Abstract
Aim of FAST-MI 2010
To gather data on characteristics, management and outcomes of patients hospitalised for acute myocardial infarction (AMI) at the end of 2010 in France.
Interventions
To provide cardiologists and health authorities national and regional data on AMI management every 5 years.
Setting
Metropolitan France. 213 academic (n=38), community (n=110), army hospitals (n=2), private clinics (n=63), representing 76% of centres treating AMI patients. Inclusion from 1 October 2010.
Population
Consecutive patients included during 1 month, with a possible extension of recruitment up to one additional month (132 centres); 4169 patients included over the entire recruitment period, 3079 during the first 31 days; 249 additional patients declining participation (5.6%).
Startpoints
Consecutive adults with ST-elevation and non-ST-elevation AMI with symptom onset ≤48 h. Patients with AMI following cardiovascular procedures excluded.
Data capture
Web-based collection of 385 items (demographic, medical, biologic, management data) recorded online from source files by external research technicians; case-record forms with automatic quality checks. Centralised biology in voluntary centres to collect DNA samples and serum. Long-term follow-up organised centrally with interrogation of municipal registry offices, patients' physicians, and direct contact with the patients.
Data quality
Data management in Toulouse University. Statistical analyses: Université Paris Descartes, Université de Toulouse, Université Pierre et Marie Curie-Paris 06, Paris.
Endpoints and linkages to other data
In-hospital events; cardiovascular events, hospital admissions and mortality during follow-up. Linkage with Institute for National Statistics.
Access to data
Available for research to any participating clinician upon request to executive committee (fastmi2010@yahoo.fr).
Keywords: Registry, myocardial infarction, management, outcomes
Background
Cardiovascular mortality has recently declined, even in countries with a low prevalence of coronary artery disease, such as France.1 This is attributed to both improved primary prevention and improved treatment of established cardiovascular disease.
Observational data collected from cohorts of patients presenting with acute myocardial infarction (AMI) give the opportunity to determine whether the practice guidelines derived from the results of clinical trials have translated into changes in everyday practice. They also permit to make a link between different management strategies and clinical outcomes. Registries such as the international Global Registry of Acute Coronary Events (GRACE),2 3 the Euro Heart Survey programme4 or the USA, Swedish or British Myocardial Ischaemia National Audit Project (MINAP) national registries5–7 have been particularly helpful to improve our knowledge in this field. Some of these registries, however, suffer from a relative lack of representativeness, while others are highly representative but cannot collect extensive clinical data.
Every 5 years since 1995, cohorts of patients hospitalised for AMI in France over a 1-month period (see online supplementary files) have been constituted, to assess their management and outcomes.8–10 All of these registries included at least 60% of all institutions taking care of patients with AMI, and the patients were followed for at least 1 year.
In 2005, the FAST-MI registry was designed to collect extensive data in patients hospitalised for AMI and to follow the cohort thus constituted for a period of up to 10 years.8 A distinctive feature of FAST-MI is that all medications delivered at the acute stage, as well as the doses used are recorded. In addition a bio-bank collecting serum and DNA samples is also constituted for the purpose of pharmacogenetic studies.11 FAST-MI 2010 was set-up to conduct a new survey with similar objectives as the 2005 registry.
Aim
The aims of FAST-MI 2010 were to provide an extensive description of the population of patients admitted for AMI throughout the French territory, to determine whether differences in terms of population characteristics existed across regions, to assess the management of the patients suffering from AMI, and to determine the implementation of practice guidelines in a real world setting. Other objectives were to assess the correlations between management strategies and in-hospital outcomes, to determine the correlations between genetic polymorphisms and morbidity-mortality in relation with the effects of medications, and to determine the relationships between biomarkers and morbidity-mortality after MI. Also, FAST-MI will enable historic comparisons with the previous French cohorts constituted since 1995 and will document the long-term (up to 10 years) outcomes of patients presenting with AMI at the end of 2010, in relation with early and long-term management.
Organisation and funding
Type of study
Prospective, multi-centre observational study in continental France and Corsica. FAST-MI 2010 is a registry of the French Society of Cardiology, supported by the Société Française de Médecine d'Urgence, SAMU de France, the Collège National des Cardiologues des Hôpitaux and the Collège National des Cardiologues Français.
