Summary
Integrin adhesion receptors are structurally dynamic proteins that adopt a number of functionally relevant conformations. We have produced a conformation-dependent anti-α5 monoclonal antibody (SNAKA51) that converts α5β1 into a ligand-competent form and promotes fibronectin binding. In adherent fibroblasts, SNAKA51 preferentially bound to integrins in fibrillar adhesions. Clustering of integrins expressing this activation epitope induced directional translocation of α5β1, mimicking fibrillar adhesion formation. Priming of α5β1 by SNAKA51 increased the accumulation of detergent-resistant fibronectin in the extracellular matrix, thus identifying an integrin conformation that promotes matrix assembly. The SNAKA51 epitope was mapped to the calf-1/calf-2 domains. We propose that the action of the antibody causes the legs of the integrin to change conformation and thereby primes the integrin to bind ligand. These findings identify SNAKA51 as the first anti-integrin antibody to selectively recognize a subset of adhesion contacts, and they identify an integrin conformation associated with integrin translocation and fibronectin matrix formation.
Keywords: integrin, fibronectin, conformation, monoclonal antibody, matrix assembly
Introduction
Integrins are heterodimeric cell surface receptors that bind to the extracellular matrix (ECM), and provide a physical link to the intracellular cytoskeleton. Integrin function depends on an ability to modulate receptor structure rapidly, and inactive, primed, and ligand-bound conformations with different affinities for ligand-binding have been characterized (Humphries, 2000; Hynes, 2002; Mould, 1996; Shimaoka et al., 2002). Integrin ligand-binding ability can be controlled both by the binding of cytoplasmic factors that induce conformational changes and by regulated positioning on the cell surface to fav3or high-avidity binding. The mechanisms responsible for transferring this signal through the integrin molecule to the extracellular head region, and for regulating ligand-binding, extracellular matrix formation, and remodelling of the cell-matrix interface, are not well understood. Several conformational changes have been suggested to underpin integrin priming, and it is possible that a series of events occurs during acquisition of ligand competency. The crystal structure of the αvβ3 integrin revealed a bent molecule where the globular head contacted the stalk region (Xiong et al., 2001). Building on this information, a switchblade model for priming was proposed in which divalent cation or ligand occupancy induces a conformational change from the bent to the extended conformation (Takagi et al., 2002). This unbending revealed β subunit activation epitopes and increased ligand-binding affinity (Beglova et al., 2002). Another conformational change associated with integrin priming is the separation of the α and β subunit legs (Kim et al., 2003; Lu et al., 2001; Takagi et al., 2001).
The first integrin crystal structure resolved the atomic details of many of the domains of the heterodimer and confirmed the predicted regions for ligand-binding (Xiong et al., 2001). In addition, conformation-dependent monoclonal antibodies have been valuable for studying the link between receptor shape and activity. The majority of antibodies that modulate the integrin activation state bind to the head region of the integrin (Humphries et al., 2003b). These antibodies allosterically alter the structure of the ligand-binding pocket in the α subunit β propeller and β subunit A-domain through local conformational changes. These local effects can stimulate or inhibit ligand-binding depending on the location of the antibody epitope and the conformation induced. The binding of ligand to the integrin can also affect the expression of certain antibody epitopes. Many of the antibodies that increase ligand-binding or recognize active integrin have ligand-induced binding sites (LIBS) (Bazzoni et al., 1995; Mould et al., 1995b).
Integrins can be localized in different adhesion structures on the cell surface, termed focal complexes, focal adhesions, fibrillar adhesions, and 3D-matrix adhesions. These contacts reflect different stages of interaction of cells with the ECM, and each is formed and disrupted in a dynamic, cyclical manner as cells translocate through sequential recruitment and loss of cytoskeletal and signaling molecules (Geiger et al., 2001; Webb et al., 2004). While focal adhesions provide robust anchorage via transcellular actomyosin-containing stress fibres, fibrillar adhesions are the major sites of fibronectin matrix deposition. Ligated α5β1 integrin molecules translocate centripetally out of focal adhesions generating fibrillar adhesions. This directional movement along the actin cytoskeleton stretches and organizes bound fibronectin into fibrils of the extracellular matrix (Pankov et al., 2000; Zamir et al., 2000).
For integrins to function as vehicles for extracellular matrix deposition, their activity needs to be highly controlled. This control appears to be through conformational modulation (Humphries et al., 2003a; Sims et al., 1991). In this study, we tested the hypothesis that α5β1 integrins associated with fibronectin matrix formation have a particular conformational property. We have identified a unique subpopulation of α5β1 integrins located in fibrillar adhesions that have a specific conformation recognized by a novel anti-α5 antibody. Integrins in this conformation can undergo directional translocation in fibrillar adhesions and promote fibronectin matrix formation.
Materials and methods
Antibodies
The antibodies used were the mouse anti-human integrin β1 antibodies TS2/16 (activating; a gift from Francisco Sanchez-Madrid, Universidad Autonoma de Madrid, Spain), 12G10 (activating) (Mould et al., 1995b), and K20 (nonfunction-modulating, Immunotech); rat anti-human integrin β1 antibodies mAb13 (inhibitory) (Akiyama et al., 1989) and 9EG7 (activating, Pharmingen); rat anti-human integrin α5 mAb16 (inhibitory) (Akiyama et al., 1989) and mAb11 (nonfunction-modulating) (Miyamoto et al., 1995); mouse anti-human integrin α5 antibodies SNAKA52 (inhibitory) and JBS5 (inhibitory, Serotec); mouse anti-human integrin αV L230 (inhibitory, ATCC); and polyclonal rabbit anti-human fibronectin Rb745 (Cukierman et al., 2001).
Production of hybridomas
α5β1 integrin was isolated from placenta by two consecutive affinity purifications using mAb13 and mAb16 columns (Mould et al., 1995a). Hybridomas were produced by selection from a fusion of spleen cells from immunized mice and 653 myeloma cells (Mould et al., 1991). Media from the resulting hybridomas was screened by ELISA, and antibody was purified using protein G-Sepharose.
ELISA
α5β1 or α4β1, diluted to 1 μg/ml in PBS, was used to coat an Immulon-3 assay plate (Dynatech) overnight at 4°C. Wells were blocked with 5% (w/v) BSA in TBS (150 mM NaCl, 25 mM Tris HCl, pH 7.4), and incubated at room temperature for 30 minutes. Culture medium from the hybridomas or antibody was added and incubated at room temperature for 1 hour. Wells were washed 3 times with PBS+, then peroxidase-conjugated anti-mouse immunoglobulin (Dako) in PBS+ was added. Plates were incubated for 30 minutes at room temperature and washed 3 times with PBS+. 0.1% (w/v) 2,2′-azino-bis (3-ethylbenzthiazoline 6-sulphonic acid) (ABTS) in 0.1 M sodium acetate, 0.05 M NaH2PO4, pH 5.0, 0.01% (v/v) H2O2 was added, and absorbance readings were determined at 405 nm on a Dynatec MR4000 plate reader.
