Abstract
Background
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract diseases in infancy and early childhood. Despite its importance as a pathogen, there is no licensed vaccine against RSV yet. The attachment glycoprotein (G) of RSV is a potentially important target for protective antiviral immune responses. Recombinant baculovirus has been recently emerged as a new vaccine vector, since it has intrinsic immunostimulatory properties and good bio-safety profile.
Methods
We have constructed a recombinant baculovirus-based RSV vaccine, Bac-RSV/G, displaying G glycoprotein, and evaluated immunogenicity and protective efficacy by intranasal immunization of BALB/c mice with Bac-RSV/G.
Results
Bac-RSV/G efficiently provides protective immunity against RSV challenge. Strong serum IgG and mucosal IgA responses were induced by intranasal immunization with Bac-RSV/G. In addition to humoral immunity, G-specific Th17- as well as Th1-type T-cell responses were detected in the lungs of Bac-RSV/G-immune mice upon RSV challenge. Neither lung eosinophilia nor vaccine-induced weight loss was observed upon Bac-RSV/G immunization and subsequent RSV infection.
Conclusion
Our data demonstrate that intranasal administration of baculovirus-based Bac-RSV/G vaccine is efficient for the induction of protection against RSV and represents a promising prophylactic vaccination regimen.
Keywords: Respiratory syncytial virus, Glycoprotein, Recombinant baculovirus, Protective immunity
INTRODUCTION
Respiratory syncytial virus (RSV) is the most important viral pathogen of causing serious bronchiolitis and pneumonia in infants and young children worldwide. RSV is also receiving increasing recognition as an important cause of lower respiratory tract illness in immunocompromised patients, the young children, and the elderly (1-3). Despite the importance of RSV as a respiratory pathogen, there is no licensed vaccine currently available against RSV infection.
In the 1960s, the immunization of children with formalin inactivated-RSV (FI-RSV) vaccine not only failed to protect, but also yielded enhanced pulmonary disease in vaccinated infants following RSV infection (4,5). Studies with BALB/c mice have become a useful model for RSV pathogenesis, since FI-RSV-enhanced disease is also observed in vaccinated BALB/c mice. It is likely that the augmented lung disease and the development of pulmonary eosinophilia are attributed to an excessive Th2 type immune response (6).
The RSV G protein is one of the major protective antigens and good inducer of strong serum and mucosal neutralizing antibody responses. It also has single immunodominant I-Ed epitope spanning RSV G amino acid 183 to 198 and largely induces a specific subset of CD4 T cells (7,8). Previously, we have reported that RSV G protein fragment (spanning amino acid residues 131-230) delivered by recombinant adenoviral vector successfully elicited long-term protective immunity against RSV infection in mice (9).
Baculoviruses are enveloped viruses possessing a rod-shaped nucleocapsids in which double stranded circular DNA genome of 88-135Kbp is packaged (10). Baculoviruses are generally utilized as a vector for the high level production of recombinant proteins but they could be also employed in gene transfer to mammalian cells (11-13). Several research groups have demonstrated that vaccination with recombinant baculovirus can induce high-level humoral and cell-mediated immunity against various antigens, suggesting that baculovirus could be used as a vaccine carrier (14,15). In addition, it has been reported that immunization with a recombinant baculovirus expressing the hemagglutinin gene of influenza virus elicited a strong innate immune response and protected mice against influenza virus challenge (16). Moreover, it is known that there is no baculovirus-specific immunity in mammals that might interfere the action of baculovirus-based vaccine (17-19).
In the present study, a recombinant baculovirus displaying the RSV G protein (Bac-RSV/G) was constructed. Our results clearly show that strong humoral and cellular immune responses were induced by intranasal administration of Bac-RSV/G in a mouse model. Importantly, complete protection against RSV challenge without vaccine-induced illness was observed in Bac-RSV/G-immune mice, suggesting that vaccination of Bac-RSV/G could be utilized as a prophylactic vaccination regimen against RSV infection.
MATERIALS AND METHODS
Cells and preparation of RSV stock
Baculoviruses were propagated in Spodoptera frugiperda 9 (Sf9) insect cells using SF-900 serum-free medium (Invitrogen) at 27℃. RSV A2 strain was propagated in HEp-2 cells (ATCC, Manassas, VA) in Dulbecco's modified Eagle's medium (Life Technologies, Gaithersburg, MD) supplemented with 3% heat-inactivated fetal calf serum, 2 mM glutamine, 20 mM HEPES, nonessential amino acid, penicillin, and streptomycin and titrated for infectivity by plaque assay as described elsewhere (20).
