Abstract
The term synovioma was coined by Smith in 1927, and later in 1936 Knox suggested the name synovial sarcoma. It occurs primarily in the paraarticular regions, usually in close association with tendon sheaths, bursae, and joint capsules. On rare occasions it may be seen in areas without any apparent relationship to synovial structures as in parapharyngeal region or the abdominal cavity. The first description of synovial sarcoma in the head and neck region was by Pack and Ariel in 1950. The majority of these tumors seem to take origin from paravertebral connective tissue spaces and manifest as solitary retropharyngeal or parapharyngeal masses near the carotid bifurcation. Synovial sarcoma has been reported in soft palate, tongue, maxillofacial region, angle of mandible, sternoclavicular region, scapular region, and the esophagus. We report a case of 28-year-old male patient with synovial sarcoma in mandibular region with biphasic pattern.
Keywords: Biphasic pattern, head and neck, synovial sarcoma
INTRODUCTION
Synovial sarcoma is a clinically and morphologically defined entity accounts for 8–10% of all soft tissue malignancies making it the fourth most common soft tissue malignancy after malignant fibrous histiocytoma, liposarcoma, and rhabdomyosarcoma. Earlier cases of synovial sarcoma were diagnosed under misnomers such as adenosarcoma, perithelial sarcoma, synovial sarcoendothelioma, sarcomesothelioma, and mesothelioma of joints. Steur (1893) and Lejars and Rubens-Duval (1910) are credited with first accurate description of synovial sarcoma.[1]
The head and neck synovial sarcomas account for 6.8% of all synovial sarcomas occurring in the body. The first reported case of head and neck synovial sarcoma was described by Jernstrom in 1954 in a case involving the pharynx.[2] The most common sites involved in the head and neck region include the hypopharynx, postpharyngeal region, and the parapharyngeal space. A few cases in the tongue, soft palate, mandible, buccal mucosa, and floor of the mouth have been described in the literature.[3–7] It sometimes appears in locations unrelated to synovium, and thus its origin remains unknown. It is now generally accepted that synovial sarcomas originate from undifferentiated or pluripotent mesenchymal cells with a dual differentiation capacity, both epithelial and mesenchymal.[8]
We are hereby reporting a very rare case of biphasic synovial sarcoma in mandibular region occurring after hemimandibulectomy. The patient was earlier treated for the ameloblastoma of the jaw and hemimandibulectomy was done. In our literature search, we did not come across any example of any connective tissue malignancy occurring after the removal of an odontogenic epithelial tumor, although there were few case reports of coexistence of different types of tumors in the salivary glands.
CASE REPORT
A 28-year-old male patient reported to the outpatient department of the Sharad Pawar Dental College and hospital with chief complaint of swelling in the region of angle of mandible on right side since 1 year. Patient also complained of occasional pus discharge intraorally and tingling sensation on the lower lip, as well as loss of appetite and weight loss.
Patient gave a history of surgical procedure on the right side 3 years back for ameloblastoma. Hemimandibulectomy was carried out on the right side and reconstruction was done with reconstruction plates. The patient was disease free for a period of 2 years when suddenly he noticed a peanut-sized swelling 1 year back and reported that the increase in the size of the swelling was gradual. The swelling grew to the present size of 4 × 3 cm over a period of 1 year.
Extraoral examination revealed facial asymmetry due to the presence of swelling on the right side. The overlying skin was normal. Further examination revealed a single, round to oval, well-defined swelling measuring 4 × 3 cm on right side of the face. The swelling was present just anterior to the region of the angle of the mandible extending anteriorly 2 cm short of angle of mouth. Superioinferiorly the swelling was extending 1.5 cm below the region of lower border of mandible. No draining sinuses were found extraorally [Figure 1].
Figure 1.
