Abstract
Proteases hydrolyze peptide bonds, thereby controlling the function of proteins and peptides on the posttranslational level. In the cardiovascular system, proteases play pivotal roles in the regulation of blood pressure, coagulation and other essential physiological processes. Accordingly, proteases are prime targets for therapeutic interventions and diagnostics. Proteases are part of complex proteolytic networks comprised of enzymes, inhibitors, activators, substrates and cleavage products. Analyzing these networks on a system-wide level is essential to understanding cardiovascular function and how dysregulation can lead to pathological conditions. Mass spectrometry-based quantitative and dynamic proteomics approaches are leading the way to enhance our knowledge of proteolytic networks such as the renin-angiotensin-system. Here, we critically review proteomics tools utilized in protease biology and provide an overview on how these methods can be used to characterize and validate protease function.
Keywords: peptides, biomarker, proteomics, cardiovascular diseases, mass spectrometry
Introduction
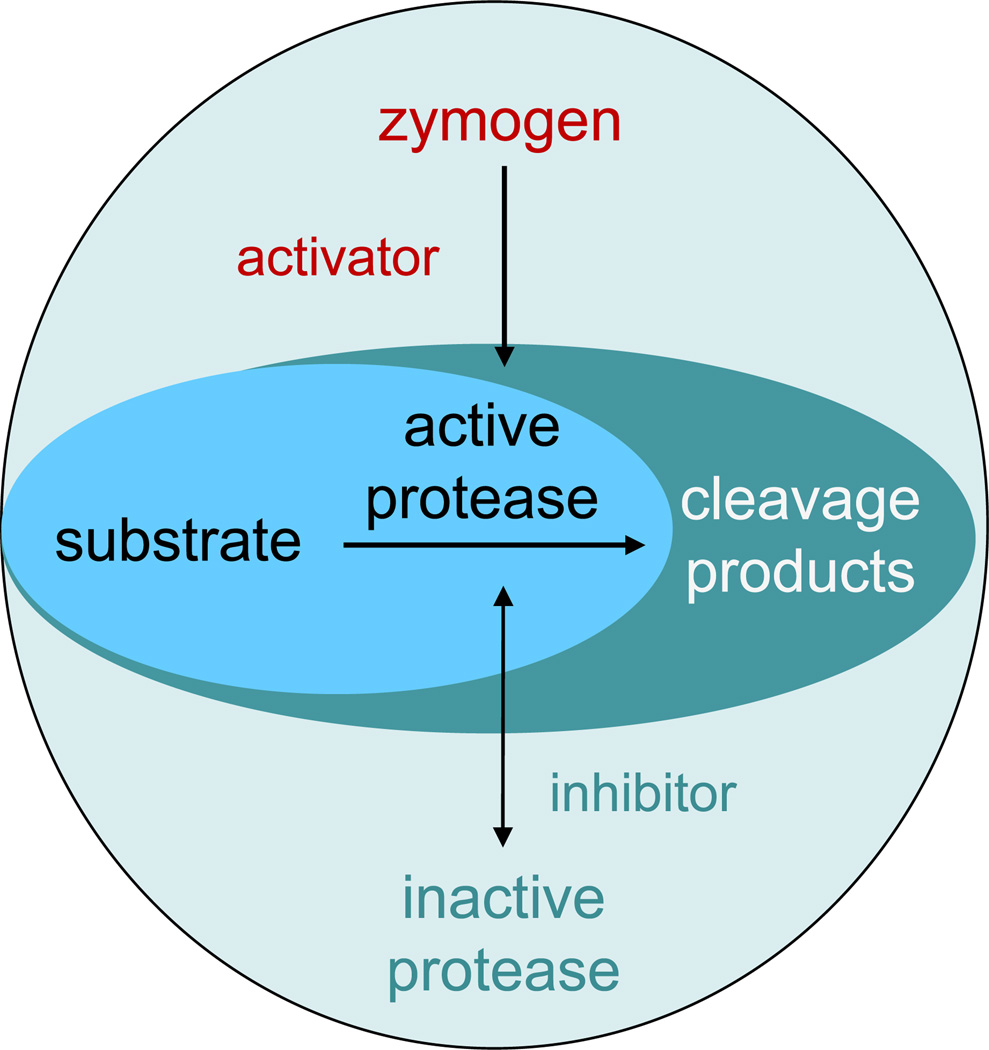
Proteases catalyze the hydrolysis of peptide bonds, which results in the cleavage of protein and peptide chains and thereby lead to an irreversible change of protein structure. The fundamental nature of this process makes proteolysis a powerful post-translational modification that can control protein function and abundance. Digestive proteases in the gastrointestinal tract break down proteins fairly indiscriminately, while peptidases involved in cell signaling catalyze very specific cleavage reactions to regulate the abundances of bioactive peptides. Uncontrolled proteolysis could have disastrous physiological consequences, therefore a multitude of mechanisms exist to tightly regulate proteolytic processing. One of the more basic regulatory principles is substrate specificity, in which the three-dimensional structure of the protease determines which substrates are accessible to the active site. Other regulatory mechanisms include the activation of proteases from inactive precursors (i.e., zymogens) and the limitation of protease activities to specific pH ranges and compartments (e.g., lysosomal proteases). Another regulatory element is the spatial and temporal interplay of proteolytic network components: Devoid all required factors (proteases, substrates and their respective activators and inhibitors) present, reactions may not occur (Figure 1).
Figure 1.
A simplified view of the essential functional relationships between members of a proteolytic network. Activators and inhibitors can modulate the activity state of proteases. For cleavage reactions to occur, the spatial and temporal distributions of activated proteases and substrates need to overlap.