K562 cell adhesion to fibronectin
Fibronectin (Miekka et al., 1982), diluted to 2 μg/ml in PBS+, was coated onto an Immulon-2 U-bottom plate overnight at 4°C. The surface was blocked with 10 mg/ml heat-denatured BSA for 1 hr at room temperature. The plate was washed once with PBS+ before addition of DMEM, 25 mM HEPES buffer containing 20 μg/ml antibody or 100 nM phorbol 12-myristate 13-acetate (PMA). K562 cells were added at 2.5×104 cells/well in DMEM, 25 mM HEPES. The plate was incubated for 30 minutes at 37°C, 10% CO2. Medium and unbound cells were removed by inversion of the plate and four PBS+ washes. 0.2% (w/v) crystal violet, 20% (v/v) methanol solution was added and incubated for 20 minutes. The plate was washed twice by immersion in water, dried before addition of 10% (w/v) SDS, and incubated for 20 minutes before reading absorbance at 600 nm on an Emax plate reader (Molecular Devices).
Production of recombinant Fc-tagged integrins
DNA constructs
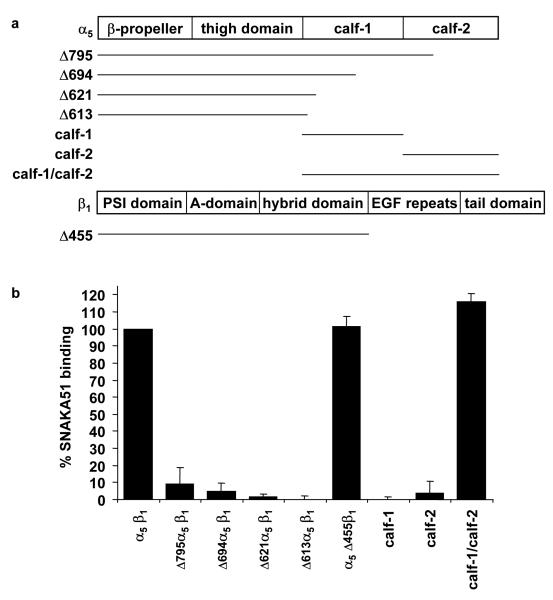
All human α5-Fc and β1-Fc DNA constructs used here have been described previously (Coe et al., 2001). C-terminally truncated versions of α5-Fc [residues 1-613 (Δ613), 1-621 (Δ621), 1-694 (Δ694), 1-795 (Δ795)] and β1-Fc [residues 1-455 (Δ455)] were produced. The β1-Fc ADMIDAS mutant (D138A) constructs used were generated as described by Mould et al. (Mould et al., 2003a). For production of α5-Fc calf domain constructs, DNA was amplified by PCR, inserting a 5′ Hind III and a 3′ Sal I restriction site, and ligated into pEE12.2hFc. The leader sequence of a murine antibody (Kabat et al., 1987) was also incorporated at the 5′ end of each construct to facilitate protein secretion. Calf-1 contained α5 residues 606-749, calf-2 contained residues 746-951, and calf-1/calf-2 contained residues 606-951.
Expression of α5β1-Fc proteins
CHOL731H cells (Cockett et al., 1991) were grown to ~95% confluence, and 20 μg of α5-Fc and β1-Fc DNA (or α5-Fc calf domain construct alone) was used to transfect cells using LipofectAMINE 2000™ reagent (Invitrogen) according to the manufacturer’s instructions. After 4 days, culture supernatant was harvested by centrifugation at 1000 × g for 5 minutes.
Solid-phase assays
Placental α5β1 integrin, diluted to 1 μg/ml in PBS+, was used to coat an Immulon-3 assay plate (Dynatech) overnight at 4°C, or anti-β1 integrin (K20) 2 μg/ml was coated overnight at 4°C. Wells were blocked with 5% (w/v) BSA in TBS for 2 hours. K20 coated plates were used to tether 0.5 μg/ml placental integrin incubated for 2 hours at room temperature. For capturing recombinant Fc-tagged integrin, goat anti-human γ1 Fc antibody (Jackson ImmunoResearch Laboratories), diluted to 2.6 μg/ml in PBS+, was coated into a immunoassay plate (Costar) overnight at 4°C. Wells were blocked with 5% (w/v) BSA in TBS for 2 hours. Transfection culture supernatants containing integrin-Fc protein were incubated in the wells for 1 hour, and wells were subsequently washed three times with TBS, 1 mM MnCl2, 1 mg/ml BSA (buffer A). Ligand and antibodies (10 μg/ml) were added in combination in buffer A. Biotinylated FnIII (6-10) was added at 0.1 μg/ml and unlabelled FnIII (6-10) at 20 μg/ml (Danen et al., 1995). Cations or EDTA were added at 2 mM. The plate was incubated at 30°C for 3 hours. Wells were washed three times with buffer A, then ExtrAvidin Peroxidase (Sigma) in buffer A was added and incubated for 20 minutes. Alternatively, peroxidase-conjugated anti-mouse or anti-rat immunoglobulin (Dako) was used and incubated for 1 hour. The plate was washed four times with buffer A, ABTS substrate was added, and absorbance was determined at 405 nm.
Indirect immunofluorescence staining
Primary human foreskin fibroblasts (hFF) were a gift from S. Yamada (NIDCR, NIH) and were used at passages 9-18. Cells were plated on 12 mm glass coverslips in DMEM with 10% FCS in a 24-well dish at 5000 cells/well and cultured overnight. The cells were fixed for 20 minutes with 4% (w/v) paraformaldehyde, 5% (w/v) sucrose in PBS. Cells were stained with anti-fibronectin Rb745, Alexa 594-conjugated anti-αV integrin L230, Alexa 488-conjugated anti-α5 antibodies SNAKA51, SNAKA52, or mAb11, and Cy2-conjugated anti-rat IgG and AMCA-conjugated anti-rabbit IgG secondary antibodies (Jackson ImmunoResearch Laboratories). Stained samples were mounted in GEL/MOUNT™ (Biomeda Corp.) containing 1 mg/ml 1,4-phenylendiamine (Fluka) to reduce photobleaching. Immunofluorescence images were obtained with a 63x/1.40 oil objective on a Zeiss Axiophot microscope equipped with a Photometrix CH 350 cooled CCD camera. Digital images and image overlays were obtained using MetaMorph 4.6 software (Universal Imaging Corp.).