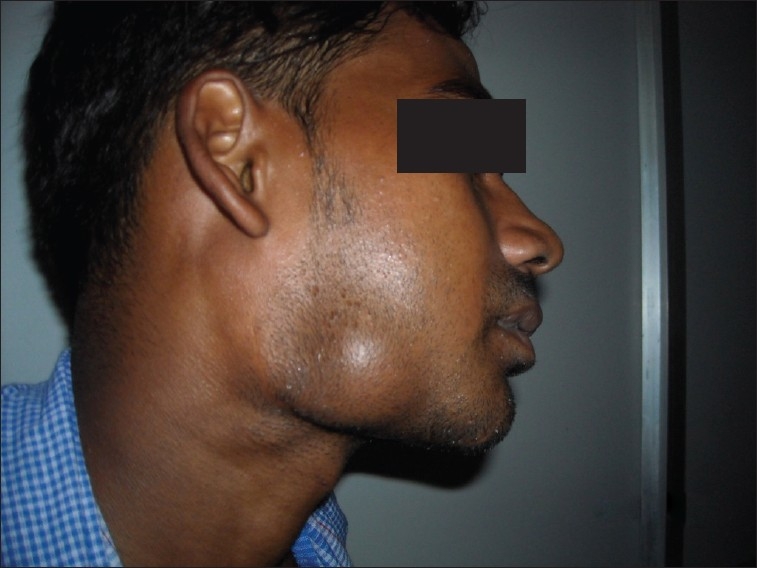
Extraoral extent of the lesion anteriposterorly and superioinferiorly
On palpation the swelling was mobile and not fixed to the underlying tissues. There was no raised temperature of the involved skin. The swelling was non-tender, and all the inspectory findings were reconfirmed.
Lymph node examination revealed enlargement of the jugulodigastric, retromandibular, and submandibular lymph nodes with the largest lymph node measuring 26 × 24 mm.
Intraorally there were no significant findings. The teeth and the mandible on the affected side were missing and no draining sinuses were detected intraorally.
Orthopantomograph revealed the absence of the mandible on the right side and the presence of reconstruction plates. No other significant finding was noted [Figure 2].
Figure 2.
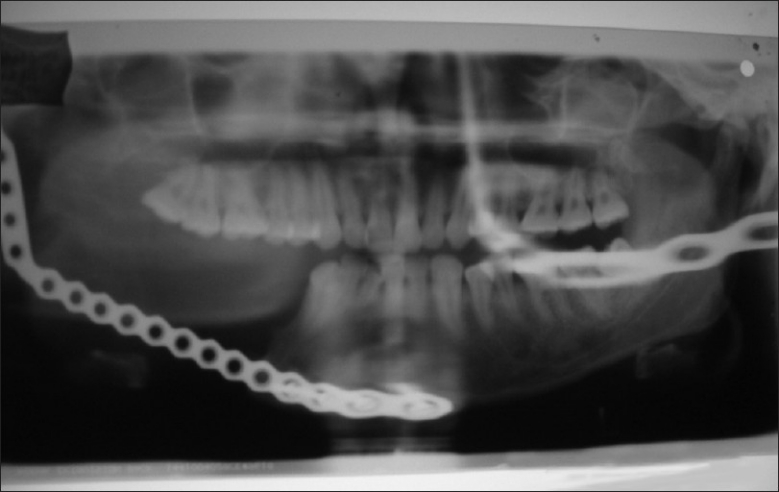
Radiograph showing reconstruction of jaw on right side but no lesional changes are observed
A provisional clinical and radiological diagnosis of recurrence of ameloblastoma or malignant ameloblastoma was made.
Fine needle aspiration cytology was performed, and smear showed presence of few basaloid cells with nuclear palisading present in clusters with myxoid material in the background; a diagnosis of ameloblastic fibroma was rendered.
Incisional biopsy revealed the presence of morphologically different types of cells resembling those of epithelium interspersed in a highly mature connective tissue stroma.
The epithelial cells had large, round, vesicular nuclei and pale staining cytoplasm. Some of the epithelial cells formed glandular structures that included a central area of cystic degeneration. In some areas, epithelial cells were seen lining cyst-like spaces in abundant connective tissue stroma [Figures 3 and 4].
Figure 3.