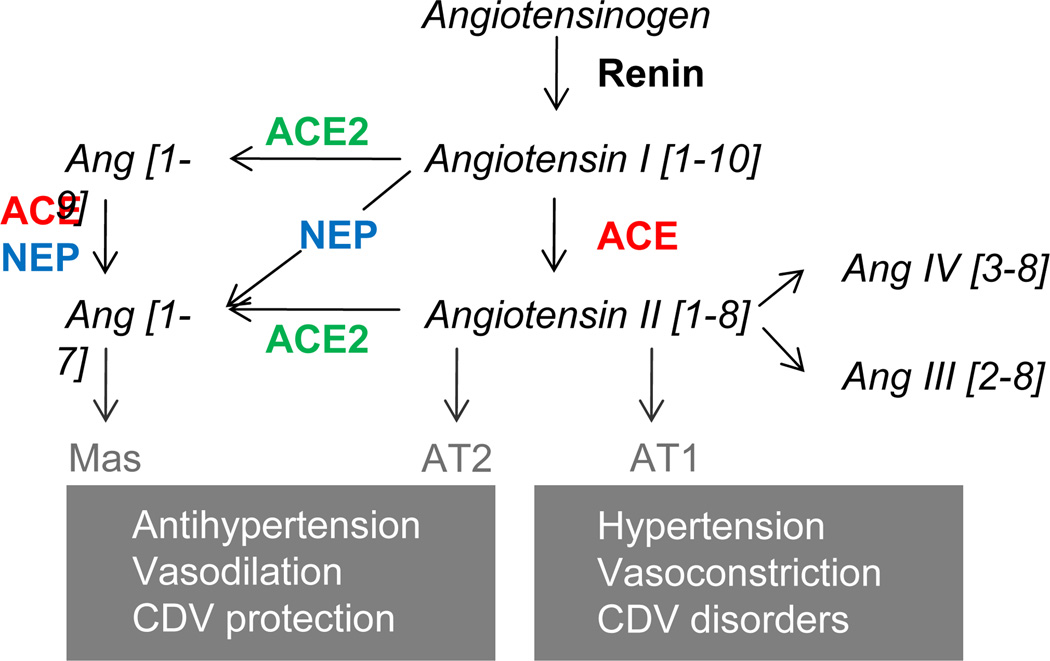
The renin-angiotensin-system can serve as an example how a proteolytic network regulates a physiological process1: Briefly, angiotensinogen is cleaved by renin to produce angiotensin I, which in turn is cleaved by angiotensin-converting enzyme (ACE) to form the effector peptide angiotensin II. Binding of angiotensin II to the AT1 receptor mediates vasoconstriction, while binding to AT2 results in vasodilation (Figure 2). The renin-angiotensin-system tightly controls arterial blood pressure and assures the uninterrupted perfusion of vital organs with oxygen and nutrients. Recent discoveries of alternative angiotensin-processing pathways illustrate that the renin-angiotensin-system is more complex than previously thought2. Dysregulation of the renin-angiotensin-system can lead to pathological conditions such as hypertension, which is a major risk factor for congestive heart failure, stroke and myocardial infarction. Due to their pivotal roles, proteolytic enzymes have emerged as prime targets for the pharmaceutical treatment of cardiovascular diseases3. The increasing awareness of the modulatory effect of the biological context mandates that protease function is analyzed on a system-wide level. Mass spectrometry (MS)-based proteomics provides a unique technological platform to capture the complexity and dynamic nature of proteolytic networks. Here, we review the current state of proteomics approaches utilized in protease biology and highlight strategies particularly suited to characterize protease activities in the context of cardiovascular disorders.
Figure 2.
Processing of angiotensin peptides by ACE, ACE2 and neprilysin as part of the rennin-angiotensin system. Renin cleaves angiotensinogen to produce angiotensin I. ACE converts angiotensin I to angiotensin II. In a second processing axis, angiotensin I is cut by ACE2 resulting in Ang [1–9], which is cleaved by either ACE or neprilysin (NEP) to produce Ang [1–7] (bracketed numbers refer to the amino acid positions within the peptide sequences). Ang [1–7] can also result from processing of angiotensin I by NEP or angiotensin II by ACE2. Binding of angiotensin II to the AT1 receptor activates vasoconstriction. In contrast, binding to the AT2 receptor mediates vasodilation, which can also be initiated by the binding of Ang [1–7] to the Mas receptor.
Mass spectrometry-based methods to unravel proteolytic networks
The renin-angiotensin-system demonstrates how MS-based proteomics can help filling the need for a detailed understanding of proteolytic networks. While renin and ACE were the original proteases of the renin-angiotensin-system, the updated view includes additional proteases such as ACE2, chymase, neprilysin and aminopeptidases (A and N) 4–7. Likewise, additional bioactive peptides such as the angiotensin metabolites Ang III [2–8], Ang IV [3–8] and Ang [1–7] that were previously considered functionally inactive, are now included8. MS-based technologies played a leading role in the identification of these novel system constituents.
Historically, protease function is assessed on an individual basis by in vitro enzyme assays after biochemical purification. The advent of new analytical technologies makes it now feasible to investigate protease activity and function in complex environments9–13. Generally, these approaches can be categorized based on whether they are designed to (i) identify constituents of the proteolytic network (proteases, substrates), (ii) screen for protease inhibitors/activators/modulators or (iii) characterize the dynamics of proteolytic processing. In Table 1, we provide an overview of the most commonly applied strategies in the field and which aspects of proteolysis they address. To choose the appropriate method, it is essential to define what aspect of protease biology needs to be addressed. Figure 1 depicts the functional relationships between members of proteolytic networks and can serve as a guide to develop research strategies: Is the protease of interest catalytically active? How does the zymogen differ from the active form of the protease? Which molecules can modulate the activity and substrate specificity of the protease? What are the endogenous substrates of the protease? What are the spatial and temporal distributions of the protease and other components of the proteolytic network? Many of these questions can be readily answered by online resources: the MEROPS database (http://merops.sanger.ac.uk) curates published information about peptidases, their substrates and inhibitors and offers indications about overall substrate specificity25; PMAP (http://www.proteolysis.org) combines five databases (ProteaseDB, SubstrateDB, CutDB, ProfileDB and PathwayDB) and a computational toolkit (including cleavage site predictions) to create an integrated reasoning environment to analyze proteolytic networks26.
Table 1.