Funding and data propriety
The study is sponsored by the French Society of Cardiology and funded by unrestricted grants of the following companies: MSD, and AstraZeneca, the Daiichi-Sankyo-Eli-Lilly alliance, Glaxo-Smith-Kline, Novartis, and sanofi-aventis. Complementary grants will be sought for dedicated research projects within the main study.
Conduct of the registry and legal issues
The cardiologists who participated in the registry were not supposed to modify their therapeutic approach in any way. In the centres participating in the bio-bank, however, an additional 10–60 ml blood sample was collected at the time the routine blood sample was drawn.
Written informed consent was provided by each patient for participating in the study. The data from patients dying early (ie, before informed consent was obtained), were collected and recorded in the database, unless opposed to by the patient's next of kin when informed.
The study was conducted in compliance with Good Clinical Practice, French law and the French data protection law. The protocol was reviewed and approved by the Committee for the Protection of Human Subjects (CPP) of Saint Louis University Hospital Paris Ile de France IV. The data recorded and the way they are handled and stored were reviewed and approved by the Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé (CCTIRS) and the Commission Nationale Informatique et Liberté (CNIL). Clinicaltrials.gov identifier: NCT01237418.
Patients
Inclusion criteria
Each patient had to meet the following criteria:
1) Man or woman aged 18 years or over.
-
2) Admitted within 48 h of symptom onset to an intensive care unit (ICU) or a Cardiology department for an AMI characterised by elevation of troponins or CK-MB associated with at least one of the following:
– symptoms compatible with myocardial ischaemia,
– development of new abnormal Q waves,
– ST-T changes compatible with myocardial ischaemia (ST segment elevation or depression, T wave inversion);
3) Having agreed to take part into the study.
ST-segment elevation myocardial infarction (STEMI) was diagnosed when ST elevation ≥1 mm was seen in at least two contiguous leads in any location on the index or qualifying ECG, or when presumed new left bundle branch block or documented new Q waves were observed.
In the absence of ST-segment elevation, patients meeting the inclusion criteria were considered to have NSTEMI.
Patients who died very early after admission and for whom cardiac markers were not measured were included if they had compatible signs or symptoms associated with typical, unequivocal ST changes. Patients admitted after resuscitation of a cardiac arrest were included only if the cardiac arrest had been preceded by chest pain suggestive of AMI, having justified a call to emergency services.
Patients participating in clinical trials could be included in the registry, and information on trial participation was recorded.
Exclusion criteria
Exclusion criteria were: AMI with symptom onset >48 h from first call, unstable angina, iatrogenic AMI immediately following cardiovascular procedures (patients with late stent thrombosis, defined as stent thrombosis occurring after hospital discharge could be included), and diagnosis of AMI refuted in favour of alternative diagnoses, such as acute myocarditis.
Participating centres
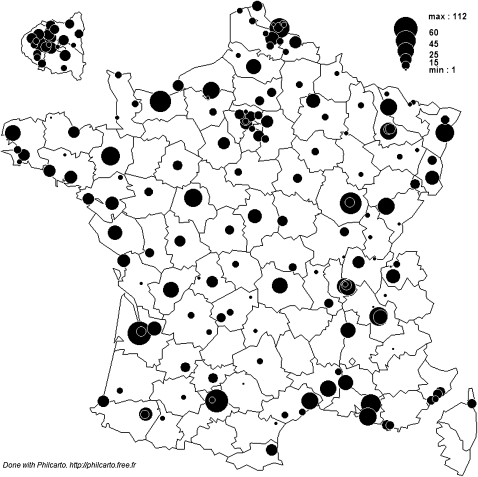
A list of all intensive care or coronary care units admitting patients with AMI in academic institutions, general hospitals, army hospitals and private clinics was established at the beginning of 2010. The physicians in charge were then asked to participate in the study. In all, 279 centres were listed, 224 of which initially accepted to participate in the study and 213 actively participated, including one general hospital which received no AMI patient during the 1-month study period. Participation rate was 76% and the centres were distributed across the whole country (figure 1).
Figure 1.
Geographical distribution of the centres participating in FAST-MI 2010.
In addition, approximately 100 further centres could receive AMI patients at their emergency rooms, but referred these patients to other institutions. These centres were not asked to participate in the registry, as the patients were included at the ‘receiving’ institutions.