Epitope mapping (sandwich ELISA)
α5β1-Fc was captured in wells from transfection supernatants via anti-human γ1 Fc antibody as detailed above. Wells were washed three times with buffer A. SNAKA51 was diluted to 10 μg/ml in buffer A and added to the wells for 2 hours. Wells were washed three times, and peroxidase-conjugated anti-mouse antibody (Jackson ImmunoResearch Laboratories), diluted in buffer A, was added for 30 minutes. Wells were washed four times with buffer A, incubated with ABTS substrate, and the absorbance was determined at 405 nm.
Antibody clustering and chasing
hFF were plated on glass coverslips in DMEM, 1% (v/v) fibronectin-depleted FCS containing 25 μg/ml cycloheximide. After overnight incubation, cells were labelled in vivo by incubation in the same medium containing 10 μg/ml anti-α5 antibody for 20 minutes. After two washes with warm medium, cells were incubated with 2 μg/ml goat anti-mouse IgG or goat anti-rat IgG in the same medium for 30 minutes (clustering and chasing period). All incubations were at 37°C in a humidified chamber with 10% (v/v) CO2. At the end of the labelling and chasing periods, samples were fixed and antibody-containing clusters were visualized with CY2-conjugated donkey anti-goat IgG and unclustered antibody with CY2-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories). Focal adhesions were stained with Alexa-594-conjugated anti-αV antibody L230. Samples were mounted and imaged as for immunofluorescence staining.
Fibronectin incorporation into the extracellular matrix
Human salivary gland (HSG) cells were plated into a 6-well dish at 1×105 cells/well and cultured overnight. The medium was changed, and cells were incubated a further 24 hours. The cells were then treated with a range of concentrations of biotinylated fibronectin or 10 μg/ml biotinylated fibronectin with or without 25 μg/ml antibody or a range of antibody concentration and incubated overnight or for different time periods. The cells were extracted with 20 mM Tris HCl, pH 8.5, 1% (w/v) deoxycholic acid, 2 mM N-ethylmaleimide, 2 mM iodoacetic acid, 2 mM EDTA, 2 mM PMSF (DOC buffer), extruded through a 23-gauge needle five times and centrifuged at 20,000 g for 20 minutes at 4°C. The pellet was washed once with DOC buffer and prepared for SDS polyacrylamide electrophoresis. Samples were resolved on 4-12% Tris-glycine gradient gels (Novex) and electrotransfered onto nitrocellulose membranes. The membranes were blocked with 5% (w/v) nonfat milk in TBS, 0.05% (w/v) Tween-20 for 30 minutes, immunoblotted with streptavidin peroxidase (Boehringer Mannheim), together with anti-pan cytokeratin (Sigma), using secondary peroxidase-conjugated anti-mouse immunoglobulin (Amersham Pharmacia Biotech). Immunoblots were visualized using the ECL system and Hyperfilm X-ray film (Amersham Pharmacia Biotech).
Results
Identification of a novel antibody that primes α5β1 ligand-binding
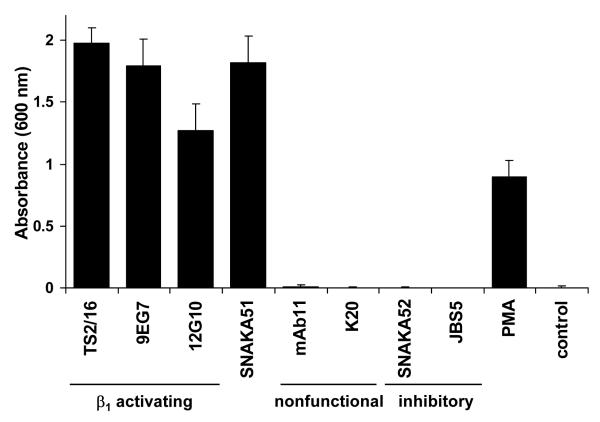
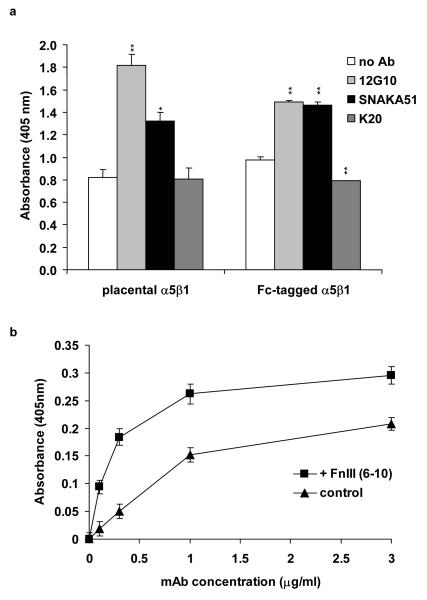
Monoclonal antibodies have emerged as a valuable tool for studying the various conformational classes adopted by integrins. To develop additional reagents for probing integrin conformation, we generated antibodies directed against the human placental α5β1 integrin and identified their subunit specificity by ELISA. Two mice were immunised with α5β1 integrin from which approximately 500 colonies were tested, from the cell fusion products and 12 positive colonies were cloned. An ELISA assay using immobilised α4β1 and α5β1 integrin was used to identify subunit specificity. Antibodies were then tested in a K562 cell adhesion assay to assess their ability to stimulate the α5β1 integrin to bind fibronectin. As previously established, K562-expressed α5β1 integrin was predominantly in an inactive state, and addition of stimulatory anti-β1 integrin antibodies (TS2/16, 9EG7, and 12G10) or PMA was required to promote cell adhesion (Fig. 1). Nonfunction-modulating anti-α5 (mAb11) and anti-β1 (K20) as well as inhibitory anti-α5 (JBS5 and SNAKA52) antibodies did not support cell adhesion. A new anti-α5 antibody, SNAKA51 with no immunoreactivity against α4β1, promoted cell adhesion to a similar extent to TS2/16, 9EG7, and 12G10, suggesting that this antibody is also able to stimulate the α5β1 integrin (Fig. 1). From this fusion, SNAKA51 was the only antibody produced that promoted cell adhesion. To confirm the ability of SNAKA51 to stimulate α5β1 integrin function in a biochemical system, solid-phase ligand-binding assays were employed. A biotinylated fibronectin fragment containing type III repeats 6 to 10 [FnIII (6-10)] was used as the ligand. This fragment contains both the RGD and synergy sequence required for α5β1 integrin binding and was used at a concentration at which an antibody effect on ligand binding would be detectable (Mould et al., 1997). SNAKA51 was found to increase ligand-binding to both placental and recombinant α5β1 integrin above the no-antibody control and the control anti-β1 antibody (K20), as did the activating anti-β1 antibody (12G10) (Fig. 2a). This increase in ligand-binding by SNAKA51 was antibody dose-dependent (data not shown).