Commonly applied techniques to study proteolytic reactions.
| Method | Description Application |
Protease | Substrate | Inhibitor | Process |
|---|---|---|---|---|---|
| General techniques for protease research | |||||
| Yeast two-hybrid systems | Yeast libraries carrying cloned open reading frames (ORF)14 Sequencing of positive PCR amplification products Identification of protein interactions on a large scale Two target ORFs are analyzed per experiment |
• | • | • | |
| Phage-display | Affinity selection of clones and screening of peptide libraries9 DNA sequencing of remaining amplification products High throughput screening of protein interactions and inhibitors One target protein or peptide is analyzed per experiment |
• | • | • | |
| 2D differential gel electrophoresis (2D DIGE) |
Gel-based protein separation of fluorescent labeled samples15 Proteins are imaged by fluorescence and identified by MS Quantitation of proteins and posttranslational modifications Comparison of two samples with internal standards |
• | • | ||
| Multidimensional protein identification technique (MudPIT) |
Peptide products are separated by SCX and RP-HPLC13 Identification of peptide products by MS Comparison of an unlimited number of samples |
• | • | ||
| Isotope-coded affinity tag (ICAT) |
Labeling of cysteine-residues in proteins followed by digestion16 Relative quantitation of peptide products by MS Comparison of two samples |
• | |||
| Isobaric tags for relative and absolute quantitation (iTRAQ) |
Isobaric amine-specific tagging of peptide products17 Relative and absolute quantitation of protease activity by MS Comparison of up to eight samples |
• | |||
| Stable isotope labeling with amino acids in cell culture (SILAC) |
Expressed proteases/peptide products labeled by amino acids18 Identification and quantitation of peptide products by MS Comparison of an unlimited number of samples |
• | |||
| Multiple reaction monitoring (MRM) |
Multiple reaction monitoring of known peptide fragments by targeted MS19 Absolute quantitation of known peptides Comparison of an unlimited number of samples |
• | • | ||
| Techniques specifically designed for protease research | |||||
| Cellular libraries of peptide substrates (CLiPS) |
Combinatorial approach to measure substrate hydrolysis11 Identification of substrates by quantitative screening of whole-cell fluorescence |
• | • | ||
| Positional scanning synthetic libraries |
Screening of tetra-peptide libraries by proteolysis-dependent signal intensities10 Screen for P1–P4 substrate specificities |
• | |||
| Colloidal barcoding bead- based protease profiling |
Screening of combinatorial libraries using polyelectrolyte-coated fluorescent silica reporter particles20 Identification of consensus proteolytic cleavage sites |
• | • | • | • |
| Near infrared (NIR) fluorogenic reporters |
NIR fluorescence signal upon cleavage of protease-sensitive peptide linkers21 In vivo imaging and quantitation of protease activities |
• | • | • | • |
| C- and N-term enrichment of cleavage products |
Negative or positive enrichment of C- or N-terminal peptide cleavage products22 Identification of proteolytic peptides and cleavage sites Comparison of two samples |
• | |||
| Activity-based probes (ABPs) |
Chemical probes with affinity and fluorescent tags23 Report on the structure and reactivity of enzyme active sites in cells and tissues |
• | • | • | |
| Proteinase activity labeling employing 18O- enriched water (PALeO) |
18O-labeling of proteolytic peptides during hydrolysis24 Quantitation and identification of protease activity, peptide substrates and cleavage products Comparison of an unlimited number of samples |
• | • | • | • |
MS-based proteomics has emerged as the premier tool to identify and quantify proteins and peptides27–29. Briefly, in a typical proteomics identification workflow, proteins are separated by one- or two-dimensional gel electrophoresis, digested by exogenous proteases (i.e., trypsin). Resulting peptide fragments are recovered, fractionated by reverse-phase chromatography and their molecular masses measured by MS. Selected peptides are fragmented in tandem MS experiments and resulting fragmentation data submitted to search engines (i.e., Mascot) that match them to fragmentation patterns predicted from protein databases30. Alternatively, in a peptidomics workflow the digestion step is omitted and peptide products formed by endogenous proteases are directly isolated31. Peptide sequence assignments are particularly challenging in peptidomics-type experiments, however recent advances in bioinformatics are starting to approach this problem32.
How to develop a quantitative view of proteolytic processing?
While MS readily provides protein and peptide identities, MS-measurements are inherently poorly quantitative33. Accurate quantitation of protein and peptide expression levels is a prerequisite to capture the dynamic nature of proteolysis and to gain functional insights. Quantitation by MS is achieved by either label-free or stable isotope labeling methods. Measurements of chromatographic ion intensity (e.g., peak areas) and spectral counting are the most commonly applied label-free approaches34. Label-free methods provide relative quantitation for an unlimited number of samples. Stable isotope labeling strategies, in contrast, can provide relative and absolute quantitation, however the specifics of the labeling reactions can limit the number of samples interrogated. Over recent years, a broad variety of stable isotope labeling methods have been developed34,35. Here, we will focus on the most prominent examples that have been utilized in the context of protease biology. In general, the presented techniques are capable of simultaneously identifying and quantifying novel and known proteases and their substrates. They can also be used to test the efficacy of protease inhibitors.
Isotope-coded affinity tags (ICAT) are one of the best-known techniques to measure protein abundances. ICAT reagents are comprised of three functional components: (i) a reactive group specific toward cysteinyl residues, (ii) a stable isotope label, and (iii) a biotin affinity tag16. The affinity tag allows for the selective enrichment of cysteine containing peptides, thus reducing sample complexity. The stable isotope label introduces either a light or heavy tag, which in turn allows for the comparative analysis of protein expression levels across two states. After labeling with ICAT reagents, two samples are combined, digested and cysteine-containing peptides enriched by avidin affinity chromatography. LC-MS/MS analysis yields both protein quantity and identity. The ICAT approach has been successfully used to identify novel protease substrates in complex cellular environments and to quantify protease activity36. The exclusive reliance on cysteine-containing peptides limits the applicability of the ICAT approach as a general quantitation approach.
This shortcoming has been addressed by the next generation of chemical labeling strategies that tag peptide N-termini and lysine side chains using N-hydroxy-succinimide (NHS) chemistry. The TMT (tandem mass tag)37 and iTRAQ (isobaric tags for relative and absolute quantitation)38 strategies share as an important design feature an isobaric stable isotope moiety, which renders differentially labeled samples indistinguishable during chromatographic and mass spectrometric analyses. Only upon MS/MS fragmentation, low molecular weight reporter ions are released and their relative ion abundances can be used for quantitation. Currently, there are four and eight reporter ions available for iTRAQ38,39 and two and six for TMT37,40, each allowing for multiplexed analysis in single LC-MS/MS experiments. iTRAQ labeling has been successfully applied to quantify peptides generated by endogenous proteases41 and to monitor protease activity in cell cultures42.