The participating centres comprised: 111 general hospitals, 49 private clinics, 13 private not-for-profit clinics, 38 academic hospitals, and 2 army hospitals.
Recruitment
Patients were recruited consecutively from ICU/cardiology departments over a period of one month (from 1 October 2010). A physician was responsible for study recruitment in each centre. A list of all patients admitted within 48 h of symptom onset for a suspicion of ACS was established at each institution, and inclusion/exclusion criteria were checked. A computerised case record form (CRF) was filled-in for each eligible patient, based on the hospital records and additional specific questionnaires. Data were recorded on-line by dedicated research technicians from a contract research organisation (CRO) (ICTA, Fontaine-lès-Dijon, France) who went to each centre on a weekly basis. In the case of incomplete data from the source patient files, the research technicians contacted the local investigator to obtain the missing information.
Recruitment could extend up to 2 months in the centres willing to do so. The population included during the first month by the totality of participating centres is used as illustrative of current management and outcomes on the scale of the whole country, while the whole population will be used to answer specific medical interrogations on a larger sample.
The data
What is recorded?
Cardiovascular and non-cardiovascular medical history, risk factors, and clinical course, including symptoms, admission and worst Killip class, therapeutic management in the pre-hospital setting, during the first 48 h, during the hospital stay and at discharge, were recorded. Left ventricular ejection fraction, when assessed at entry and at any time during the hospital stay was recorded. Routine laboratory results on admission were collected. All in-hospital complications were recorded. In all, the electronic CRF comprised 385 items, to which was added information on medications administered before the index event, pre-hospital, during the first 24 and 48 h and at hospital discharge; for oral medications, doses were recorded.
In 131 centres, a biology substudy collected blood and serum samples for the assessment of DNA, RNA (120 Centres) and centralised measurement of biological markers (77 Centres).
Patients' follow-up
Follow-up data are collected by research technicians based at the French Society of Cardiology (SFC) under the supervision of the Unité de Recherche Clinique (URC-EST) of Assistance Publique-Hôpitaux de Paris (APHP), using the following sequential procedure, which was implemented for the follow-up of patients participating in the FAST-MI 2005 registry: (1) consulting the registry offices of the patients' birthplaces for death certificates; (2) contacting the patients' general practitioners and/or cardiologists; and (3) contacting the patients or their relatives. In many instances, written contact is followed by telephone interviews with the patients or their family.
For each reported event leading to hospitalisation or death, hospital discharge reports are sought and analysed by at least one physician from the research team. All cases of cardiovascular events are centrally reviewed by at least one physician. We will specifically collect information on the occurrence of acute coronary syndromes, myocardial revascularisation, hospitalisation for heart failure, stroke, bleeding events requiring hospitalisation, hospitalisation for cancer. Cases in which the final diagnosis appears unclear or debatable are reviewed by a three-member critical events committee.
Data quality
The quality of the data was insured by automated data checks when the electronic CRF were filled-in, and by the fact that all the data entered were verified, and modified if necessary, by external research assistants. This methodology had already been used for the FAST-MI 2005 survey with research assistants from the same CRO and an external audit in three of 21 regions showed data concordance in >90% of the cases.
Completeness of the data was adequate for most variables (eg, missing values <4% for height and weight; <2% for admission blood pressure and heart rate; <0.2% for risk factors or cardiovascular history; GRACE score calculated in 94.5%).
Once entered into the electronic CRF, data were stored in a central database at the SFC, Paris. Data management is ensured in conjunction between the SFC and the cardiology departments of Toulouse and HEGP university hospitals.
Use of the data and cross-linkage
Data are available for research to any participating clinician submitting an analysis plan to the executive committee (fastmi2010@yahoo.fr). In addition, any centre can claim access to its own data.
The database will be cross-linked with the French Institute for National Statistics and the French national death database (CepiDC) to gather additional information on the cause of death.
Finally, to evaluate changes in practice in France over the past fifteen years, individual data from the 1995, 2000, 2005 and 2010 registries will be merged in a common database.
Statistical analyses
For quantitative variables, means, standard deviations, and minimum and maximum values are calculated. In addition, medians with the IQR are calculated for some variables. Discrete variables are presented as percentages. Comparisons are made with chi-square or Fisher' s exact tests for discrete variables, and by unpaired T tests, Mann–Whitney U tests, Wilcoxon sign-rank tests or one-way analyses of variance for continuous variables. Odds and hazard ratios are given with their 95% CIs.