Figure 1. K562 cell adhesion to fibronectin is promoted by SNAKA51 and other stimulatory anti-β1 antibodies.
K562 cells were allowed to attach to a fibronectin-coated surface (2 μg/ml) in the presence of the indicated anti-integrin antibodies (10 μg/ml), PMA (100 nM), or no antibody (control). Unattached cells were removed, and remaining cells were fixed and stained with crystal violet. Cell attachment was quantified by absorbance measured at 600 nm.
Figure 2. SNAKA51 promotes ligand-binding to α5β1 integrin, and SNAKA51 binding to α5β1 integrin is increased in the presence of ligand.
Using a solid-phase ligand-binding assay, the binding of biotinylated FnIII (6-10) (0.1 μg/ml) to α5β1 integrin was measured. a) Binding of FnIII (6-10) to directly coated placental α5β1 integrin and anti-Fc captured recombinant integrin in the presence and absence of SNAKA51, 12G10, or K20. Statistical analysis was performed using a 2-tailed t-test in comparison to the no antibody control. * p<0.005, **p<0.0005. b) Dose-dependent binding of biotinylated SNAKA51 to K20 tethered placental α5β1 integrin in the presence or absence of FnIII (6-10) (20 μg/ml).
To determine whether the epitope recognized by SNAKA51 was affected by conformational changes in the integrin induced by ligand-binding or cations, the amount of antibody bound to the α5β1 integrin was measured in the presence and absence of the FnIII (6-10) fragment or in different cation-containing buffers. The addition of FnIII (6-10) increased the amount of SNAKA51 binding to α5β1 over a range of antibody concentrations, for example by nearly 5-fold at an antibody concentration of 0.1 μg/ml (Fig. 2b), indicating that the SNAKA51 epitope is a ligand-induced binding site (LIBS). SNAKA51 binding was increased by the addition of manganese, but not affected by calcium, magnesium or EDTA (data not shown). Using an ELISA measuring the amount of antibody bound to α5β1 integrin, SNAKA51 showed significantly reduced binding to α5β1 integrin, for example 24% at 1 μg/ml, in comparison to both JBS5 and SNAKA52, which both bound equally well to the integrin, and 77% in comparison to 12G10 (data not shown).
These findings indicated that SNAKA51 increases α5β1 ligand-binding and recognizes a subpopulation of the purified integrin through binding to a cation-independent LIBS epitope.
SNAKA51 specifically stains fibrillar adhesions
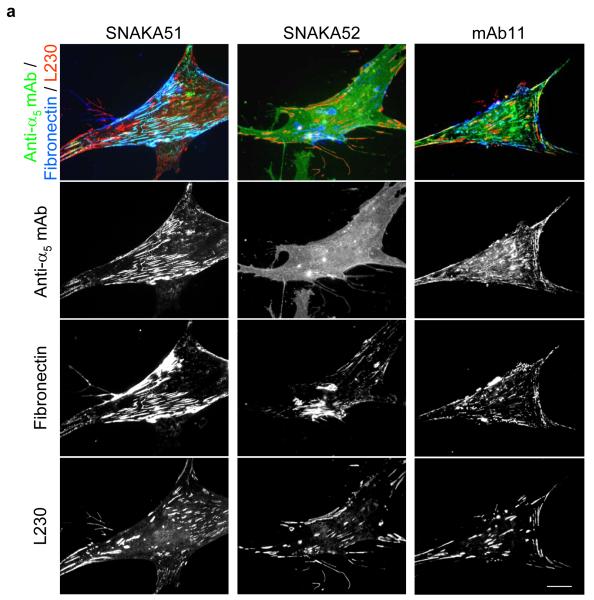
Since the SNAKA51 antibody appears to recognize a subpopulation of the α5β1 integrin, we examined its localization on the cell surface. Human foreskin fibroblasts were fixed and stained with a series of anti-α5 antibodies, anti-fibronectin (Rb745), and anti-αV integrin antibody (L230), the latter as a marker for focal adhesions. SNAKA51 colocalized with fibronectin in fibrillar adhesions (Fig. 3a, left panels). Inhibitory α5 antibody SNAKA52 gave diffuse staining across the whole cell surface (Fig. 3a, center panels), which was similar to other inhibitory α5 or β1 integrin antibodies (JBS5 and mAb13, data not shown). The nonfunction-modulating α5 antibody mAb11 stained a combination of these two distributions, which included both integrins that were distributed across the cell surface and those that localized in adhesion complexes (Fig. 3a, right panels). These findings indicate that the subpopulation of integrin recognized by SNAKA51 is specifically localized to fibrillar adhesions and is probably in either a ligand-bound or ligand-competent conformation. This population is distinct from those recognized by both the SNAKA52 and mAb11 antibodies.
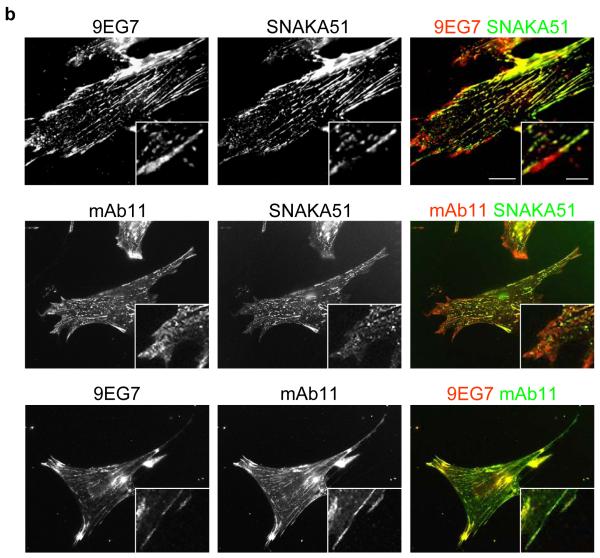
Figure 3. SNAKA51 only colocalizes with fibronectin fibers and primed β1 integrin in fibrillar adhesions.