Construction and production of recombinant baculoviruses
The coding sequence of RSV G protein from RSV A2 strain was amplified from cDNA by PCR and cloned into the EcoR I and Xho I sites of pFastBac-1 vector (Fig. 1A). The recombinant baculovirus was subsequently generated by using the Bac-to-Bac® system (Invitrogen) following the manufacturer's instructions. The recombinant baculoviruses were purified from supernatants of infected Sf9 insect cells with 25% (w/v) sucrose in 5 mM NaCl, 10 mM EDTA in a SW28 rotor (Beckman, USA) at 24,000 rpm for 75 min at 4℃. The supernatant was decanted, and the pellet was resuspended in phosphate-buffered saline (PBS) and centrifuged for 4 h at 24,000 rpm, 4℃. The viral pellet was resuspended in PBS and titrated by plaque assays on Sf9 cells.
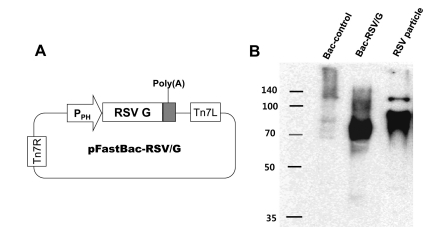
Figure 1.
Construction and characterization of Bac-RSV/G vaccine. (A) The shuttle vector, pFastBac-RSV/G, was designed as shown in the diagram and constructed as described in the Materials and Methods. The vector was used to generate Bac-RSV/G recombinant baculovirus. (B) The presence of the RSV G protein on Bac-RSV/G was confirmed by western blotting. Viral particles were prepared as described in the Materials and Methods, and subjected to western blotting with an RSV G-specific monoclonal antibody.
Immunization and challenge
Female BALB/c mice were purchased from Charles River Laboratories Inc. (Yokohama, Japan). Mice kept under specific-pathogen-free conditions. For immunization, 6- to 8-week-old mice were inoculated with baculoviruses via the intranasal (i.n.) route. For i.n. immunizations, mice were lightly anesthetized by ether/chloroform inhalation, and 2×108 PFU of Bac-RSV/G or Bac-control in a volume of 70µl was applied to the left nostril. Three to four weeks after second immunization, the mice were challenged i.n. with 1×106 PFU of live RSV A2 strain. All animal studies were performed according to the guidelines of our Institutional Animal Care and Use Committee (Approval No. 2010-9-4).
ELISA
Blood was obtained from the retro-orbital plexus with a heparinized capillary tube, collected in an Eppendorf tube, and centrifuged, and serum was stored at -20℃. RSV G protein-specific antibody titers in immunized mice were measured by a direct enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated overnight with 100µl/well of 0.5µg/ml of purified G protein fragment diluted in PBS and then blocked with PBS containing 1% skim milk and 0.05% Tween-20 for 2 h. Sera were then added in serial dilutions and incubated for 2 h. The plates were washed five times with PBS containing 0.05% Tween 20 and incubated for 30 min with various dilutions of horseradish peroxidase-conjugated affinity-purified rabbit anti-mouse total IgG, secondary antibody (Zymed Laboratories, San Francisco, CA). The plates were washed five times and developed with 3,3',5,5'-tetramethylbenzidine, and the reaction was stopped with 1 M H3PO4 and analyzed at 450 nm with a Thermo ELISA plate reader. The wells receiving no serum were used to calculate cut-off values.