Multivariate analyses of predictors of short-term outcome (in-hospital, and 30 days) will use multiple logistic regression analysis.
Long-term survival curves will be estimated using the Kaplan Meier method and comparisons will be made using log-rank tests. Independent correlates of survival will be determined using a multivariate Cox model. Variables included in the final multivariate models will comprise those with a significance level <0.15 in the univariate analyses, unless otherwise stated. For specific analyses regarding management options, propensity scores will be calculated using multiple logistic regression analyses and propensity-score-matched cohorts will be constructed.
For genetic analyses, all single-nucleotide polymorphisms evaluated will be tested for deviation from Hardy–Weinberg equilibrium with the use of χ2 tests.
Statistical analyses are performed at Université Paris Descartes (Nicolas Danchin, MD, PhD), Université of Toulouse (Jean Ferrières, MD, PhD, Vincent Bataille, PhD), and Université Pierre et Marie Curie Paris 06 (supervised by Tabassome Simon, MD, PhD), using the SAS V.8.2 (SAS Institute), STATA (V.9), NCSS, or IBM-SPSS V.20.0 (IBM) softwares.
Strengths and weaknesses
The main strength of the present registry is that detailed data were recorded to characterise the patients, their management and outcomes. Overall more than 385 fields had to be filled-in, not counting the totality of the medications used (including doses for oral medications). The percentage of institutions participating in the registry was high. We could not include, however, centres from overseas French territories, so that our data are illustrative of Metropolitan France only.
Though the cardiologists were asked not to modify their therapeutic approach, we have no way to make sure that participating in the registry had absolutely no impact on practices. As we committed not to divulge the data of individual centres to anyone beside the clinicians in the centres themselves, however, there was no incentive for them to change their habits.
Contrary to mandatory registries, FAST-MI was based upon the voluntary participation of the centres. It therefore cannot pretend to represent an exhaustive collection of AMI patients during the study period. Of note, most centres not participating were low-volume centres, so that our data are likely skewed towards management practices that are mostly used in larger-volume centres. Major bias, however, is unlikely. In a population of AMI patients collected from medico-administrative databases during the first 6 months of 2006, mean age was 65 years12; in 2010, national data based on the ICD I21, I22 and I23 codes found a mean age of 68.0 years and 33% women (data from the Institut National de Veille Sanitaire). Overall, it is likely that some patients, (eg, very elderly) were hospitalised outside cardiology departments, or may have been admitted to smaller hospitals and therefore not included in our registry. The clinical outcomes in FAST-MI must be interpreted in this context. Temporal comparisons with previous French surveys using the same methodology, however, should not be affected by this potential bias, which was common to all previous surveys.
Results
Inclusion process
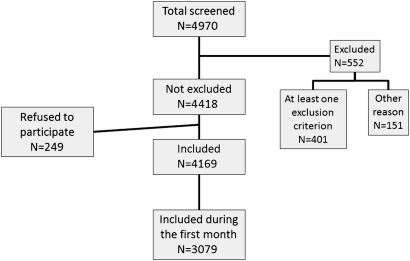
The inclusion process is summarised in figure 2. From a population of 4970 patients screened for participation in the registry, 552 were excluded because they had one or more exclusion criteria, had no myocardial infarction or were not admitted during the survey timeframe, and 249 (5.6%) did not consent to participate; 3079 were included during the first 31 days. Further analyses will describe the population admitted to the totality of participating centres, during the first 31 days.
Figure 2.
Flow chart of the inclusion process.
Baseline characteristics
Mean age of the whole population was 66±14 years, and STEMI patients were 4.5 years younger than patients with NSTEMI; 27% of the patients were women. The prevalence of risk factors was related to the type of infarction with more patients with hypertension, hyperlipidaemia, and diabetes mellitus in the NSTEMI population, and more current smokers among STEMI patients. The current episode of MI was the first clinical manifestation of coronary artery disease in 75% of the patients (table 1).
Table 1.
