automaticity led to an increase in the slope of phase 4 depolarization leading to spontaneous beating in SVC preparations (Figure 6). Therefore, the presence of phase 4 depolarization in APs of SVC sleeve preparations suggests a role for enhanced automaticity in the genesis of atrial arrhythmias of SVC origin.
Antiarrhythmic effects of ranolazine in SVC sleeves
Previous studies in canine PV sleeves have shown that ranolazine (5–10 μM) is capable of suppressing late phase 3 EAD and DAD-induced triggered activity generated by parasympathetic and/or sympathetic stimulation.9–13, 15 Inhibition of peak INa may contribute to the effect of ranolazine to suppress DAD activity in SVC. The effect of peak INa blockers to eliminate DAD activity is well established.34 In addition to blocking peak INa, all sodium channel blockers also block late INa, usually at lower concentrations. Song et al31 showed that TTX suppresses DADs caused by enhanced late INa. Lidocaine is expected to do the same. Flecainide, another Na channel blocker also suppresses DADs by inhibiting late INa.35 Rosen and Danilo34 have previously shown that other peak sodium channel blockers (tetrodotoxin, lidocaine etc) inhibit ouabain-induced DADs. A likely explanation for these findings is that TTX and other Na channel blockers suppress DADs by reducing Na-dependent diastolic calcium overload leading to inhibition of ITi.31, 35 as previously discussed. The effect of ranolazine to suppress pacing-induced hyperpolarization and associated enhancement of phase 4, is best explained by an effect of the drug to inhibit parasympathetic nerve activity. In addition to blocking NaV1.5 cardiac sodium channels, ranolazine has been reported to block NaV1.7 and NaV1.8 neuronal sodium channels.36, 37 Our knowledge regarding the effect of ranolazine on nerve excitation is limited, but is known to depend on the Na channel isoforms expressed in the neuronal cells. Ranolazine has been shown to inhibit NaV1.7 and 1.8 neuronal Na channel currents36, 38 as well as on NaV1.1 neuronal sodium channel current.39 To what extent these Na channel isoforms contribute to the firing of vagal nerves remains to be established. The effects of ranolazine on vagal nerve excitability is also not well established. Because ranolazine has been shown to have no effect on heart rate,40 a major effect of ranolazine on vagal tone seems unlikely.
Our data demonstrate that in SVC sleeve preparations, ranolazine can diminish arrhythmias by: 1) effectively eliminating EADs- and DADs-induced triggered activity induced by isoproterenol, ACh, high calcium or their combination; 2) suppressing phase 4 depolarization (spontaneous or induced by fast pacing); and 3) possibly by inhibiting intracardiac parasympathetic nerve activity. These actions of ranolazine are in addition to those previously described involving suppression of AF by potent atrial-selective depression of peak INa-dependent parameters. Thus, ranolazine, previously shown to be useful in suppressing AF triggers arising from PV sleeves, may also be useful in suppressing AF triggers originating in SVC sleeves.
PV versus SVC sleeves
Our data show that compared to canine PV sleeve preparations, SVC sleeve preparations exhibit phase 4 depolarization (always absent in PV sleeve), display a smaller Vmax and AP amplitude, but longer APDs. It remains to be established if similar differences are present in other species, including the human heart.
Late phase 3 EAD and DAD–induced triggered activity following exposure to isoproterenol, high calcium or their combination were observed in both PV and SVC sleeve preparations; however, isoproterenol-induced automaticity was observed much more frequently in SVC preparations.
Conclusions
Late phase 3 EAD- and DAD-mediated extrasystoles as well as automatic beats arising from SVC sleeves may serve as triggers of some atrial arrhythmias, including atrial tachycardia and atrial fibrillation. Ranolazine can effectively suppress these triggers.
Acknowledgments
We are grateful to Judith Hefferon for assistance with preparation of the illustrations and to Robert J. Goodrow Jr, for technical assistance
Funding Sources: Supported by a grant from Gilead Sciences, Inc., grant HL47678 (CA) from NHLBI, and New York State and Florida Masonic Grand Lodges.