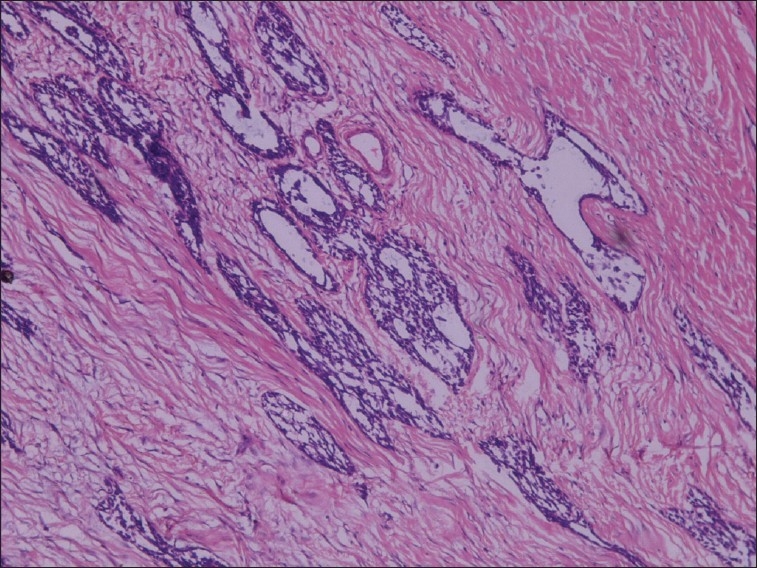
Epithelial islands showing cystic degeneration and mature connective tissue stroma (H and E stain, 10×)
Figure 4.
Hyperchromatic connective tissue stroma with streaming pattern and epithelial cells lining duct-like spaces (H and E stain, 10×)
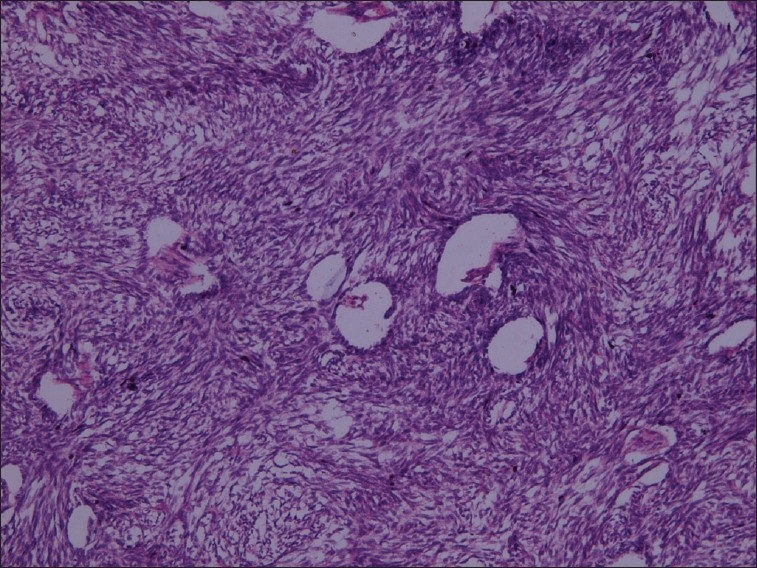
There was abundant fibrous stroma that contained plump spindle-shaped cells with oval nuclei that was hyperchromatic. In some areas a herring bone pattern was seen [Figure 5].
Figure 5.
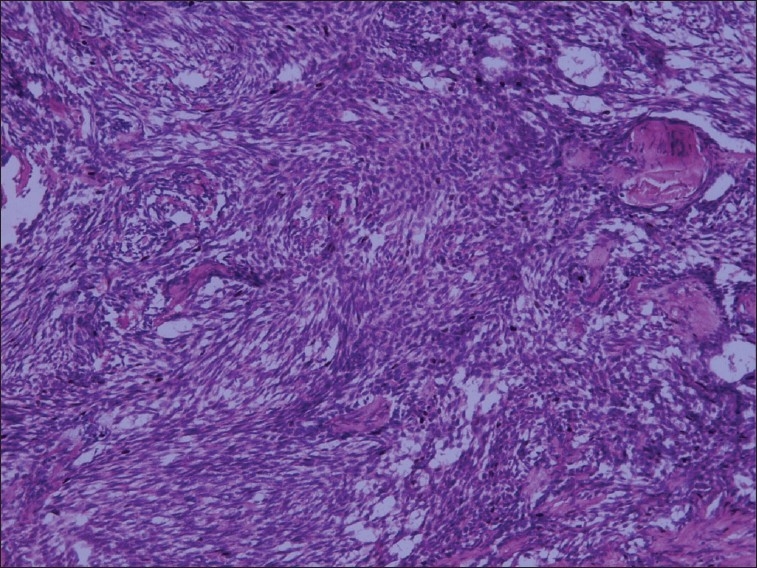
Spindle-shaped cells and oval to round epithelial cells (H and E stain, 10×)
A histopathological differential diagnosis of ameloblastic fibrosarcoma or carcinosarcoma was made.