SILAC (stable isotope labeling by amino acids) has been developed as a metabolic labeling method alternative to chemical labeling approaches. SILAC has been applied in cell culture systems18 and more recently in entire organisms43. SILAC relies on the in vitro incorporation of essential amino acids with substituted stable isotope nuclei (e.g., Arg and Lys labeled with 13C, 15N). Heavy and light versions of the amino acids are incorporated into every protein in the system, which, in turn, allows for comparative expression analyses. SILAC has been used to determine which substrates are trapped by a catalytically inactivated protease44 and more recently to quantify Granzyme B catalyzed proteolysis45. Compared to chemical derivatization strategies, SILAC offers more efficient and reproducible labeling.
High levels of sample complexity and large cleavage products are a challenge for proteomic analyses. To reduce complexity and determine cleavage sites within large proteins, investigators developed strategies to enrich for N-terminal cleavage products that are of interest46. In positive enrichment methods, α-amino groups that are newly formed by proteolysis are captured by chemical or enzymatic processes47, 48. In depletion methods, internal peptides are removed by either altered chromatographic properties22 or by chemical means49. Recently, a positive enrichment strategy termed C terminomics has been introduced for the until then elusive C-terminal cleavage products50. Combining N- and C-terminal peptide data provides complementary information and increased coverage of cleavage sites. However, such enrichment steps considerably extend sample processing and analysis time.
Another strategy to address sample complexity is targeted MS. These types of experiments are typically performed on triple quadrupole instruments operating in multiple reaction monitoring (MRM) mode. MRM assays determine the abundances of peptide analytes by measuring the intensities of their characteristic MS/MS fragment ions and can be used in relative and absolute quantitation mode. Compared to other MS approaches, MRM provides higher sensitivity and specificity28. MRM assays have been used to study the pharmacokinetics of enalapril, an ACE inhibitor used in the treatment of hypertension and congestive heart failure51. The MRM approach was also used to simultaneously quantify seven angiotensin II receptor antagonists in plasma of patients undergoing cardiovascular treatment52. Despite their broad usage in pharmacological studies, MRM assays have so far not been employed in protease research, which can partially be attributed to the laborious assay development that requires optimized LC and MS conditions for each analyte19.
How to determine the substrate specificity and activity state of proteases?
Proteases are typically low abundant molecules, which makes their functional characterization by MS-based workflows challenging. Therefore, activity-based probes (ABPs) have been devised as chemical warheads directed at the active sites of enzymes. ABP-based profiling has been used to detect and capture the subset of catalytically active proteases in complex biological systems and to give insights into the catalytic mechanism and substrate specificity of enzymes53. ABPs report on the functional state of proteases by selectively labeling enzymes that share active site features (affinity, reactivity). Currently, ABPs have been developed for serine-, cysteine-, aspartyl- and metallo-proteases23. One of the most important design features of ABPs is their ability to distinguish active proteases from inactive precursors (zymogens)54 or proteases whose activity is modulated by other mechanisms55. ABP-profiling thereby provides functional information that is beyond standard protein expression data. ABPs with various combinations of affinity- and fluorescent tags have been developed to study diseases in vivo56, 57. In conjunction with downstream analytic technologies like MS, ABP-profiling offers the unique advantage to enrich for proteome fractions that share functional properties. ABP-profiling can generally be classified between (i) gel-based methods and (ii) LC-MS strategies58, 59. The LC-MS-based platform uses biotin-tagged probes that capture intact enzymes on streptavidin beads. On-bead digestion and subsequent LC-MS analysis provide protein identification60. In the bottom-up variation of the strategy, the enrichment step occurs on the peptide level after all proteins have been digested61. Shortcomings of ABP-profiling include the limited specificity and availability of functional probes. Also, some of the probes are toxic and cannot be used in vivo. Finally, ABPs inactivate captured proteases and therefore enzyme function cannot be further studied.
How to gain insights into the dynamics of proteolytic processing?
In contrast to the methods described above that primarily aim at the identification of protease network components, few experimental approaches exist that capture the dynamics of proteolytic processing62. Classically, chromo- or fluorogenic protease cleavage assays are used to measure the kinetics of individual proteolytic reactions. Fluorescence-based assays rely on the activation or dequenching of fluorophores upon proteolytic cleavage. The design of such assays requires precise knowledge of the targeted cleavage sites, so that appropriate constructs can be synthesized that specifically report substrate-to-product conversions63. Introduction of fluorescent reporter functionalities may alter substrate-enzyme interaction kinetics. Also, cleavage-assays are likely to fall short when complex proteolytic reactions are being investigated, i.e. when a protease recognizes multiple, alternate cleavage sites on the same substrate molecule. MS can fill this critical technological gap by its ability to detect and identify unknown peptide metabolites on a large-scale in a virtually unbiased way. MS-based monitoring of enzymatic reactions has been described for electrospray and MALDI-ionization techniques64. For example, in the MALDI-MES strategy (MS-assisted enzyme screening)65 endogenous proteases are captured from crude samples and immobilized on functionalized beads. The beads are subsequently incubated with substrate solutions, whose compositions are monitored over time by MALDI-MS analyses. Enzyme immobilization and the use of separate substrate solutions minimize interferences from the biological matrix (e.g., salt, proteins) during MS-analysis. Villanueva et al. showed that even against complex biological backgrounds (i.e., serum) ex vivo incubation of endogenous proteases and substrates can yield characteristic protease activity signatures that can be used as cancer biomarkers66. In the PALeO approach (protease activity labeling employing 18O-enriched water)24, ex vivo incubations occur in the presence of 18O-enriched water. Hydrolysis of peptide bonds results in the concomitant incorporation of solvent 18O-atoms into the C-termini of nascent cleavage products, which can be readily detected by MS based on their characteristic isotope patterns67. Interestingly, some substrate-protease interactions extend beyond the initial cleavage reaction: Cleavage products formed by serine proteases (e.g., trypsin) have been shown to rebind to the protease and reform acyl-enzyme intermediates. In the presence of H218O, hydrolysis of the acyl-enzyme intermediate facilitates the incorporation of a second 18O-atom into the C-terminus68. 18O-based strategies such as PALeO can therefore provide additional information regarding aspects of protease-substrate interaction and enzymatic mechanism. The 18O-label allows positively selecting cleavage products and disregarding signals derived from the biological matrix background. Strategies such as PALeO are important steps towards mapping proteolytic networks in more global and dynamic ways. Studying complex proteolytic networks, however, remains an analytical challenge and accurate quantitative information is needed. Such efforts can be aided by providing non-degradable quantitation standards (e.g., all-d-amino acid peptides) and defined protease substrates69. Stable isotope labeling of these peptides (e.g., by acid-catalyzed 18O-exchange) allows to track their degradation in complex mixtures70.