Footnotes
Conflict of Interest Disclosures: Dr. Antzelevitch received a research grant and serves as a consultant to Gilead Sciences, Inc. Dr. Belardinelli is employed by Gilead Sciences, Inc.
References
- 1.Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS, Chen SA. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 2.Shah D, Haissaguerre M, Jais P, Hocini M. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol. 2003;26:1631–1635. doi: 10.1046/j.1460-9592.2003.t01-1-00243.x. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Kholova I, Kautzner J. Morphology of atrial myocardial extensions into human caval veins: a postmortem study in patients with and without atrial fibrillation. Circulation. 2004;110:483–488. doi: 10.1161/01.CIR.0000137117.87589.88. [DOI] [PubMed] [Google Scholar]
- 5.Macedo PG, Kapa S, Mears JA, Fratianni A, Asirvatham SJ. Correlative anatomy for the electrophysiologist: ablation for atrial fibrillation. Part I: pulmonary vein ostia, superior vena cava, vein of Marshall. J Cardiovasc Electrophysiol. 2010;21:721–730. doi: 10.1111/j.1540-8167.2010.01728.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang XH, Liu X, Sun YM, Shi HF, Zhou L, Gu JN. Pulmonary vein isolation combined with superior vena cava isolation for atrial fibrillation ablation: a prospective randomized study. Europace. 2008;10:600–605. doi: 10.1093/europace/eun077. [DOI] [PubMed] [Google Scholar]
- 7.Arruda M, Mlcochova H, Prasad SK, Kilicaslan F, Saliba W, Patel D, Fahmy T, Morales LS, Schweikert R, Martin D, Burkhardt D, Cummings J, Bhargava M, Dresing T, Wazni O, Kanj M, Natale A. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18:1261–1266. doi: 10.1111/j.1540-8167.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 8.Corrado A, Bonso A, Madalosso M, Rossillo A, Themistoclakis S, Di BL, Natale A, Raviele A. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Cardiovasc Electrophysiol. 2010;21:1–5. doi: 10.1111/j.1540-8167.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 9.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burashnikov A, Sicouri S, Di Diego JM, Belardinelli L, Antzelevitch C. Synergistic effect of the combination of dronedarone and ranolazine to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson DJ, Rogers JC, Powell T, Brown HF. Effect of catecholamines on the ventricular myocyte action potential in raised extracellular potassium. Acta Physiol Scand. 1993;148:177–186. doi: 10.1111/j.1748-1716.1993.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 12.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicouri S, Carlsson L, Antzelevitch C. Electrophysiologic and antiarrhythmic effects of AZD1305 in canine pulmonary vein sleeves. J Pharmacol Exp Ther. 2010;334:255–259. doi: 10.1124/jpet.110.166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicouri S, Gianetti B, Zygmunt AC, Cordeiro JM, Antzelevitch C. Antiarrhythmic effects of simvastatin in canine pulmonary vein sleeve preparations. J Am Coll Cardiol. 2011;57:986–993. doi: 10.1016/j.jacc.2010.08.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sicouri S, Cordeiro JM, Talarico M, Antzelevitch C. Antiarrhythmic effects of losartan and enalapril in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2011;22:698–705. doi: 10.1111/j.1540-8167.2010.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2009;20:803–810. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 18.Chen YJ, Chen YC, Yeh HI, Lin CI, Chen SA. Electrophysiology and arrhythmogenic activity of single cardiomyocytes from canine superior vena cava. Circulation. 2002;105:2679–2685. doi: 10.1161/01.cir.0000016822.96362.26. [DOI] [PubMed] [Google Scholar]
- 19.Murphy DA, Johnstone DE, Armour JA. Preliminary observations on the effects of stimulation of cardiac nerves in man. Can J Physiol Pharmacol. 1985;63:649–655. doi: 10.1139/y85-108. [DOI] [PubMed] [Google Scholar]