Human fibroblasts were fixed and stained using indirect immunofluorescence for: a) α5 integrin [SNAKA51 (left panels) or SNAKA52 (center panels) or mAb11 (right panels), green], fibronectin (blue) and αV integrin as a focal adhesion marker (L230, red); or b) Top panel β1 integrin in a primed conformation (9EG7, red) and α5 integrin (SNAKA51, green), middle panel total β1 integrin (mAb11 red) and α5 integrin (SNAKA51, green), bottom panel β1 integrin in a primed conformation (9EG7, red) and total β1 integrin (mAb11 green). Bar 20 μm, insert bar 5μm.
Ligand-competent β1 integrin, detected with the 9EG7 antibody, has been shown to translocate out from focal adhesions into fibrillar adhesions, suggesting that it detects α5β1 (Pankov et al., 2000). Since this integrin localizes to focal adhesions before translocating out into fibrillar adhesions, we compared the localization of SNAKA51-positive with 9EG7-positive integrins. 9EG7 stained integrins throughout the focal adhesions and out into the fibrillar adhesions, whereas SNAKA51 labelled integrins that were at the distal edge of the focal adhesion away from the cell perimeter and along the fibrillar adhesions (Fig. 3b). Similar results to the 9EG7 staining were obtained with 12G10 (data not shown). The integrin in the focal adhesion staining was shown to be α5β1 since mAb11 and 9EG7 showed complete colocalization. These findings indicate that the α5 subunit undergoes a conformational change during the transition from focal adhesions to fibrillar adhesions. The SNAKA51 antibody therefore identifies a unique subpopulation of α5β1 integrin compared to other anti-integrin antibodies.
The α5β1 integrin priming signal is transduced through its legs to the β1 A-domain
Elucidating the nature of the α5β1 integrin conformation in fibrillar adhesions should help elucidate how the bidirectional control of matrix deposition and intracellular signalling are coordinated. To understand how the SNAKA51 antibody modulates α5β1 function, we initially mapped its epitope. Recombinant truncated α5β1 integrins were expressed as Fc-tagged proteins (Coe et al., 2001; Ridgway et al., 1996) (Fig. 4a). SNAKA51 recognized recombinant integrin containing full-length extracellular α5 and β1 subunits. However, any deletion from the carboxyl-terminus of the α5 construct, including truncation into the calf-2 domain, abolished binding (Fig. 4b, Δ795, Δ694, Δ621, Δ613). An integrin containing a truncated β1 leg (Δ455β1), together with full-length α5, bound SNAKA51 to a similar degree as the full-length protein, confirming the epitope location in the α5 subunit leg (Fig. 4b). The calf-1 and -2 domains were expressed as Fc-tagged proteins, either together as one construct (residues 606-951) or as individual domains (calf-1 residues 606-749 or calf-2 residues 746-951). The SNAKA51 antibody only recognized the construct containing both calf-1 and calf-2 domains and did not bind to either of the individual domains (Fig. 4b). The epitope for SNAKA51 is therefore within the calf domains; it is conceivable that the calf domains require each other for their correct folding and that the antibody epitope might be in either calf or at the calf-1/calf-2 junction.
Figure 4. The SNAKA51 epitope maps to the calf domains of the α5 subunit.
a) Diagrammatic representation of the extracellular domains of the α5 and β1 integrin subunits indicating the position of truncations for the various recombinant constructs used in this study. b) The SNAKA51 binding site on the α5 integrin subunit was mapped using a sandwich ELISA of Fc-tagged truncated or individual domains of recombinant integrin. The Fc-tagged protein was specifically captured using anti-Fc antibodies, and then binding of SNAKA51 to the integrin was measured using enzyme-conjugated anti-mouse secondary antibody.
Since the SNAKA51 epitope maps to the α5 leg region of the integrin, the conformational change that it triggers could either be transduced directly up the α5 subunit or it could cross to the β1 leg and then up to the ligand-binding site in the integrin head region. The α5β1 Fc-tagged integrin has a high constitutive level of ligand-binding (Fig. 5), which makes it difficult to observe any further activation. Therefore, we employed a mutant integrin containing the point mutation D138A in the ADMIDAS cation-binding site in the β1 subunit, which we have shown recently to be constitutively inactive. The ligand-binding ability of this mutant can be rescued with the stimulatory anti-β1 antibody 12G10, demonstrating that it is competent to bind ligand (Mould et al., 2003a). Mutant integrin was produced in both the full-length β1 and Δ455β1 constructs. SNAKA51 and 12G10 promoted ligand-binding by the full-length ADMIDAS mutant, with 12G10 having a more potent effect (Fig. 5, α5β1 D138A). The greater effect of 12G10 is probably due to its epitope being spatially close to the ligand binding pocket and SNAKA51 having to induce a long distance allosteric effect. In the truncated β1 subunit construct, SNAKA51 stimulation was abolished, while 12G10 was still able to activate the integrin (α5 Δ455β1 D138A). These results indicate that the priming signal from the SNAKA51 epitope is transduced between the integrin legs from α5 to β1 and then up to the β A-domain, where conformational changes promote the integrin to bind ligand. In agreement with this conclusion, 12G10 and SNAKA51 had a reciprocal effect, with the binding of one increasing the epitope expression of the other (data not shown).
Figure 5. Induction of priming by SNAKA51 is dependent on the β1 leg and modulates β1 A-domain ligand-binding ability.
Binding of biotinylated FnIII (6-10) to either wild-type or ADMIDAS mutant (D138A) recombinant α5β1 integrin, containing either a full-length or truncated (Δ455) β1 subunit, was measured in the presence or absence of anti-integrin antibodies.
Clustering of active α5β1 leads to integrin translocation
The α5β1 integrin has been shown to translocate from focal adhesions across the cell surface and form fibrillar adhesions (Pankov et al., 2000). Because the SNAKA51 antibody recognizes integrin in a specific conformation that is localized to fibrillar adhesions, we hypothesized that antibody addition might induce formation of these adhesion structures. Human fibroblasts were treated with cycloheximide and cultured in the absence of fibronectin to prevent any ligand-related α5β1 integrin translocation. These cells were treated with anti-α5 antibodies, and then the antibodies were clustered with secondary antibody. Clustered integrin was then chased to observe integrin movement compared to unclustered antibody, fixing either after the initial labelling or after antibody-chasing without clustering. SNAKA51 initially labelled the entire cell surface, but after clustering it translocated along the cell membrane away from anti-αV (L230)-labelled focal adhesions and formed linear fibrillar adhesion-like structures along the cell surface (Fig. 6a). In contrast, the inhibitory (SNAKA52) and nonfunction-modulating (mAb11) antibodies remained unorganized on the cell surface even after clustering (Fig. 6b, c). Cells treated with anti-α5 antibody and chased in the absence of the clustering secondary antibody did not exhibit the translocation away from the focal adhesions observed for clustered SNAKA51. Consequently clustering of the SNAKA51-positive subpopulation, even in the absence of ligand, led to directional integrin translocation. These results indicate a requirement for integrins to adopt a specific activated conformation for translocation to occur.