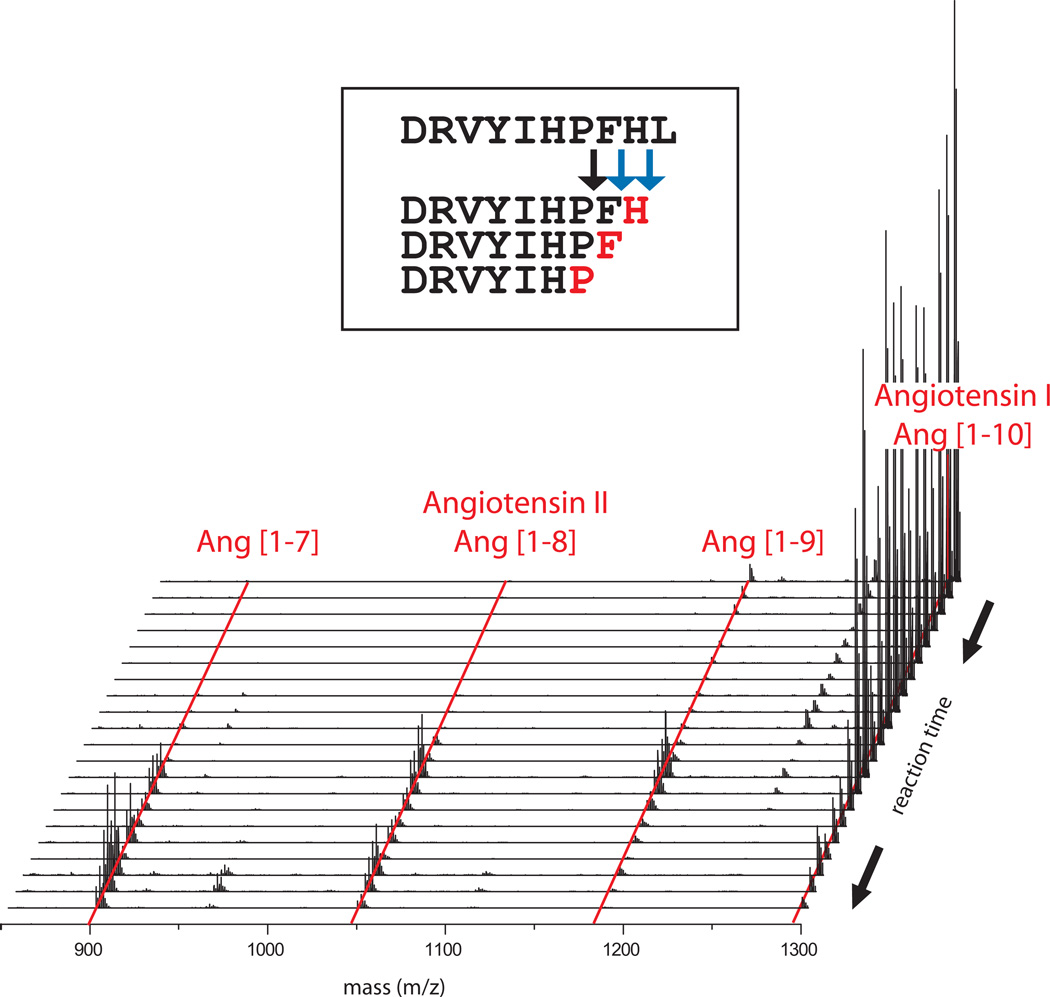
The emerging field of dynamic proteomics promises to greatly facilitate the elucidation of complex proteolytic networks. Already, there is ample evidence that such approaches can shed new light into even well studied subject areas such as the renin-angiotensin-system. Using MALDI-MES, Schlüter et al. determined that renal Cathepsin G can generate angiotensin II71. Using the PALeO-assay, we demonstrated that endothelin-converting enzyme-1 (ECE-1), a member of the neprilysin protease family, can also generate angiotensin II (Figure 3) (M. Hardt, unpublished data, 2011). ECE-1-mediated peptide processing included additional cleavage reactions that could only be resolved by temporal analysis72. Further, the data reconfirmed that pH conditions influence the substrate specificity and activity profile of ECE-173. The existence of alternative angiotensin II biosynthesis pathways illustrates that the renin-angiotensin-system is far more complex than previously thought.
Figure 3.
PALeO, a dynamic MS-based protease assay, shows that ECE-1 converts angiotensin I to multiple biologically active metabolites including angiotensin II. The waterfall-plot shows the subsequent generation of Ang[1–9], angiotensin II and Ang[1–7] at extracellular pH (7.4). Red letters in the insert specify sites of proteolytic 18O-incorporation. Blue arrows indicate novel cleavage sites detected by PALeO, while the black arrow denotes the previously known cut site.68.
Future perspectives
Proteomics and peptidomics technologies continue to positively impact protease research by aiding in the identification and quantitation of proteases, their substrates and inhibitors. Advances in MS-instrument technology that improve mass accuracy and sensitivity will further increase identification rates74. Advances in MS-quantitation methods will help shift the analytical focus from static to dynamic measurements and assist in moving the field from identification- to activity-based workflows. In addition, improved quantitation techniques will enhance comparative studies that, for example, investigate the effect of perturbations (e.g., pathological conditions) on proteolytic networks.