- 20.Lu Z, Scherlag BJ, Niu G, Lin J, Fung KM, Zhao L, Yu L, Jackman WM, Lazzara R, Jiang H, Po SS. Functional properties of the superior vena cava (SVC)-aorta ganglionated plexus: evidence suggesting an autonomic basis for rapid SVC firing. J Cardiovasc Electrophysiol. 2010;21:1392–1399. doi: 10.1111/j.1540-8167.2010.01787.x. [DOI] [PubMed] [Google Scholar]
- 21.Bridge JH, Davidson CJ, Savio-Galimberti E. A novel mechanism of pacemaker control that depends on high levels of cAMP and PKA-dependent phosphorylation: a precisely controlled biological clock. Circ Res. 2006;98:437–439. doi: 10.1161/01.RES.0000214324.34563.31. [DOI] [PubMed] [Google Scholar]
- 22.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 23.Nattel S. Atrial electrophysiology and mechanisms of atrial fibrillation. J Cardiovasc Pharmacol Ther. 2003;8:S5–11. doi: 10.1177/107424840300800102. [DOI] [PubMed] [Google Scholar]
- 24.Tamargo J, Caballero R, Delpon E. Pharmacological approaches in the treatment of atrial fibrillation. Curr Med Chem. 2004;11:13–28. doi: 10.2174/0929867043456241. [DOI] [PubMed] [Google Scholar]
- 25.Ghias M, Scherlag BJ, Lu Z, Niu G, Moers A, Jackman WM, Lazzara R, Po SS. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54:2075–2083. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Song Y, Shryock JC, Belardinelli L. A slowly inactivating sodium current contributes to spontaneous diastolic depolarization of atrial myocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1254–H1262. doi: 10.1152/ajpheart.00444.2009. [DOI] [PubMed] [Google Scholar]
- 27.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA, Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 28.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–H2039. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- 32.Baruscotti M, DiFrancesco D, Robinson RB. Na(+) current contribution to the diastolic depolarization in newborn rabbit SA node cells. Am J Physiol Heart Circ Physiol. 2000;279:H2303–H2309. doi: 10.1152/ajpheart.2000.279.5.H2303. [DOI] [PubMed] [Google Scholar]
- 33.Lakatta EG, Vinogradova T, Lyashkov A, Sirenko S, Zhu W, Ruknudin A, Maltsev VA. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart’s pacemaker. Ann N Y Acad Sci. 2006;1080:178–206. doi: 10.1196/annals.1380.016. [DOI] [PubMed] [Google Scholar]
- 34.Rosen MR, Danilo P., Jr Effects of tetrodotoxin, lidocaine, verapamil and AHR-266 on ouabain induced delayed afterdepolarizations in canine Purkinje fibers. Circ Res. 1980;46:117–124. doi: 10.1161/01.res.46.1.117. [DOI] [PubMed] [Google Scholar]
- 35.Fredj S, Lindegger N, Sampson KJ, Carmeliet P, Kass RS. Altered Na+ channels promote pause-induced spontaneous diastolic activity in Long QT syndrome type 3 myocytes. Circ Res. 2006;99:1225–1232. doi: 10.1161/01.RES.0000251305.25604.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol. 2008;73:940–948. doi: 10.1124/mol.107.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estacion M, Waxman SG, Dib-Hajj SD. Effects of ranolazine on wild-type and mutant hNav1.7 channels and on DRG neuron excitability. Mol Pain. 2010;6:35. doi: 10.1186/1744-8069-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajamani S, Shryock JC, Belardinelli L. Block of tetrodotoxin-sensitive, Na(V)1.7 and tetrodotoxin-resistant, Na(V)1.8, Na+ channels by ranolazine. Channels (Austin) 2008;2:449–460. doi: 10.4161/chan.2.6.7362. [DOI] [PubMed] [Google Scholar]
- 39.Kahlig KM, Lepist I, Leung K, Rajamani S, George AL. Ranolazine selectively blocks persistent current evoked by epilepsy-associated Nanu1.1 mutations. Br J Pharmacol. 2010;161:1414–1426. doi: 10.1111/j.1476-5381.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao G, Walsh E, Shryock JC, Messina E, Wu Y, Zeng D, Xu X, Ochoa M, Baker SP, Hintze TH, Belardinelli L. Antiadrenergic and hemodynamic effects of ranolazine in conscious dogs. J Cardiovasc Pharmacol. 2011;57:639–647. doi: 10.1097/FJC.0b013e31821458e8. [DOI] [PMC free article] [PubMed] [Google Scholar]