Figure 6. Clustering of SNAKA51-bound α5 integrin induces integrin translocation out of focal adhesions and across the cell surface.
Fibroblasts were plated on glass coverslips and incubated overnight in fibronectin-depleted medium with cycloheximide. Cells were labelled with antibody for 20 minutes, using SNAKA51 (a), SNAKA52 (b), or mAb11 (c). Unbound antibody was rinsed away, and the cells were either fixed (‘no chasing’), or the cell-bound antibody was clustered with goat anti-mouse or anti-rat IgG and incubated for a further 30 minutes (‘30’ chasing’) before fixation. For unclustered α5 integrin, cells were stained with anti-mouse or anti-rat IgG (green). For clustered α5 integrin, cells were stained with anti-goat IgG (green). In addition, all samples were stained with anti-αV antibody L230 (red) as a focal adhesion marker. Bar 20 μm.
Integrin priming by SNAKA51 increases the incorporation of fibronectin into the matrix
Translocation of the α5β1 integrin is the driving force for fibronectin fibrillogenesis (Pankov et al., 2000). The translocation ability of the clustered SNAKA51-positive α5β1 integrin conformer into fibrillar adhesion-like structures and its ability to bind fibronectin suggested that the integrin needs to be in this conformation when it drives fibronectin fibrillogenesis. To test this hypothesis, we measured the ability of cells treated with anti-α5 antibodies to produce fibronectin matrix. Fibronectin incorporated into the extracellular matrix can be characterized separately from soluble and non-matrix associated fibronectin by the use of the detergent deoxycholate. Human salivary gland (HSG) cells (which do not deposit an extensive fibronectin matrix) were treated with a range of concentrations of biotinylated fibronectin. The cells were then extracted with deoxycholate-containing buffer, and the insoluble fraction was analyzed by Western blotting. Biotinylated fibronectin was detected using peroxidase-conjugated avidin and compared to the amount of cytokeratin in the same sample to control for sample loading. Little or no fibronectin was incorporated into the matrix up to a concentration of 5 μg/ml exogenous fibronectin (Figure 7a). A concentration of 10 μg/ml biotinylated fibronectin was used for further assays. The incorporation of fibronectin into the matrix was measured over time, or in the presence of different integrin antibodies. Fibronectin was easily detectable in the insoluble fraction after 3 hours (Figure 7b). SNAKA51 antibody substantially increased the incorporation of biotinylated fibronectin into the insoluble fraction of the HSG cells compared to the no antibody treated control (Fig. 7c). The activating β1 integrin antibody TS2/16 and inhibitory α5 integrin antibody SNAKA52 were used as positive and negative controls, respectively. Fibronectin incorporation was suppressed by the inhibitory α5 antibody (SNAKA52). As little as 1 μg/ml of SNAKA51 was capable of increasing fibronectin incorporation into the matrix, and the level detected was not increased by addition of more antibody (Figure 7d). Therefore, SNAKA51 has the ability to activate the α5β1 integrin and promote directional integrin translocation associated with the incorporation of more fibronectin into the matrix.
Figure 7. SNAKA51 promotes fibronectin incorporation into the deoxycholate-insoluble matrix fraction.
Human salivary gland cells (HSG) were treated overnight with a) a range of concentrations of biotinylated fibronectin, or biotinylated fibronectin (10 μg/ml) and b) harvested at different time points, or c) with anti-integrin antibodies (25 μg/ml), or d) a range of concentrations of anti-α5 (SNAKA51). The cells were extracted with deoxycholate buffer, and the insoluble matrix fraction was collected and Western-blotted. Upper panel indicates biotinylated fibronectin incorporation into the insoluble matrix fraction. Lower panel indicates cytokeratin as internal loading controls for the HSG cells.
Discussion
In this study, we have tested the hypothesis that a specific α5β1 integrin conformation is associated with the process of fibronectin matrix formation. We produced monoclonal antibodies directed against the α5β1 integrin and identified an anti-α5 antibody (SNAKA51) that stimulated α5β1 and promoted cell adhesion and ligand-binding. This antibody had a manganese-sensitive LIBS epitope and bound to a subpopulation of integrin located in fibrillar adhesions. The molecular stimulation initiated by this antibody appears to be transferred from its epitope on the α5 calf domains, through the β1 leg, and up to the β A-domain, thereby increasing ligand-binding. By clustering the integrin via this activation epitope, directional translocation of the integrin away from the focal adhesions was stimulated, thereby mimicking normal α5β1 integrin translocation during fibrillar adhesion formation. Induction of the SNAKA51-positive primed integrin conformation increased the incorporation of soluble fibronectin into matrix by cells. Our results demonstrate that a specific α5β1 integrin conformer is required for translocation and formation of fibrillar adhesions and fibronectin matrix.
We generated and characterized an anti-α5 antibody (SNAKA51) that increased K562 adhesion to fibronectin. SNAKA51 promoted cell adhesion and in vitro ligand-binding to a similar extent as activating β1 antibodies. Thus, SNAKA51 is a member of a rare group of activating integrin antibodies that bind to the α subunit of non-A domain-containing integrins. Several stimulatory anti-αIIb activating antibodies have been described that bind either to the β-propeller domain (PT25-2 and D33C) (Gulino et al., 1990; Puzon-McLaughlin et al., 2000; Tokuhira et al., 1996) or to a site close to the heavy/light chain border in the membrane proximal region (PMI-1) (Calvete et al., 1991; Loftus et al., 1987). An activating αL antibody, NKI-L16, binds to the bottom of the thigh domain (Huang and Springer, 1995; Keizer et al., 1988). Another activating α5 antibody has been identified, but its binding site has not been described in any detail (Chiarugi et al., 2003). The wide range of domains containing the activating antibody epitopes is consistent with dynamic structural changes taking place throughout the molecule during priming.