Due to their key roles in diseases, proteases have tremendous potential as therapeutic targets and biomarkers. Taking advantage of the catalytic properties could lead to more specific and sensitive diagnostic tools compared to what is achievable with non-catalytic biomarkers. Proteases such as ACE are prime examples for how proteolytic enzymes have been targeted in drug discovery3. MS provides valuable tools to these endeavors. However, it is important to keep in mind that a single analytical platform is unlikely to explain complex and dynamic proteolytic systems by themselves. In vivo validation, such as classical overexpression and knock out experiments, are needed to confirm the biochemical roles of proteases. Novel live imaging probes are changing the validation process. Near infrared (NIR) fluorescence that penetrates through tissue offers the ability to noninvasively monitor the spatial and temporal distributions of enzyme activities21. NIR-probes have been used to visualize specific proteolytic cleavages in intact organisms75 and validate in vivo targets of protease inhibitors76. NIR-based imaging has an immense potential for revolutionizing the drug development process and clinical practice. Knowledge gained from activity-based and dynamic proteomics can accelerate the development of these exciting technologies.
Conclusion
MS-based technologies are increasingly recognized as methods of choice for studying proteolytic enzymes. Here, we provided an overview of the most prominent examples in the field and illustrated how these methods contribute to cardiovascular research. In the study of the renin-angiotensin-system, system-wide proteomics analyses were essential to discover alternative pathways generating vasodilatory and vasoconstrictive peptides. Similarly, activity-based proteomics workflows provided unprecedented details of the dynamics of proteolytic processing that assisted in the discovery of novel pharmaceutical targets. Despite these success stories, it is important to consider that proteomics approaches have shortcomings: Most quantitative MS-strategies are technically limited to small sample numbers, which renders them less suitable to clinical studies with large cohorts. Emerging strategies such as targeted MRM-assays can increase throughput but at the cost of the discovery research component. Single-handedly, none of the presented MS-technologies provides a complete picture of complex protease networks. Likewise, MS-based techniques are not directly suitable for in vivo measurements. These limitations postulate the careful integration of MS-based approaches with other experimental techniques such as live imaging. MS-based proteomics has proved its utility for elucidating protease function in biological systems. The rapidly evolving field promises to continue contributing to our understanding of the renin-angiotensin-system, blood coagulation and other cardiovascular systems that entail complex proteolytic networks.
Acknowledgments
Funding Sources: This work was partially supported by a grant from the National Institute of Dental and Craniofacial Research (R01 DE 019796) to Markus Hardt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None.
References
- 1.Skeggs LT, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira AJ, Raizada MK. Genomic and proteomic approaches for targeting of angiotensin-converting enzyme2 for cardiovascular diseases. Curr Opin Cardiol. 2008;23:364–369. doi: 10.1097/HCO.0b013e328303b79b. [DOI] [PubMed] [Google Scholar]
- 3.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 4.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 5.Dive V, Chang C-F, Yiotakis A, Sturrock ED. Inhibition of zinc metallopeptidases in cardiovascular disease--from unity to trinity, or duality? Curr Pharm Des. 2008;15:3606–3621. doi: 10.2174/138161209789271889. [DOI] [PubMed] [Google Scholar]
- 6.Takai S, Shiota N, Sakaguchi M, Muraguchi H, Matsumura E, Miyazaki M. Characterization of chymase from human vascular tissues. Clin Chim Acta. 1997;265:13–20. doi: 10.1016/s0009-8981(97)00114-9. [DOI] [PubMed] [Google Scholar]
- 7.Acharya KR, Sturrock ED, Riordan JF, Ehlers MR. Ace revisited: a new target for structure-based drug design. Nat Rev Drug Discov. 2003;2:891–902. doi: 10.1038/nrd1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustafa T, Lee JH, Chai SY, Albiston AL, McDowall SG, Mendelsohn FA. Bioactive angiotensin peptides: focus on angiotensin IV. J Renin Angiotensin Aldosterone Syst. 2001;2:205–210. doi: 10.3317/jraas.2001.032. [DOI] [PubMed] [Google Scholar]
- 9.Balestrieri ML, Napoli C. Novel challenges in exploring peptide ligands and corresponding tissue-specific endothelial receptors. Eur J Cancer. 2007;43:1242–1250. doi: 10.1016/j.ejca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Barrios AM, Craik CS. Scanning the prime-site substrate specificity of proteolytic enzymes: a novel assay based on ligand-enhanced lanthanide ion fluorescence. Bioorg Med Chem Lett. 2002;12:3619–3623. doi: 10.1016/s0960-894x(02)00786-2. [DOI] [PubMed] [Google Scholar]
- 11.Boulware KT, Daugherty PS. Protease specificity determination by using cellular libraries of peptide substrates (CLiPS) Proc Natl Acad Sci USA. 2006;103:7583–7588. doi: 10.1073/pnas.0511108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cusick ME, Klitgord N, Vidal M, Hill DE. Interactome: gateway into systems biology. Hum Mol Genet. 2005;14:R171–R181. doi: 10.1093/hmg/ddi335. [DOI] [PubMed] [Google Scholar]
- 13.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 14.Parrish JR, Gulyas KD, Finley RL., Jr Yeast two-hybrid contributions to interactome mapping. Curr Opin Biotechnol. 2006;17:387–393. doi: 10.1016/j.copbio.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 16.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 17.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 19.Picotti P, Rinner O, Stallmach R, Dautel F, Farrah T, Domon B, Wenschuh H, Aebersold R. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat Methods. 2010;7:43–46. doi: 10.1038/nmeth.1408. [DOI] [PubMed] [Google Scholar]