Preparation of lung lymphocytes and flow cytometric analysis
The lungs were perfused with 5 ml of PBS containing 10 U/ml heparin (Sigma-Aldrich, St. Louis, MO) through the right ventricle using a syringe fitted with 25-gauge needle. The lungs were then removed and placed in RPMI medium supplemented with glutamine, gentamicin, penicillin G, and 10% FBS (HyClone, Logan, UT). The tissue was then processed through a steel screen to obtain a single-cell suspension, and particulate matter was removed by passage through a 70-µm Falcon cell strainer (BD Labware, Franklin Lakes, NJ). Freshly explanted BAL fluid or lung cells were purified by density gradient centrifugation and stained in PBS-3% FBS-0.09% NaN3 using fluorochrome-conjugated antibodies. The antibodies used were anti-CD4 (clone RM4-5), anti-CD44 (clone IM7) or anti-CD43 (clone 1B11). Both antibodies were purchased from BD PharMingen (San Diego, CA). After staining, cells were fixed in PBS-2% (wt/vol) paraformaldehyde, and events were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). To enumerate the number of cytokine-producing cells, intracellular cytokine staining was performed as described elsewhere (21). In brief, 2×106 freshly explanted lung lymphocytes were cultured in a culture tube. Cells were left untreated or stimulated with 10µM G (183-195) peptide (WAICKRIPNKKPG) and then incubated for 5 h at 37℃ in 5% CO2. Brefeldin A (5µg/ml) (Sigma-Aldrich) was added for the duration of the culture period to facilitate intracellular cytokine accumulation. Cells were then stained for surface markers, washed, fixed, permeabilized with fluorescence-activated cell sorter buffer containing 0.5% saponin (Sigma-Aldrich, Seoul, Korea), and stained for cytokines. The antibodies used were anti-IFN-γ (clone XMG1.2) or anti-IL-17A (TC11-18H10.1). Dead cells were excluded on the basis of forward and side light scatter patterns. Data were collected using CELLQuest software (BD Biosciences) and analyzed with CELLQuest and WinMDI version 2.9 software (Scripps Research Institute, La Jolla, CA). Lung supernatants were also collected for analysis with the FlowCytomix (eBioscience), according to the protocol. Kits containing antibody beads (IL-4, IL-5, IL-6, IL-10, IL-13) were used to measure cytokine levels in each of the samples.
Bronchoalveolar lavage (BAL) and eosinophil assay
At 5 days post-challenge, a subset of mice from each group was sacrificed and tracheotomy was performed. The lung airways were washed with 0.8 ml of PBS containing 1% fetal bovine serum (FBS). The collected bronchoalveolar lavage (BAL) cells were used for measuring eosinophils. Approximately 1×105 cells from BAL fluid were incubated for 15 min with anti-Fc receptor antibody (2.4G2) to prevent nonspecific binding. Next, cells were stained with specific antibodies at final concentration of 1µg/100µl in PBS-3% FBS-0.09% NaN3. The antibodies used were PE-conjugated anti-Siglec-F (E50-2440), APC-conjugated anti-CD45 (30-F11), and FITC-conjugated CD11c (HL3). Both antibodies were purchased from BD PharMingen (San Diego, CA). After staining, cells were fixed in PBS-2% (wt/vol) paraformaldehyde, and events were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA).
RSV titer in lung
Four or five days after RSV challenge, subsets of mice were euthanized and the lungs were removed into Eagle's modified essential medium. The tissues were then processed through a steel screen to obtain a single-cell suspension, and particulate matter was removed by passage through a 70-µm cell strainer (BD Labware, Franklin Lakes, NJ). The supernatants were collected, and RSV titers in the supernatants were measured by standard plaque assay on subconfluent HEp-2 monolayers. The data are expressed as the PFU per gram of lung tissue.
Statistical analysis
Comparison of differences was conducted by using an unpaired, two-tailed Student t test. The difference was considered statistically significant when the p value was ≤0.05.
RESULTS
Generation of recombinant baculovirus displaying RSV G
The recombinant baculovirus expressing RSV G coding sequences under the control of the polyhedrin promoter, Bac-RSV/G, was generated using Bac-to-Bac system with a pFastBac-1 plasmid as shown in Fig. 1A. To examine whether Bac-RSV/G express and display RSV G in the virus particle, western blotting analysis was performed with sucrose gradient-purified baculoviruses. Using the G-specific monoclonal antibody, specific bands of approximately 70 kDa to 90 kDa were detected in the purified Bac-RSV/G particles, but not in Bac-control virus particles (Fig. 1B). As a positive control, purified RSV particles produced in HEp-2 cells were used and slightly higher molecular weight bands were detected (Fig. 1B). The size difference might be due to different patterns of glycosylation between insect cells and human cells.