Main baseline characteristics
| Whole population (N=3079), N (%) | NSTEMI (N=1306), N (%) | STEMI or presumed new LBBB (N=1773), N (%) | p Value | |
| Centre | 0.038 | |||
| Academic | 1077 (35) | 426 (33) | 651 (37) | |
| General hospital | 1257 (41) | 558 (43) | 699 (39) | |
| Private clinic | 597 (19) | 262 (20) | 335 (19) | |
| Private, not for profit | 138 (4.5) | 53 (4) | 85 (5) | |
| Army | 10 (0.3) | 7 (0.5) | 3 (0.2) | |
| Centre with PCI on site | 2789 (91) | 1125 (86) | 1664 (94) | <0.001 |
| Age | 65.6±14.4 | 68.2±13.6 | 63.7±14.6 | <0.001 |
| Age >75 years | 960 (31) | 488 (37) | 472 (26) | <0.001 |
| Sex (female) | 829 (27) | 388 (30) | 441 (25) | 0.003 |
| Risk factors | ||||
| Hypertension | 1653 (54) | 805 (62) | 848 (48) | <0.001 |
| Hypercholesterolaemia | 1328 (43) | 619 (47) | 709 (40) | <0.001 |
| Diabetes mellitus | 653 (21) | 346 (26.5) | 307 (17) | <0.001 |
| Current smoking | 1035 (34) | 328 (25) | 707 (40) | <0.001 |
| Family history | 768 (25) | 318 (24) | 450 (25) | 0.513 |
| Cardiovascular history | ||||
| No known CAD | 2293 (74.5) | 838 (64) | 1455 (82) | <0.001 |
| Previous MI | 498 (16) | 293 (22) | 205 (12) | <0.001 |
| Previous PCI | 489 (16) | 303 (23) | 186 (10.5) | <0.001 |
| Previous CABG | 212 (7) | 112 (9) | 100 (6) | 0.026 |
| History of heart failure | 146 (5) | 94 (7) | 52 (3) | <0.001 |
| History of stroke or TIA | 139 (4.5) | 68 (5) | 71 (4) | 0.112 |
| Peripheral artery disease | 244 (8) | 154 (12) | 90 (5) | <0.001 |
| Medications used before event | ||||
| Aspirin | 696 (23) | 402 (31) | 294 (17) | <0.001 |
| Thienopyridine | 398 (13) | 263 (20) | 135 (7) | <0.001 |
| Statin | 864 (28) | 468 (36) | 396 (22) | <0.001 |
| β-blocking agent | 738 (24) | 399 (31) | 339 (19) | <0.001 |
| ACE-inhibitor or angiotensin II receptor blockers | 1030 (33.5) | 552 (40.5) | 478 (28) | <0.001 |
CABG: coronary artery bypass graft surgery; CAD, coronary artery disease; GRACE, Global registry of acute coronary events; LBBB, left bundle branch block; MI, myocardial infarction; NSTEMI, non-ST-segment-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction; TIA, transient ischaemic attack.
About one out of four patients were on statins, β-blockers, or aspirin at the time of their AMI.
Initial presentation and early management
Typical chest pain was present in 83%; 17% presented with Killip class II or higher; the location of AMI was anterior in 37% of the STEMI patients. Mean GRACE score was 142±37 (table 2).
Table 2.