- 20.Marcon L, Battersby BJ, Rühmann A, Ford K, Daley M, Lawrie GA, Trau M. 'On-the-fly' optical encoding of combinatorial peptide libraries for profiling of protease specificity. Mol Biosyst. 2010;6:225–233. doi: 10.1039/b909087h. [DOI] [PubMed] [Google Scholar]
- 21.Baruch A, Jeffery DA, Bogyo M. Enzyme activity--it's all about image. Trends Cell Biol. 2004;14:29–35. doi: 10.1016/j.tcb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Gevaert K, Goethals M, Martens L, van Damme J, Staes A, Thomas GR, Vandekerckhove J. Exploring proteomes and analyzing protein processing by mass spectrometric identification of sorted N-terminal peptides. Nat Biotech. 2003;21:566–569. doi: 10.1038/nbt810. [DOI] [PubMed] [Google Scholar]
- 23.Cravatt BF, Wright A, Kozarich J. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 24.Robinson S, Niles RK, Witkowska HE, Rittenbach KJ, Nichols RJ, Sargent JA, Dixon SE, Prakobphol A, Hall SC, Fisher SJ, Hardt M. A mass spectrometry-based strategy for detecting and characterizing endogenous proteinase activities in complex biological samples. Proteomics. 2008;8:435–445. doi: 10.1002/pmic.200700680. [DOI] [PubMed] [Google Scholar]
- 25.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igarashi Y, Heureux E, Doctor KS, Talwar P, Gramatikova S, Gramatikoff K, Zhang Y, Blinov M, Ibragimova SS, Boyd S, Ratnikov B, Cieplak P, Godzik A, Smith JW, Osterman AL, Eroshkin AM. PMAP: databases for analyzing proteolytic events and pathways. Nucleic Acids Res. 2009;37:D611–D618. doi: 10.1093/nar/gkn683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson T, Mann M, Aebersold R, Yates JR, Bairoch A, Bergeron JJM. Mass spectrometry in high-throughput proteomics: ready for the big time. Nat Meth. 2010;7:681–685. doi: 10.1038/nmeth0910-681. [DOI] [PubMed] [Google Scholar]
- 28.Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat Biotech. 2010;28:710–721. doi: 10.1038/nbt.1661. [DOI] [PubMed] [Google Scholar]
- 29.Mallick P, Kuster B. Proteomics: a pragmatic perspective. Nat Biotech. 2010;28:695–709. doi: 10.1038/nbt.1658. [DOI] [PubMed] [Google Scholar]
- 30.Gevaert K, Vandekerckhove J. Protein identification methods in proteomics. Electrophoresis. 2000;21:1145–1154. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1145::AID-ELPS1145>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov VT, Yatskin ON. Peptidomics: a logical sequel to proteomics. Expert Rev Proteomics. 2005;2:463–473. doi: 10.1586/14789450.2.4.463. [DOI] [PubMed] [Google Scholar]
- 32.Menschaert G, Vandekerckhove TTM, Baggerman G, Schoofs L, Luyten W, van Criekinge W. Peptidomics coming of age: a review of contributions from a bioinformatics angle. J Proteome Res. 2010;9:2051–2061. doi: 10.1021/pr900929m. [DOI] [PubMed] [Google Scholar]
- 33.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 34.Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem. 2007;389:1017–1031. doi: 10.1007/s00216-007-1486-6. [DOI] [PubMed] [Google Scholar]
- 35.Gevaert K, Impens F, Ghesquière B, van Damme P, Lambrechts A, Vandekerckhove J. Stable isotopic labeling in proteomics. Proteomics. 2008;8:4873–4885. doi: 10.1002/pmic.200800421. [DOI] [PubMed] [Google Scholar]
- 36.Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci USA. 2004;101:6917–6922. doi: 10.1073/pnas.0305862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson A, Schäfer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AKA, Hamon C. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 38.Ross P, Huang Y, Marchese J, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin D. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Choe L, D’Ascenzo M, Relkin NR, Pappin D, Ross P, Williamson B, Guertin S, Pribil P, Lee KH. 8-plex quantitation of changes in cerebrospinal fluid protein expression in subjects undergoing intravenous immunoglobulin treatment for Alzheimer’s disease. Proteomics. 2007;7:3651–3660. doi: 10.1002/pmic.200700316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayon L, Hainard A, Licker V, Turck N, Kuhn K, Hochstrasser DF, Burkhard PR, Sanchez J-C. Relative quantification of proteins in human cerebrospinal fluids by MS/MS using 6-plex isobaric tags. Anal Chem. 2008;80:2921–2931. doi: 10.1021/ac702422x. [DOI] [PubMed] [Google Scholar]
- 41.Hardt M, Witkowska HE, Webb S, Thomas LR, Dixon SE, Hall SC, Fisher SJ. Assessing the effects of diurnal variation on the composition of human parotid saliva: quantitative analysis of native peptides using iTRAQ reagents. Anal Chem. 2005;77:4947–4954. doi: 10.1021/ac050161r. [DOI] [PubMed] [Google Scholar]
- 42.Dean R, Smith D, Overall CM. Proteomic identification of cellular protease substrates using isobaric tags for relative and absolute quantification (iTRAQ). Chapter 21. Curr Protoc Protein Sci. 2007 doi: 10.1002/0471140864.ps2118s49. [DOI] [PubMed] [Google Scholar]
- 43.Kruger M, Moser M, Ussar S, Thievessen I, Luber CA, Forner F, Schmidt S, Zanivan S, Fässler R, Mann M. SILAC mouse for quantitative proteomics uncovers kindling-3 as an essential factor for red blood cell function. Cell. 2008;134:353–364. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Neher SB, Villen J, Oakes EC, Bakalarski CE, Sauer RT, Gygi SP, Baker TA. Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Mol Cell. 2006;22:193–204. doi: 10.1016/j.molcel.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Plasman K, van Damme P, Kaiserman D, Impens F, Demeyer K, Helsens K, Goethals M, Bird PI, Vandekerckhove J, Gevaert K. Probing the efficiency of proteolytic events by positional proteomics. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.003301. M110.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agard NJ, Wells JA. Methods for the proteomic identification of protease substrates. Curr Opin Chem Biol. 2009;13:503–509. doi: 10.1016/j.cbpa.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmer JC, Enoksson M, Wildfang E, Zhu W, Igarashi Y, Denault J-B, Ma Y, Dummitt B, Chang Y-H, Mast AE, Eroshkin A, Smith JW, Tao WA, Salvesen GS. Profiling constitutive proteolytic events in vivo. Biochem J. 2007;407:41–48. doi: 10.1042/BJ20070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wildes D, Wells JA. Sampling the N-terminal proteome of human blood. Proc Natl Acad Sci USA. 2010;107:4561–4566. doi: 10.1073/pnas.0914495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonald L, Robertson DH, Hurst JL, Beynon RJ. Positional proteomics: selective recovery and analysis of N-terminal proteolytic peptides. Nat Methods. 2005;2:955–957. doi: 10.1038/nmeth811. [DOI] [PubMed] [Google Scholar]