Intranansal Bac-RSV/G immunization induces humoral immune responses
To investigate the immunogenicity of Bac-RSV/G, the groups of mice were immunized i.n. with Bac-RSV/G, Bac-control, or PBS. RSV G-specific antibody levels in sera from the immune mice at 2 weeks after priming and 2 weeks after boosting were determined by ELISA. The specific antibody responses were barely detectable in all groups of mice at 14 days after priming (data not shown). Following booster immunization, however, the mean titers of serum antibodies increased significantly only in the group of mice immunized with Bac-RSV/G (Fig. 2A).
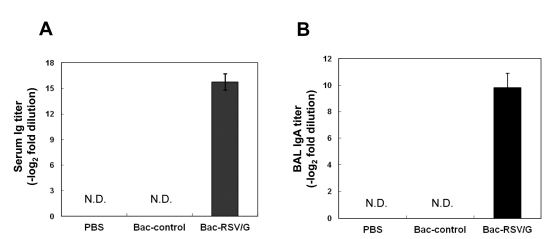
Figure 2.
Characterization of humoral immune responses induced by Bac-RSV/G vaccine. (A) BALB/c mice were immunized twice with 2×108 PFU of recombinant baculovirus (Bac-RSV/G or Bac-control) via intranasal route, and systemic anti-RSV IgG antibody titers were measured by serum ELISA two weeks after boosting. (B) Secretory IgA titers were measured in the BAL fluid for each immune group five days after RSV challenge. The results represent Log2 endpoint values from four to five individual mice. Data are representative of at least three independent experiments and data are average±SEM, n=4~5 mice per group. N.D., not detected.
Secretory IgA has been shown to directly mediate local immunity against aerial pathogens, implying an important role for antibody in protection in the upper respiratory tract (22). Thus, for an effective RSV vaccine development, the induction of secretory IgA on respiratory mucosal surface is critical. To examine whether Bac-RSV/G vaccination elicits IgA response in the respiratory tract, bronchoalveolar lavage (BAL) was performed at day 5 after RSV challenge and levels of IgA were determined by RSV-specific ELISA. Each group of mice immunized with Bac-control or PBS did not exhibit any specific IgA response, whereas Bac-RSV/G-immune mice showed significantly enhanced level of specific IgA response (Fig. 2B). These results suggest that intranasal immunization of Bac-RSV/G effectively induce both RSV G-specific serum IgG and respiratory IgA.
Intranasal Bac-RSV/G immunization induces G-specific Th1 and Th17 responses
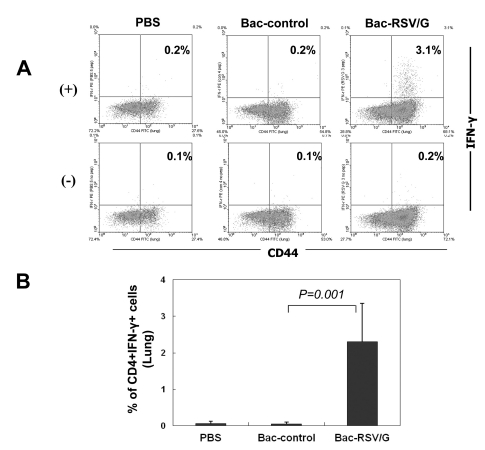
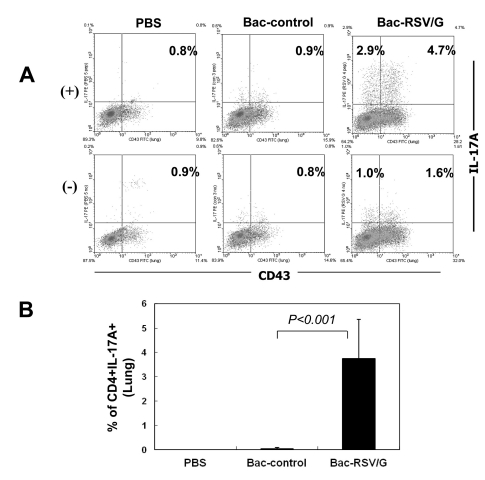
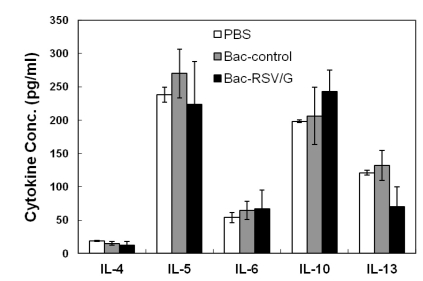
Since the RSV G contains I-Ed-restricted CD4 T-cell epitope, we examined whether Bac-RSV/G immunization induces specific CD4 T cells or not. To this end, Bac-RSV/G, Bac-control, or PBS immune mice were challenged with RSV. CD4 T cell responses were evaluated at 5 days after challenge by measuring the production of IFN-γ in lung lymphocytes that had been stimulated with I-Ed-restricted G (183-195) epitope peptide ex vivo. As shown in Fig. 3, G-specific CD4+IFN-γ+cells were detected in the lungs of Bac-RSV/G-immunized group (~2.5% of gated CD4 T cells on average), while few specific cells (<0.2% of CD4 T cells) were observed in the lungs of Bac-control or PBS-immunized group. We next investigated whether immunization of Bac-RSV/G induces other CD4+ T cell subset such as Th2 and