Index acute myocardial infarction, initial presentation and management
| Whole population (N=3079), N (%) | NSTEMI (N=1306), N (%) | STEMI or presumed new LBBB (N=1773), N (%) | p Value | |
| Admission parameters | ||||
| Typical chest pain | 2568 (83) | 1036 (79) | 1532 (86) | <0.001 |
| Initial Killip class | <0.001 | |||
| I | 2457 (83) | 1023 (81) | 1434 (84) | |
| II | 301 (10) | 124 (10) | 177 (10) | |
| III | 153 (5) | 91 (7) | 62 (4) | |
| IV | 63 (2) | 21 (2) | 42 (2) | |
| GRACE score | 142±37 (n=2879) | 138±38 (n=1216) | 145±36 (n=1663) | <0.001 |
| Time delays (minutes) | ||||
| Time from onset to first call or contact Median [25th; 75th percentiles] | 90 [30; 300] | 105 [30; 390] (n=1257) | 74 [30; 240] (n=1727) | <0.001 |
| Procedures and management | ||||
| Coronary angiography | 2884 (94) | 1185 (91) | 1699 (96) | <0.001 |
| PCI | 2380 (77) | 859 (66) | 1521 (86) | <0.001 |
| Fibrinolysis in STEMI patients | ||||
| No | 1532 (86) | |||
| Pre-hospital | 137 (8) | |||
| In-hospital | 104 (6) | |||
| Primary PCI in STEMI patients | ||||
| Performed | 1073 (64) | |||
| Intended | 1171 (70) | |||
| Coronary artery bypass grafting | 89 (3) | 66 (5.1) | 23 (1.3) | <0.001 |
| Medications in first 48 h | ||||
| Aspirin | 2956 (96) | 1256 (96) | 1700 (96) | 0.686 |
| Clopidogrel | 2474 (80) | 1139 (87) | 1335 (75) | <0.001 |
| Prasugrel | 743 (24) | 179 (14) | 564 (32) | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 1062 (34.5) | 318 (24) | 744 (42) | <0.001 |
| Low molecular weight heparin | 1887 (61) | 784 (60) | 1103 (62) | 0.220 |
| Unfractionated heparin | 1705 (55) | 656 (50) | 1049 (59) | <0.001 |
| Fondaparinux | 518 (17) | 247 (19) | 271 (15) | 0.008 |
| Bivalirudin | 102 (3) | 21 (2) | 81 (5) | <0.001 |
| Statin | 2704 (88) | 1114 (85) | 1590 (90) | <0.001 |
| β-blocking agents | 2439 (79) | 1015 (78) | 1424 (80) | 0.079 |
| ACE-I or ARB | 1949 (63) | 804 (62) | 1145 (65) | 0.086 |
ARB, angiotensin II receptor blocker; CABG: coronary artery bypass graft surgery; CAD, coronary artery disease; GRACE, Global registry of acute coronary events; LBBB, left bundle branch block; MI, myocardial infarction; NSTEMI, non-ST-segment-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction; TIA, transient ischaemic attack.
The median time from symptom onset to first call was 90 min, and was shorter (74 min) in STEMI patients. In STEMI patients, median time from qualifying ECG to primary PCI was 110 min, and time from ECG to intravenous fibrinolysis was 22 min.
Overall, 94% of the patients underwent coronary angiography, and PCI was used in 77%. In STEMI patients, 14% were treated with fibrinolytic agents and 64% had primary PCI with an additional 6% referred for intended primary PCI but no PCI actually performed.
In-hospital complications
The mean duration in ICCU was 4.5 days, and mean hospital stay was 8 days. Ten per cent of the patients had a maximal Killip class III or IV at any time during the acute phase. Recurrent MI and stroke were rare. Overt bleeding was found in 7% of the patients, with 4% needing blood transfusion. TIMI major bleeding was present in 2.3% and TIMI minor bleeding in 2.7% (table 3).
Table 3.
In-hospital evolution and complications
| Whole population (N=3079), N (%) | NSTEMI (N=1306), N (%) | STEMI or presumed new LBBB (N=1773), N (%) | p Value | |
| Maximal Killip class during stay: | <0.001 | |||
| I | 2435 (79) | 1025 (78.5) | 1410 (79.5) | |
| II | 331 (11) | 142 (11) | 169 (11) | |
| III | 191 (6) | 104 (8) | 87 (5) | |
| IV | 122 (4) | 35 (3) | 87 (5) | |
| LVEF (%) | 51.4±11.4 (n=2601) | 54±11 (n=1092) | 50±11 (n=1509) | <0.001 |
| Recurrent myocardial infarction | 35 (1) | 16 (1) | 19 (1) | 0.691 |
| Stroke | 14 (0.5) | 2 (0.2) | 12 (0.7) | 0.033 |
| Any bleeding or transfusion | 333 (11) | 151 (12) | 182 (10) | 0.252 |
| TIMI major bleeding | 71 (2.3) | 28 (2.1) | 43 (2.4) | 0.607 |
| TIMI minor bleeding | 82 (2.7) | 28 (2.1) | 54 (3.0) | 0.125 |
| In-hospital death | 104 (3.4) | 25 (1.9) | 79 (4.5) | <0.001 |
LVEF, left ventricular ejection fraction; ICU, intensive care unit; TIMI, Thrombolysis in Myocardial Infarction.
In-hospital death was 3.4% in the whole population, higher in STEMI patients (4.5% vs 1.9%, p<0.001).