Earlier slides of the patient were retrieved from the archives of the department and it reconfirmed the first diagnosis of ameloblastoma.
The patient underwent surgery with wide local excision and removal of involved lymph nodes.
The excised specimen was further submitted for histopathological screening. The features were consistent with the earlier findings of incisional biopsy. The lymph node examination revealed tumor infiltration of the regional lymph nodes.
The excised specimen was subjected to immunohistochemical analysis. Immunohistochemical staining revealed positivity for pancytokeratin in epithelial cells and spindle cells, and vimentin was positive for only the fibrous stroma [Figures 6–8]. A weak reactivity was observed with epithelial membrane antigen and carcinoembryonic antigen. A final diagnosis of biphasic synovial sarcoma was made because the spindle cells and epithelial cells were both positive for pancytokeratin.
Figure 6.
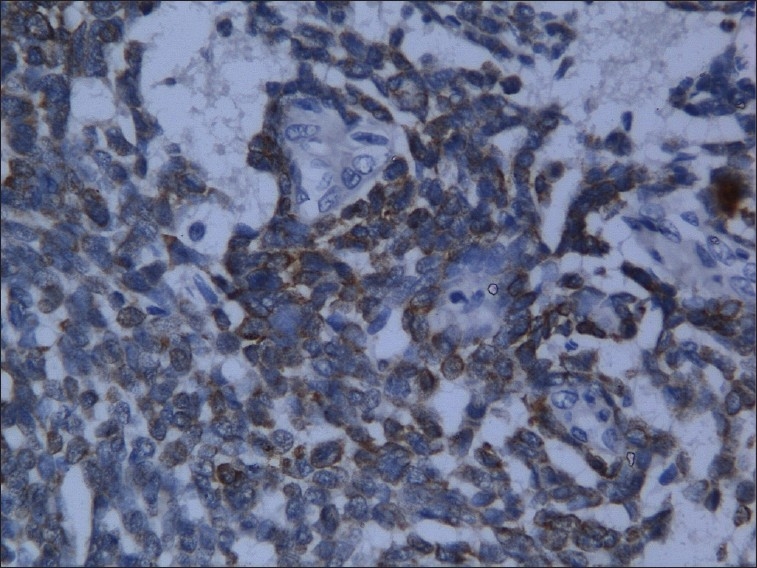
Epithelial cells positive for pancytokeratin (H and E stain, 40×)
Figure 8.
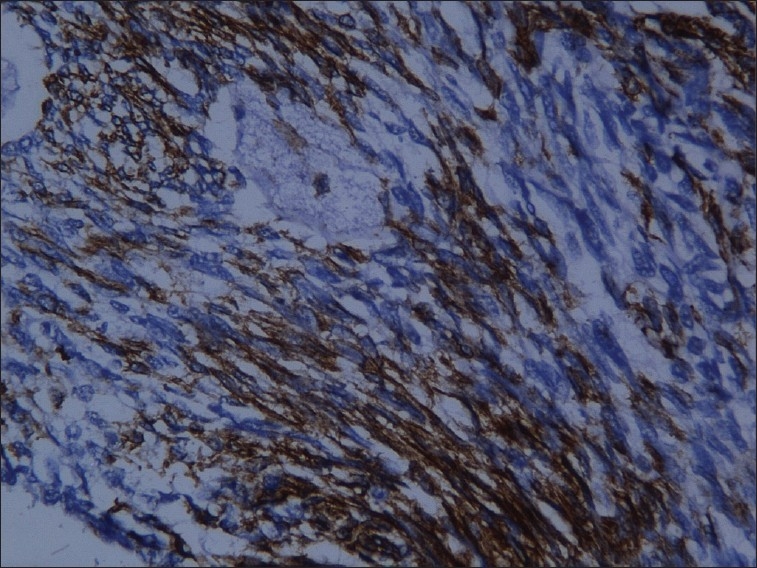
Connective tissue cells positive for vimentin (H and E stain, 20×)
Figure 7.