Th17. Bac-RSV/G-vaccinated mice showed increased frequency of G-specific IL-17-producing CD4 T cells in the lungs after RSV challenge (~3.7% of gated CD4 T cells on average; Fig. 4). However, the levels of Th2-type cytokines such as IL-4, IL-5, IL-10, and IL-13 measured by multiplex antibody-based bead assays were not significantly different in the lungs from all groups of mice (Fig. 5). Together, these results indicated that mixed G-specific Th1/Th17-cell responses were induced by Bac-RSV/G vaccination.
Figure 3.
G-specific Th1-cell response in Bac-RSV/G-immune mice. (A) Mice were i.n. immunized with Bac-RSV/G, Bac-control, or PBS and challenged with RSV A2 four weeks after boosting. Lung mononuclear cells were prepared from the lungs of the same group of mice (n=4~5) five days after challenge and stimulated with G183-195 peptide (+) or without peptide (-). Cells were stained for CD4, CD44, and IFN-γ, and analyzed by flow cytometry. Cells gated for CD4 are shown in each dot plot and the percentages represent the frequency of G-specific IFN-γ-positive cells. (B) Average data represent mean±SD (n=5). The results are a representative of two independent experiments.
Figure 4.
G-specific Th17-cell response in Bac-RSV/G-immune mice. (A) Mice were immunized with Bac-RSV/G, Bac-control, or PBS i.n. and challenged with RSV four weeks after boosting. Lung mononuclear cells were prepared from the lungs of the same group of mice (n=4~5) five days after challenge and stimulated with G183-195 peptide (+) or without peptide (-). Cells were stained for CD4, CD43, and IL-17A, and analyzed by flow cytometry. Cells gated for CD4 are shown in each dot plot and the percentages represent the frequency of G-specific IL-17A-positive cells. (B) Average data represent mean±SD (n=5). The results are a representative of two independent experiments.
Figure 5.
Bac-RSV/G immunization does not induce Th2 type cytokines in the lung. Mice were immunized and challenged as in Fig. 3. Lungs from mice were harvested five days after RSV challenge, and cytokine levels in the lung supernatant were assessed by multiplex antibody-based assay (FlowCytomix). Bars represent the mean of 4~5 mice per group and data are a representative of two independent experiments.
Protective efficacy of Bac-RSV/G vaccine against RSV challenge
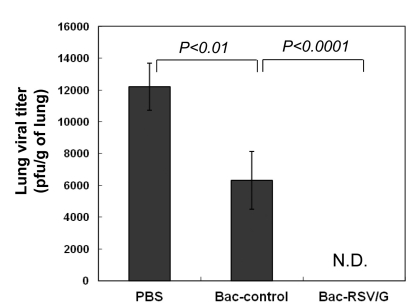
To determine whether Bac-RSV/G has protective efficacy against RSV infection, mice were challenged with live RSV A2 virus at four weeks after booster immunization. While there was active RSV replication in the lungs of the Bac-control or PBS immune mice, immunization of Bac-RSV/G prevented any detectable RSV replication in the lungs during the peak of infection (Fig. 6). Interestingly, immunization of Bac-control resulted in partial decrease of viral replication at the peak. This might be due to nonspecific innate immunity induced by baculovirus inoculation. It was previously reported that intranasal administration of wild-type baculovirus induces strong innate immune responses and provides protection against lethal challenge of influenza virus (16).
Figure 6.