Using modified ELISA assays, we found that SNAKA51 has a manganese-sensitive LIBS epitope which was mapped to the calf domains of the α5 subunit leg region (Fig. 8a). This epitope is distant from the ligand-binding site in the integrin head region, suggesting a long-distance conformational transduction of the stimulatory signal from this site to the ligand-binding pocket. SNAKA51 rescued ligand-binding to a deactivated β1 ADMIDAS mutant, indicating that the antibody is able to induce a conformational change in the β1 A-domain from its remote epitope. The conformational changes in the β1 A-domain required for integrin priming have been identified as shifts in the α1 and α7 helices (Luo et al., 2003; Mould et al., 2003b). Truncation of the β1 leg (Δ455β1) abolished the stimulatory activity of SNAKA51, demonstrating that the signal from the α5 calf domain is dependent on the β1 leg being present and suggests a movement between the two subunit legs. We propose that the SNAKA51 signal is transferred through the β1 leg by movement apart of the two subunits. This separation could be due to a steric effect of the antibody pushing the legs apart and may represent the upstream event normally caused by binding of cytoplasmic factors (Calderwood, 2004; Hughes et al., 1996). The integrin crystal structure reveals contacts between the calf-2 domain of the α subunit and the β subunit EGF-4 and βTD domains (Xiong et al., 2001). The leg movement we propose to be induced by SNAKA51 could break these inter-subunit contacts and cause bending in the flexible region between the EGF-4 and βTD domains of the β subunit, leading to integrin priming. These data suggest that this novel antibody binds only to integrins in a certain conformation/activity state, which occurs via the dynamic equilibrium of integrin conformers, the integrin-associated cytoplasmic complex, or ligation.
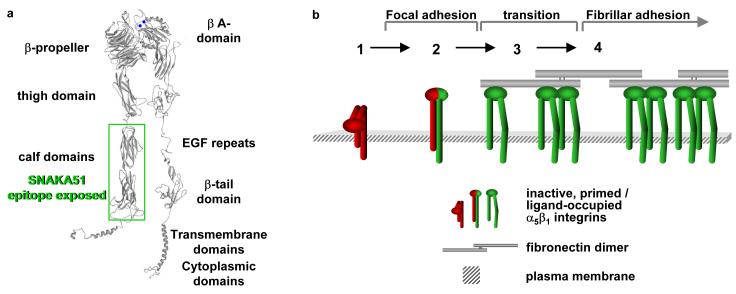
Figure 8. Model of transitions of α5β1 integrin for fibrillar adhesion formation and fibronectin fibrillogenesis.
a) α5β1 integrin predicted domain structure, with a green box (calf domains) indicating the region that contains the SNAKA51 epitope. Blue balls represent cations bound to the MIDAS and ADMIDAS sites in the β A-domain.
- Inactive integrin is diffusely located on the cell surface.
- α5β1 located in focal adhesions expresses epitopes reporting a primed β1 conformation (e.g. 9EG7). These integrins may or may not be fully bound by ligand.
- Integrin located at the distal edge of focal adhesions has additional SNAKA51 epitope expression. Clustering of this integrin promotes translocation.
- Ligated-clustered integrin translocates out of focal adhesions along the actin cytoskeleton, stretching extracellular fibronectin fibrils and driving fibrillogenesis.
The localization of SNAKA51 on fibroblasts demonstrated that its epitope is exposed on a specific population of α5β1 in a ligand-bound or ligand-competent conformation in fibrillar adhesions. This SNAKA51-positive α5β1 was located at the edge of focal adhesions where there is a concentration of fibronectin molecules that marks the initiation point for formation of fibrillar adhesions and fibronectin fibrillogenesis. The SNAKA51 binding in focal adhesions did not fully colocalize with 9EG7 or mAb11, suggesting that several intermediate conformations exist for the α5β1 integrin, and epitope expression for 9EG7 and SNAKA51 binding are indicators of different conformational states.
The clustered, SNAKA51-labelled integrin translocated away from focal adhesions in a directional manner similar to that observed in fibrillar adhesion formation (Pankov et al., 2000). This movement only occurred with clustered SNAKA51, demonstrating that a specific conformation and multiple α5β1 integrins are required for translocation. The ability to stimulate translocation was not specific to SNAKA51, as 9EG7 triggered the same effect (data not shown). Regardless, we were able to induce and mimic α5β1 integrin translocation even in the absence of ligand, indicating that clustering of a specific integrin conformation is all that is required for this function. These results are consistent with the driving force behind fibrillar adhesion formation being generated from the cytoplasmic protein complex associated with the actin cytoskeleton. This force initiates stretching of the fibronectin molecules required to expose cryptic sites used for fibrillogenesis (Pankov and Yamada, 2002; Zhong et al., 1998). In addition, the fibronectin dimer has four binding sites for α5β1 and so naturally clusters this integrin (see (Pankov and Yamada, 2002)). Clustering may be required to build a large enough cytoplasmic complex to connect to the actin cytoskeleton and drive translocation.
Different functional antibodies affect the activation state of the integrin, making it possible to alter the integrin affinity. We used fibronectin incorporation into the extracellular matrix as a readout for the priming of α5β1 ligand-binding. The HSG cells do not normally deposit an extensive fibronectin matrix. Inducing the SNAKA51-specific α5β1 integrin conformation on HSG cells promoted soluble fibronectin incorporation into the matrix, supporting the conclusion that α5β1 integrin adopts this conformation when driving fibronectin fibrillogenesis.
From current knowledge and the results obtained in this study, we propose a model in which the α5β1 integrin, which is initially diffuse on the cell surface (state 1, Fig. 8b) and perhaps in a bent conformation, is recruited to the focal adhesion where a conformational event occurs, such as unbending, that results in a primed β1 subunit (state 2). This integrin moves to the distal region of the focal adhesion where either through changes in the cytoplasmic complex or ligation it undergoes a conformational change in the α5 subunit in which the legs of the integrin are separated and the SNAKA51 epitope is revealed (state 3). This fully primed, or ligand-occupied, and clustered integrin is then actively transported out into fibrillar adhesions and drives fibronectin fibrillogenesis (state 4).
The process of fibronectin matrix formation is important for many cellular functions that involve either cellular sensing of the surrounding environment or using the matrix as a structural support. The α5β1 integrin is the part of the complex molecular motor that crosses the cell membrane barrier to drive fibronectin fibrillogenesis. We have elucidated a conformational path through the integrin structure used for integrin priming. The conformation of both α5 and β1 integrin subunits are purposely controlled, and a specific conformation is required for fibronectin matrix formation.
Acknowledgments
These studies were supported by grants from the Wellcome Trust (to MJH).