- 50.Schilling O, Barré O, Huesgen PF, Overall CM. Proteome-wide analysis of protein carboxy termini: C terminomics. Nat Methods. 2010;7:508–511. doi: 10.1038/nmeth.1467. [DOI] [PubMed] [Google Scholar]
- 51.Lu S, Jiang K, Qin F, Lu X, Li F. Simultaneous quantification of enalapril and enalaprilat in human plasma by high-performance liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study. J Pharm Biomed Anal. 2009;49:163–167. doi: 10.1016/j.jpba.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Ferreiros N, Dresen S, Alonso RM, Weinmann W. Validated quantitation of angiotensin II receptor antagonists (ARA-II) in human plasma by liquid-chromatography-tandem mass spectrometry using minimum sample clean-up and investigation of ion suppression. Ther Drug Monit. 2007;29:824–834. doi: 10.1097/FTD.0b013e31815d0f66. [DOI] [PubMed] [Google Scholar]
- 53.Barglow KT, Cravatt BF. Activity-based protein profiling for the functional annotation of enzymes. Nat Methods. 2007;4:822–827. doi: 10.1038/nmeth1092. [DOI] [PubMed] [Google Scholar]
- 54.Khan AR, James MN. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 1998;7:815–836. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobe B, Kemp BE. Active site-directed protein regulation. Nature. 1999;402:373–376. doi: 10.1038/46478. [DOI] [PubMed] [Google Scholar]
- 56.Evans MJ, Cravatt BF. Mechanism-based profiling of enzyme families. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 57.Sadaghiani AM, Verhelst SH, Bogyo M. Tagging and detection strategies for activity-based proteomics. Curr Opin Chem Biol. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 58.Greenbaum D, Baruch A, Hayrapetian L, Darula Z, Burlingame A, Medzihradszky KF, Bogyo M. Chemical approaches for functionally probing the proteome. Mol Cell Proteomics. 2002;1:60–68. doi: 10.1074/mcp.t100003-mcp200. [DOI] [PubMed] [Google Scholar]
- 59.Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics. 2001;1:1067–1071. doi: 10.1002/1615-9861(200109)1:9<1067::AID-PROT1067>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 60.Jessani N, Niessen S, Wei BQ, Nicolau M, Humphrey M, Ji Y, Han W, Noh DY, Yates JR, 3rd, Jeffrey SS, Cravatt BF. A streamlined platform for high-content functional proteomics of primary human specimens. Nat Methods. 2005;2:691–697. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- 61.Adam GC, Burbaum J, Kozarich JW, Patricelli MP, Cravatt BF. Mapping enzyme active sites in complex proteomes. J Am Chem Soc. 2004;126:1363–1368. doi: 10.1021/ja038441g. [DOI] [PubMed] [Google Scholar]
- 62.Schlüter H, Hildebrand D, Gallin C, Schulz A, Thiemann J, Trusch M. Mass spectrometry for monitoring protease reactions. Anal Bioanal Chem. 2008;392:783–792. doi: 10.1007/s00216-008-2213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tung C-H. Fluorescent peptide probes for in vivo diagnostic imaging. Biopolymers. 2004;76:391–403. doi: 10.1002/bip.20139. [DOI] [PubMed] [Google Scholar]
- 64.Liesener A, Karst U. Monitoring enzymatic conversions by mass spectrometry: a critical review. Anal Bioanal Chem. 2005;382:1451–1464. doi: 10.1007/s00216-005-3305-2. [DOI] [PubMed] [Google Scholar]
- 65.Schlüter H, Jankowski J, Rykl J, Thiemann J, Belgardt S, Zidek W, Wittmann B, Pohl T. Detection of protease activities with the mass-spectrometry-assisted enzyme-screening (MES) system. Anal Bioanal Chem. 2003;377:1102–1107. doi: 10.1007/s00216-003-2211-8. [DOI] [PubMed] [Google Scholar]
- 66.Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, Fleisher M, Lilja H, Brogi E, Boyd J, Sanchez-Carbayo M, Holland EC, Cordon-Cardo C, Scher HI, Tempst P. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J Clin Invest. 2006;116:271–284. doi: 10.1172/JCI26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharon N, Grisaro V, Neumann H. Pepsin-catalyzed exchange of oxygen atoms between water and carboxylic acids. Arch Biochem Biophys. 1962;97:219–221. doi: 10.1016/0003-9861(62)90070-x. [DOI] [PubMed] [Google Scholar]
- 68.Yao X, Afonso C, Fenselau C. Dissection of proteolytic 18O labeling: endoprotease-catalyzed 16O-to-18O exchange of truncated peptide substrates. J Proteome Res. 2003;2:147–152. doi: 10.1021/pr025572s. [DOI] [PubMed] [Google Scholar]
- 69.Villanueva J, Nazarian A, Lawlor K, Tempst P. Monitoring peptidase activities in complex proteomes by MALDI-TOF mass spectrometry. Nature Protoc. 2009;4:1167–1183. doi: 10.1038/nprot.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niles R, Witkowska HE, Allen S, Hall SC, Fisher SJ, Hardt M. Acid-catalyzed oxygen-18 labeling of peptides. Anal Chem. 2009;81:2804–2809. doi: 10.1021/ac802484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rykl J, Thiemann J, Kurzawski S, Pohl T, Gobom J, Zidek W, Schlüter H. Renal cathepsin G and angiotensin II generation. J Hypertens. 2006;24:1797–1807. doi: 10.1097/01.hjh.0000242404.91332.be. [DOI] [PubMed] [Google Scholar]
- 72.Johnson GD, Stevenson T, Ahn K. Hydrolysis of peptide hormones by endothelin-converting enzyme-1. A comparison with neprilysin. J Biol Chem. 1999;274:4053–4058. doi: 10.1074/jbc.274.7.4053. [DOI] [PubMed] [Google Scholar]
- 73.Fahnoe DC, Knapp J, Johnson GD, Ahn K. Inhibitor potencies and substrate preference for endothelin-converting enzyme-1 are dramatically affected by pH. J Cardiovasc Pharmacol. 2000;36:S22–S25. doi: 10.1097/00005344-200036051-00009. [DOI] [PubMed] [Google Scholar]
- 74.Mann M, Kelleher NL. Precision proteomics: the case for high resolution and high mass accuracy. Proc Natl Acad Sci USA. 2008;105:18132–18138. doi: 10.1073/pnas.0800788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 76.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]