Immune protection from respiratory RSV challenge by vaccination with Bac-RSV/G. Each group of immune mice was challenged with 1×106 PFU of RSV A2 at 4 weeks after immunization and the level of viral replication in the lungs was determined by plaque assay at day 5. Results are expressed as the mean±SEM from 4 to 5 mice per group. The limit of detection is 200 PFU/g of lungs. N.D., not detected.
Bac-RSV/G immunization do not promote vaccine-induced eosinophilia
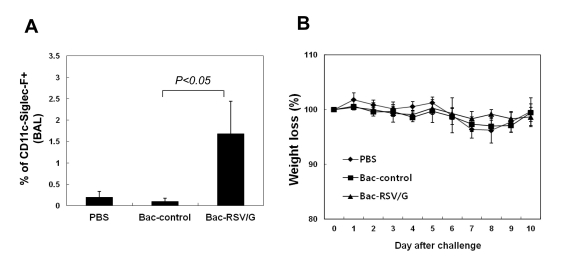
It was reported that immunization with vaccinia virus expressing the entire RSV G glycoprotein results in pulmonary eosinophilia following challenge with live RSV (6,23,24). These studies indicated that RSV G expressed vaccines have a possibility for the development of pulmonary eosinophilia. To determine whether the intranasal immunization of Bac-RSV/G potentiates eosinophilia, the levels of eosinophils in the BAL fluids of the immune mice were examined by flow cytometry 5 days after RSV challenge using antibodies to Siglec-F, CD45, and CD11c as described previously (25). Bac-RSV/G-immunized mice have a higher frequency of CD11c-SiglecF+ in the BAL as compared with Bac-control or PBS group (Fig. 7A, p<0.05), but the level of eosinophil influx in Bac-RSV/G immune mice was relatively weak (~2% of the total CD45+ BAL cells). These results suggest that intranasal Bac-RSV/G immunization barely increase the risk of vaccine-induced eosinophilia.
Figure 7.
Bac-RSV/G immunization promotes neither lung eosinophilia nor vaccine-enhanced illness. (A) Mice were immunized and challenged as in Fig. 3. BAL was performed five days after RSV challenge, and BAL cells were stained with antibodies to CD45, Siglec-F, and CD11c, and eosinophils were quantitated among CD45+-gated cells. Average percentages of Siglec-F+CD11c-cells among total CD45+ cells represent mean±SD (n=6). The results are a representative of three independent experiments. (B) The same group of immune mice were challenged with RSV and then weighed each day. Results are expressed as the mean±SEM from 5 mice for each group. All data are representative of at least two independent experiments.
To evaluate other RSV-induced pathology by Bac-RSV/G immunization, we monitored weight loss in all immune-mice after RSV challenge. Following infection with live RSV, there was no significant weight loss in Bac-RSV/G-immune mice (Fig. 7B) and disease score (data not shown). Taken together, these results suggest that intranasal Bac-RSV/G vaccination give rise to protective immunity in the absence of subsequent vaccine-enhanced disease.
DISCUSSION
To develop a safe and effective RSV vaccine, many strategies and platforms have been applied in pre-clinical and clinical phases (26). Many RSV vaccine candidates specifying target antigens employed two envelop proteins, G attachment protein and F fusion protein, because these antigens are known to induce protective immunity against live RSV infection. However, in the BALB/c mouse model, immunization of G protein expressed from recombinant vaccinia virus elicited Th2-biased responses, which was responsible for the vaccine-enhanced diseases (27). Thus, G protein has been falsely regarded as a bad target antigen for a long time, although it could induce strong neutralizing antibody responses upon immunization. Recently, it has been suggested that G protein itself is not the cause of vaccine-enhanced diseases and the proper balance between RSV-specific Th1 and Th2 responses is rather an important factor controlling both safety and efficacy of G-targeted RSV vaccine. Using appropriate platforms and/or adjuvants, this balance could be achieved with G-targeted vaccines. For example, we have recently demonstrated that mucosal immunization of recombinant adenovirus vaccine expressing the core domain of G successfully induced protective immunity without vaccine-induced diseases (9).