References
- Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–75. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–7. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol. 2002;9:282–7. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–66. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Arias J, Alvarez MV, Lopez MM, Henschen A, Gonzalez-Rodriguez J. Further studies on the topography of human platelet glycoprotein IIb. Localization of monoclonal antibody epitopes and the putative glycoprotein IIa- and fibrinogen-binding regions. Biochem J. 1991;273(Pt 3):767–75. doi: 10.1042/bj2730767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–44. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockett MI, Bebbington CR, Yarranton GT. The use of engineered E1A genes to transactivate the hCMV-MIE promoter in permanent CHO cell lines. Nucleic Acids Res. 1991;19:319–25. doi: 10.1093/nar/19.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe AP, Askari JA, Kline AD, Robinson MK, Kirby H, Stephens PE, Humphries MJ. Generation of a minimal alpha5beta1 integrin-Fc fragment. J Biol Chem. 2001;276:35854–66. doi: 10.1074/jbc.M103639200. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Danen EH, Aota S, van Kraats AA, Yamada KM, Ruiter DJ, van Muijen GN. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin alpha 5 beta 1. J Biol Chem. 1995;270:21612–8. doi: 10.1074/jbc.270.37.21612. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nature Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gulino D, Ryckewaert JJ, Andrieux A, Rabiet MJ, Marguerie G. Identification of a monoclonal antibody against platelet GPIIb that interacts with a calcium-binding site and induces aggregation. J Biol Chem. 1990;265:9575–81. [PubMed] [Google Scholar]
- Huang C, Springer TA. A binding interface on the I domain of lymphocyte function-associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1) J Biol Chem. 1995;270:19008–16. doi: 10.1074/jbc.270.32.19008. [DOI] [PubMed] [Google Scholar]
- Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem. 1996;271:6571–4. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–39. [PubMed] [Google Scholar]
- Humphries MJ, McEwan PA, Barton SJ, Buckley PA, Bella J, Paul Mould A. Integrin structure: heady advances in ligand binding, but activation still makes the knees wobble. Trends Biochem Sci. 2003a;28:313–20. doi: 10.1016/s0968-0004(03)00112-9. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Symonds EJ, Mould AP. Mapping functional residues onto integrin crystal structures. Curr Opin Struct Biol. 2003b;13:236–43. doi: 10.1016/s0959-440x(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kabat EA, Wu TT, Reid-Miller M, Perry HM, Gottesman KS. Sequences of proteins of immunological interest. 4th edition US Dept. of Health and Human Services, NIH; Washington D.C. USA: 1987. [Google Scholar]
- Keizer GD, Visser W, Vliem M, Figdor CG. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988;140:1393–400. [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–5. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Loftus JC, Plow EF, Frelinger AL, 3rd, D’Souza SE, Dixon D, Lacy J, Sorge J, Ginsberg MH. Molecular cloning and chemical synthesis of a region of platelet glycoprotein IIb involved in adhesive function. Proc Natl Acad Sci U S A. 1987;84:7114–8. doi: 10.1073/pnas.84.20.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Takagi J, Springer TA. Association of the membrane proximal regions of the alpha and beta subunit cytoplasmic domains constrains an integrin in the inactive state. J Biol Chem. 2001;276:14642–8. doi: 10.1074/jbc.M100600200. [DOI] [PubMed] [Google Scholar]
- Luo BH, Springer TA, Takagi J. Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc Natl Acad Sci U S A. 2003;100:2403–8. doi: 10.1073/pnas.0438060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miekka SI, Ingham KC, Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982;27:1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–5. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Mould AP. Getting integrins into shape: recent insights into how integrin activity is regulated by conformational changes. J Cell Sci. 1996;109(Pt 11):2613–8. doi: 10.1242/jcs.109.11.2613. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin alpha 5 beta 1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ J Biol Chem. 1995a;270:26270–7. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Mould AP, Askari JA, Akiyama SK, Yamada KM, Humphries MJ. An assessment of the efficacy of anti-integrin alpha subunit monoclonal antibody production using affinity purified beta 1-integrin dimers as immunogen. Biochem Soc Trans. 1991;19:361S. doi: 10.1042/bst019361s. [DOI] [PubMed] [Google Scholar]
- Mould AP, Askari JA, Aota S, Yamada KM, Irie A, Takada Y, Mardon HJ, Humphries MJ. Defining the topology of integrin α5β1-fibronectin interactions using inhibitory anti-α5 and anti-β1 monoclonal antibodies. J Biol Chem. 1997;272:17283–92. doi: 10.1074/jbc.272.28.17283. [DOI] [PubMed] [Google Scholar]
- Mould AP, Barton SJ, Askari JA, Craig SE, Humphries MJ. Role of ADMIDAS cation-binding site in ligand recognition by integrin alpha 5beta 1. J Biol Chem. 2003a;278:51622–29. doi: 10.1074/jbc.M306655200. [DOI] [PubMed] [Google Scholar]
- Mould AP, Barton SJ, Askari JA, McEwan PA, Buckley PA, Craig SE, Humphries MJ. Conformational changes in the integrin beta A domain provide a mechanism for signal transduction via hybrid domain movement. J Biol Chem. 2003b;278:17028–35. doi: 10.1074/jbc.M213139200. [DOI] [PubMed] [Google Scholar]
- Mould AP, Garratt AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognises a ligand-induced binding site epitope on the beta 1 subunit. FEBS Lett. 1995b;363:118–22. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–90. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Puzon-McLaughlin W, Kamata T, Takada Y. Multiple discontinuous ligand-mimetic antibody binding sites define a ligand binding pocket in integrin alpha(IIb)beta(3) J Biol Chem. 2000;275:7795–802. doi: 10.1074/jbc.275.11.7795. [DOI] [PubMed] [Google Scholar]
- Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9:617–21. doi: 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Ginsberg MH, Plow EF, Shattil SJ. Effect of platelet activation on the conformation of the plasma membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1991;266:7345–52. [PubMed] [Google Scholar]
- Takagi J, Erickson HP, Springer TA. C-terminal opening mimics ‘inside-out’ activation of integrin alpha5beta1. Nat Struct Biol. 2001;8:412–6. doi: 10.1038/87569. [DOI] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–11. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Tokuhira M, Handa M, Kamata T, Oda A, Katayama M, Tomiyama Y, Murata M, Kawai Y, Watanabe K, Ikeda Y. A novel regulatory epitope defined by a murine monoclonal antibody to the platelet GPIIb-IIIa complex (alpha IIb beta 3 integrin) Thromb Haemost. 1996;76:1038–46. [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 2004;6:154–61. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–45. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–6. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–51. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]