Medications prescribed and procedures scheduled at discharge
In the 2975 patients who were discharged alive, 36% were scheduled for a rehabilitation programme (26% for NSTEMI and 44% for STEMI patients) (table 4).
Table 4.
Medications prescribed at discharge
| Whole population (N=2962), N (%) | NSTEMI (N=1275), N (%) | STEMI or presumed new LBBB (N=1687), N (%) | p Value | |
| Aspirin | 2841 (96) | 1203 (94) | 1638 (97) | <0.001 |
| Clopidogrel | 1852 (62) | 872 (68) | 980 (58) | <0.001 |
| Prasugrel | 798 (27) | 206 (16) | 592 (35) | <0.001 |
| Dual antiplatelet therapy | 2576 (87) | 1039 (81) | 1537 (91) | <0.001 |
| Statin | 2699 (91) | 1134 (89) | 1565 (93) | <0.001 |
| Beta-blocker | 2547 (86) | 1045 (82) | 1502 (89) | <0.001 |
| ACE-I or ARB | 2361 (80) | 949 (74) | 1412 (84) | <0.001 |
CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; GRACE, Global registry of acute coronary events; LBBB, left bundle branch block; MI, myocardial infarction; NSTEMI, non-ST-segment-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction; TIA, transient ischaemic attack.
Medications prescribed at discharge comprised aspirin in 96%, with dual antiplatelet therapy in 87%, statins in 91%, beta-blockers in 86%, ACE-inhibitors or ARBs in 80%. More STEMI patients received recommended medications.
Conclusion
The FAST-MI 2010 survey gives a global picture of the way patients with STEMI and NSTEMI were managed in France at the end of 2010. Since 2005, regional networks have been implemented in most French regions, with most patients now admitted or transferred to hospitals with catheterisation laboratories. Many smaller hospitals no longer provide onsite care for STEMI patients, who are immediately transferred to larger centres using mobile intensive care units (Service d'Aide Médicale Urgente, SAMU).
Compared with the previous French surveys, the use of coronary angiography has continued to grow, with over 90% of the patients undergoing coronary angiography during their initial hospital stay.8–10 Approximately 80% of STEMI patients receive reperfusion therapy, and primary PCI has become the predominant method of reperfusion.13–15 These results are in keeping with the trend observed in other countries, and the figures are concordant with those recently reported from the Swedish registry.16 The rates of prescription of recommended medications are high, comparable to those reported in the MINAP17 and Get With the Guidelines18 registries.
Finally, complication rates and in-hospital mortality have declined markedly, in comparison with the previous French surveys performed using a similar methodology.8–10
Supplementary Material
Acknowledgments
We are indebted to all patients who accepted to participate in the registry and to all cardiologists and emergency physicians who took part in the study. We also thank Geneviève Derumeaux, MD, PhD, President of the Société Française de Cardiologie, Dominique Pateron, MD, PhD, Past-President of the Société Française de Médecine d'Urgence, Maxime Guenoun, MD, President of the Collège National des Cardiologues Français, Marc Giroud, MD, President of SAMU-urgences de France and the Collège National des Cardiologues des Centres Hospitaliers for their support. We also wish to thank the following companies which provided unrestricted grants which permitted the registry to exist: Merck, the Daiichi-Sankyo-Eli-Lilly alliance, AstraZeneca, Glaxo-Smith-Kline, sanofi-aventis, Novartis.
Footnotes
Contributors: All authors have participated in the registry, and have contributed to the design, collection, analysis of the data, writing, or critical reviewing of the manuscript.
Funding: MSD, Eli-Lilly, Daiichi Sankyo, AstraZeneca, GSK, Novartis, sanofi-aventis.
Disclosures: MH: speaker and consulting fees: AstraZeneca, Daiichi-Sankyo, Eli-Lilly. YC: speaker and consulting fees: Boehringer-Ingelheim, MSD, Pfizer, Novartis, Servier. PG: speaker and consulting fees: Boeringer-Ingelheim, The Medicines Company, sanofi-aventis, BMS, AstraZeneca, Lilly Daiichi Sankyo JF: speaker fees: Astra Zeneca, Merck, Novartis, Servier. TS: research grants: Astra-Zeneca, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, MSD, Novartis, Pfizer, sanofi-aventis, Servier; speaker and consulting fees: AstraZeneca, Bayer-Schering, Eli-Lilly, sanofi-aventis. ND: research grants: Astra-Zeneca, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, MSD, Novartis, Pfizer, sanofi-aventis, Servier and The Medicines Company; advisory panels or lecture fees: Astra-Zeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli-Lilly, Menarini, Merck-Serono, Novo-Nordisk, Servier, sanofi-aventis.