Baculovirus has recently been emerged as a new tool for vaccine vector development since it has many advantages as a vaccine platform (28-30). First, baculovirus, which has been used to overexpress recombinant proteins in insect cells, is neither replication-competent nor pathogenic in mammalian cells, making it safer vaccine vector than other mammalian viral vectors. In addition, baculovirus-based vaccines produced in insect cells are not contaminated by LPS. Second, baculovirus-based vaccine possesses several advantages upon production, such as easy manipulation, relatively simple scale-up, and high titers (11,31). Thirdly, there is no pre-existing vector immunity against baculovirus in mammals, which enables baculovirus to escape vector neutralization by pre-existing immunity during in vivo delivery (19). Lastly, baculovirus has the ability to stimulate strong innate immunity, exhibiting strong adjuvanticity itself. It has been previously shown that inoculation of wild-type baculovirus alone can stimulate the secretion of inflammatory cytokines from innate immune cells and confer protection from lethal virus infection in mice (16). Consistent with this report, intranasal inoculation of control baculovirus also provided partial protection against live RSV challenge in our study (Fig. 6). The baculovirus-mediated stimulation of innate immunity might be associated with the induction of type I interferons, mediated by both TLR9-dependent (32) and TLR9-independent pathways (33). Thus, it becomes evident that baculovirus can act as a natural adjuvant by stimulating innate immunity and be used as a safe and effective vaccine carrier.
Our data demonstrate that intranasal immunization of Bac-RSV/G vaccine induces complete protection from RSV infection, which is associated with enhanced Th1 and Th17 responses in the absence of significant Th2 responses. Although the role of Th1 or CTL responses to RSV clearance is well delineated (34), the role of Th17-mediated responses have not been fully elucidated in host defense against RSV. Th17 cells, which are characterized by massive production of IL-17, are thought to contribute to autoimmune diseases but also play a crucial role in host defense against extracellular bacteria (35,36). In addition, Th17-associated effector molecules seem to be necessary and sufficient for protection against several viral pathogens. For example, recent studies have reported that Tc17 cells are potently cytolytic against vaccinia virus-infected cells (37,38). In these studies, both IL-17-producing CD4+ and CD8+ T cells are necessary to confer protection against viral infections, exhibiting cytotoxic activity for clearance of vaccinia virus-infected cells. Mice immunized with a recombinant vaccinia virus expressing the G protein of RSV (vvG) exhibit pulmonary eosinophilia induced by Th2 type cytokines after challenge RSV infection (6). Th2 type cytokines are thought to promote eosinophil infiltration to the lungs of RSV-infected mice and may contribute to immunopathology associated with vaccine-enhanced diseases. Interestingly, we showed that the levels of Th2-type cytokines, such as IL-4, IL-5, IL-10, or IL-13, were very low in the lungs of Bac-RSV/G-immune mice following RSV challenge, not significantly different to those in the PBS control group. Several evidences suggest that IL-17 negatively regulates Th2 cytokine production (39,40). Thus, the induction of Th1/Th17 responses by Bac-RSV/G vaccination may have attenuated the development of Th2 responses. Furthermore, IL-17 may play certain roles in immune defense in the lungs, including augmentation of mucosal immunity by delivery of IgA and IgM into the airway lumen (41). Though the exact mechanism by which the Th17 responses are induced by Bac-RSV/G vaccination is not clear, the G-specific Th17 response may be associated with clearance of RSV from the lungs. Further studies will be needed to elucidate the exact role of Th17 immunity in RSV infection.
In conclusion, our present study demonstrates that baculovirus displaying RSV G protein, Bac-RSV/G, induces antigen-specific humoral and cellular immunity, and provides protection against RSV challenge without vaccine-induced immunopathology. Thus, our study provides strong evidence that Bac-RSV/G vaccine could be further developed as a mucosal RSV vaccine.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2010-0021151).
We wish to acknowledge technical support of Hoon-Ji Choi in the completion of this work.
Footnotes
The authors have no financial conflict of interest.