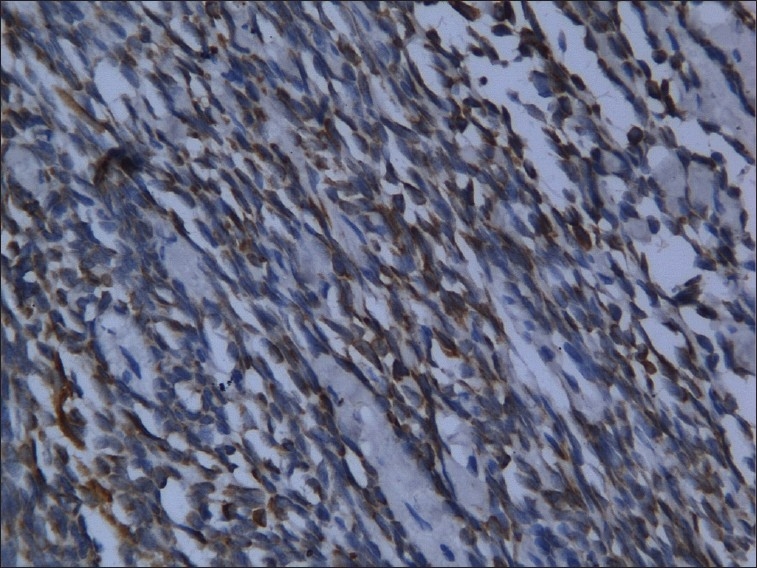
Spindle cells positive for pancytokeratin (H and E stain, 20×)
DISCUSSION
Synovial sarcoma occurs primarily in the paraarticular regions, usually in close association with tendon sheaths, bursae, and joint capsules. Sometimes it may be encountered in areas completely unrelated to synovial structures such as in the parapharyngeal region or the abdominal wall.[1]
Synovial sarcoma like most other sarcomas arises from primitive cells that have the potential to differentiate into either mesenchymal or epithelial components.[1] Mittinen and Virtanen in 1984 suggested that there are distinct differences between synovial lining cells and synovial sarcoma, and synovial sarcoma is a carcinosarcoma-like tumor with true epithelial differentiation, and the term “synovial sarcoma” apparently is a misnomer that should be abandoned.[9]
Less than 100 cases of synovial sarcoma in the head and neck sites have been reported, but the occurrence of mandibular region synovial sarcoma is extremely rare. In our review and search of literature we came across only two case reports of mandibular synovial sarcoma. Because of rarity of this neoplasm in the orofacial region, it is more likely to be misdiagnosed.[6,7]
Roth et al. reported 24 cases in 1975. These patients had a median age of 19 years (range 10–51) with a male-to-female ratio of 7:5, and these authors concluded that patients are more frequently affected in the early years of life (second to third decade).[2]
Majority of synovial sarcomas radiographically present with round or more or less lobulated swellings or masses with varying density. The underlying bone tends to be uninvolved.[1]
Hirsch et al. in 1997 described magnetic resonance images of six synovial sarcomas of the head and neck to determine their characteristic sites of origin, size, extent, intensity, and contrast enhancement. They concluded that a non-mucosal head and neck mass that is isointense to gray matter on T1-weighted images and is well defined yet heterogeneous, with septations, hemorrhage, cysts, calcification, or multilocularity, should raise suspicion of a synovial sarcoma. Because the appearance of synovial sarcomas varies and other masses may appear similar, no specific imaging characteristics define the entity.[10]
Molecular studies of synovial sarcomas in other parts of the body reveal a characteristic translocation t(X;18)(p11.2;q11.2).[11] Bridge et al in 1988 in a cytogenetic analysis of a synovial sarcoma of the base of the tongue showed a reciprocal translocation involving chromosomes X and 18 t(X;18)(pl1.2;q11.2). The same translocation was reported to be characteristic of synovial sarcomas of the extremities. They stated that this translocation in an oral synovial sarcoma confirms the unity of origin of this neoplasm with the far more common synovial sarcomas of the extremity. Karyotypic analysis may prove useful in confirming the diagnosis of this uncommon neoplasm.[12] Genetic trials were not carried out for this particular case.