Competing interests: None.
Ethics approval: Ethics approval was approved by CPP St Louis hospital, Paris.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aouba A, Pequignot F, Toullec AL, et al. Les causes médicales de décès en France en 2004 et leur évolution 1980–2004. BEH 2007;35/36:308–14 [Google Scholar]
- 2.Fox KA, Goodman SG, Anderson FA, Jr, et al. From guidelines to clinical practice: the impact of hospital and geographical characteristics on temporal trends in the management of acute coronary syndromes. The Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003;24:1414–24 [DOI] [PubMed] [Google Scholar]
- 3.Fox KA, Eagle KA, Gore JM, et al. The global registry of acute coronary events, 1999 to 2009–GRACE. Heart 2010;96:1095–101 [DOI] [PubMed] [Google Scholar]
- 4.Hasdai D, Behar S, Wallentin L, et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin; the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS). Eur Heart J 2002;23:1190–201 [DOI] [PubMed] [Google Scholar]
- 5.Herrett E, Smeeth L, Walker L, et al. The Myocardial Ischaemia National Audit Project (MINAP). Heart 2010;96:1264–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–21 [DOI] [PubMed] [Google Scholar]
- 7.Peterson ED, Roe MT, Chen AY, et al. The NCDR ACTION Registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart 2010;96:1798–802 [DOI] [PubMed] [Google Scholar]
- 8.Cambou JP, Simon T, Mulak G, et al. The French registry of Acute ST elevation or non-ST-elevation Myocardial Infarction (FAST-MI): study design and baseline characteristics. Arch Mal Coeur Vaiss 2007;100:524–34 [PubMed] [Google Scholar]
- 9.Danchin N, Vaur L, Genes N, et al. Management of acute myocardial infarction in intensive care units in 1995: a nationwide French survey of practice and early hospital results. J Am Coll Cardiol 1997;30:1598–605 [DOI] [PubMed] [Google Scholar]
- 10.Hanania G, Cambou JP, Gueret P, et al. Management and in-hospital outcome of patients with acute myocardial infarction admitted to intensive care units at the turn of the century: results from the French nationwide USIC 2000 registry. Heart 2004;90:1404–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009;360:363–75 [DOI] [PubMed] [Google Scholar]
- 12.Tuppin P, Neumann A, Danchin N, et al. Combined secondary prevention after hospitalization for myocardial infarction in France: analysis from a large administrative database. Arch Cardiovasc Dis 2009;102:279–92 [DOI] [PubMed] [Google Scholar]
- 13.Danchin N, Blanchard D, Steg PG, et al. Impact of prehospital thrombolysis for acute myocardial infarction on 1-year outcome: results from the French Nationwide USIC 2000 Registry. Circulation 2004;110:1909–15 [DOI] [PubMed] [Google Scholar]
- 14.Danchin N, Coste P, Ferrieres J, et al. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on acute ST-elevation myocardial infarction (FAST-MI). Circulation 2008;118:268–76 [DOI] [PubMed] [Google Scholar]
- 15.Danchin N, Vaur L, Genes N, et al. Treatment of acute myocardial infarction by primary coronary angioplasty or intravenous thrombolysis in the “real world”: one-year results from a nationwide French survey. Circulation 1999;99:2639–44 [DOI] [PubMed] [Google Scholar]
- 16.Jernberg T, Johanson P, Held C, et al. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011;305:1677–84 [DOI] [PubMed] [Google Scholar]
- 17.Gavalova L, Weston C, Birkhead J, et al. Myocardial Ischaemia National Audit Project (MINAP). How the NHS cares for patients with heart attack. Tenth Public Report 2011. 2011. http://www.hqip.org.uk/assets/NCAPOP-Library/MINAP-public-report-2011.pdf [Google Scholar]
- 18.Motivala AA, Cannon CP, Srinivas VS, et al. Changes in myocardial infarction guideline adherence as a function of patient risk: an end to paradoxical care? J Am Coll Cardiol 2011;58:1760–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.