References
- 1.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson RF. Impact of respiratory syncytial virus in the United States. Am J Health Syst Pharm. 2008;65(23 Suppl 8):S3–S6. doi: 10.2146/ajhp080438. [DOI] [PubMed] [Google Scholar]
- 4.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 5.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 6.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 7.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 8.Tebbey PW, Hagen M, Hancock GE. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–2357. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blissard GW, Rohrmann GF. Baculovirus diversity and molecular biology. Annu Rev Entomol. 1990;35:127–155. doi: 10.1146/annurev.en.35.010190.001015. [DOI] [PubMed] [Google Scholar]
- 11.Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors: a laboratory manual. Oxford (UK): Oxford University Press; 1992. [Google Scholar]
- 13.Lee MJ, Jin YH, Kim K, Choi Y, Kim HC, Park S. Expression of hepatitis B virus x protein in hepatocytes suppresses CD8 T cell activity. Immune Netw. 2010;10:126–134. doi: 10.4110/in.2010.10.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai B, Lu X, Meng J, Hu Q, Mao P, Lu B, Chen Z, Yuan Z, Wang H. Vaccination of mice with recombinant baculovirus expressing spike or nucleocapsid protein of SARS-like coronavirus generates humoral and cellular immune responses. Mol Immunol. 2008;45:868–875. doi: 10.1016/j.molimm.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss R, Hüser A, Ni S, Tuve S, Kiviat N, Sow PS, Hofmann C, Lieber A. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against plasmodium falciparum circumsporozoite protein. Mol Ther. 2007;15:193–202. doi: 10.1038/sj.mt.6300008. [DOI] [PubMed] [Google Scholar]
- 16.Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y, Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- 17.Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- 18.Pieroni L, Maione D, La Monica N. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum Gene Ther. 2001;12:871–881. doi: 10.1089/104303401750195845. [DOI] [PubMed] [Google Scholar]
- 19.Facciabene A, Aurisicchio L, La Monica N. Baculovirus vectors elicit antigen-specific immune responses in mice. J Virol. 2004;78:8663–8672. doi: 10.1128/JVI.78.16.8663-8672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SH, Chang J, Yang SH, Kim HJ, Kwak HH, Kim BM, Lee SH. Enhancement of antigen-specific antibody and CD8(+) T cell responses by codelivery of IL-12-encapsulated microspheres in protein and peptide vaccination. Immune Netw. 2007;7:186–196. [Google Scholar]
- 21.Lee JB, Chang J. CD43 Expression regulated by IL-12 signaling is associated with survival of CD8 T cells. Immune Netw. 2010;10:153–163. doi: 10.4110/in.2010.10.5.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003;293:3–15. doi: 10.1078/1438-4221-00241. [DOI] [PubMed] [Google Scholar]
- 23.Hancock GE, Speelman DJ, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang J. Current progress on development of respiratory syncytial virus vaccine. BMB Rep. 2011;44:232–237. doi: 10.5483/BMBRep.2011.44.4.232. [DOI] [PubMed] [Google Scholar]
- 27.Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YH, Lee LH, Shih WL, Hu YC, Liu HJ. Baculovirus surface display of sigmaC and sigmaB proteins of avian reovirus and immunogenicity of the displayed proteins in a mouse model. Vaccine. 2008;26:6361–6367. doi: 10.1016/j.vaccine.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Kondoh D, Arai E, Matsuoka H, Seki C, Tanaka T, Okada M, Ishii A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003;316:161–170. doi: 10.1016/j.virol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Yang DG, Chung YC, Lai YK, Lai CW, Liu HJ, Hu YC. Avian influenza virus hemagglutinin display on baculovirus envelope: cytoplasmic domain affects virus properties and vaccine potential. Mol Ther. 2007;15:989–996. doi: 10.1038/mt.sj.6300131. [DOI] [PubMed] [Google Scholar]
- 31.Hu YC. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol Sin. 2005;26:405–416. doi: 10.1111/j.1745-7254.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronowski AM, Hilbert DM, Sheehan KC, Garotta G, Schreiber RD. Baculovirus stimulates antiviral effects in mammalian cells. J Virol. 1999;73:9944–9951. doi: 10.1128/jvi.73.12.9944-9951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe T, Kaname Y, Wen X, Tani H, Moriishi K, Uematsu S, Takeuchi O, Ishii KJ, Kawai T, Akira S, Matsuura Y. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J Virol. 2009;83:7629–7640. doi: 10.1128/JVI.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripp RA, Oshansky C, Alvarez R. Cytokines and respiratory syncytial virus infection. Proc Am Thorac Soc. 2005;2:147–149. doi: 10.1513/pats.200502-014AW. [DOI] [PubMed] [Google Scholar]
- 35.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 37.Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, Brutkiewicz RR, Blum JS, Kaplan MH. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol. 2010;185:2089–2098. doi: 10.4049/jimmunol.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, Peebles RS., Jr A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182:5317–5321. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009;182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]