Histologically synovial sarcoma is composed of two morphologically different types of cells that form a characteristic biphasic pattern: epithelial cells resembling those of carcinoma and fibrosarcoma-like spindle cells. There are transitions between epithelial and spindle cells suggesting a close generic relationship. Histological variants of SS are subclassified into four types: (1) biphasic type with distinct epithelial and spindle cell components present in various proportions and patterns, (2) monophasic spindle cell type with little or no evidence of epithelial differentiation, (3) monophasic epithelial type, and (4) poorly differentiated type.[8] Immunohistochemically, both epithelial and spindle cells are reactive with cytokeratin and epithelial membrane antigen, while only the spindle cells are positive for vimentin.[11]
Due to the lack of cases in maxillofacial area, the information regarding appropriate therapy for this tumor is limited. However, adequate surgical excision with adequate follow-up seems to be the treatment of choice. Post-treatment recurrence rate for synovial sarcoma arising from all body sites is about 70%. Usually local recurrence is expected within the first 2 years of treatment but can appear up to 20 years. Metastasis are usually blood borne to lungs (94%).[7] Five-year survival rate is about 36–51%. Prognosis is affected by tumor size, location, patient age, histological subtype, extent, mitotic activity, and margin of resection. Despite advances in the treatment of local disease, distant metastasis remains the predominant cause of death.[8]
CONCLUSION
In summary, this report presents a very rare case report of synovial sarcoma occurring in the mandibular body region after the excision of mandible. The final diagnoses were confirmed by histological and immunohistochemical means. The patient underwent surgical resection with wide excision and removal of lymph nodes. Patient is on periodic recall and there has been no recurrence after 1 year of surgery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Enzinger FM, Weiss SW. St Louis: CV Mosby; 1983. Soft tissue tumors; pp. 659–88. [Google Scholar]
- 2.Bertolini F, Bianchi B, Pizzigallo A, Tullio A, Sesenna E. Synovial cell sarcoma of the neck.Case report and review of the literature. Acta Otorhinolaryngol Ital. 2003;23:391–5. [PubMed] [Google Scholar]
- 3.Carrillo R, El-Naggar AK, Rodriguez-Peralto JL, Batsakis JG. Synovial sarcoma of the tongue: Case report and review of the literature. J Oral Maxillofac Surg. 1992;50:904–6. doi: 10.1016/0278-2391(92)90288-b. [DOI] [PubMed] [Google Scholar]
- 4.Sun JJ, Rasgon BM, Wild TW, Hilsinger RL., Jr Synovial cell sarcoma of the maxillary sinus: A first reported case. Otolaryngol Head Neck Surg. 2003;129:587–90. doi: 10.1016/S0194-59980301392-5. [DOI] [PubMed] [Google Scholar]
- 5.Goebel WM, High CJ, Kiviat J, Stoeckel DC. Anterior buccal mucosal mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:667–71. doi: 10.1016/j.tripleo.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Koga C, Harada H, Kusukawa J, Kameyama T. Synovial sarcoma arising in the mandibular bone. Oral Oncology EXTRA. 2005;41:45–8. [Google Scholar]
- 7.Tilakratne WM. Synovial sarcoma of the mandible. J Oral Pathol Med. 2006;35:61–3. doi: 10.1111/j.1600-0714.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Zhang J, He X, Niu Y. Synovial sarcoma in the oral and maxillofacial region: Report of 4 cases and review of the literature. Oral Maxillofac Surg. 2008;66:161–7. doi: 10.1016/j.joms.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Mittinen M, Virtanen I. Synovial sarcoma: A misnomer. Am J Pathol. 1984;117:18–25. [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch RJ, Yousem DM, Loevner LA, Montone KT, Chalian AA, Hayden RE, et al. Synovial sarcomas of the head and neck: MR Findings. Am J Roentgenol. 1997;169:1185–8. doi: 10.2214/ajr.169.4.9308488. [DOI] [PubMed] [Google Scholar]
- 11.Barnes L, Eveson JW, Reichart P, Sidransky D. Lyon: IARC Press; 2005. World Health Organization classification of tumors.Pathology and genetics, malignant soft tissue tumors; p. 149. [Google Scholar]
- 12.Bridge JA, Bridge RS, Borek DA, Shaffer BS, Norris CW. Translocation t(X; 18) in orofacial synovial sarcoma. Cancer. 1988;62:935–7. doi: 10.1002/1097-0142(19880901)62:5<935::aid-cncr2820620514>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]


