Abstract
Background:
Oral submucous fibrosis is a chronic debilitating disease of oral mucosa and is characterized by generalized fibrosis of the oral soft tissues which tends to present itself clinically as palpable vertical fibrous bands. Hence, this study was conducted to evaluate histopathologically the collagen fiber bundle orientation in relation to epithelium and to find the reason for unidirectional orientation of clinically palpable fibrous bands.
Materials and Methods:
The study included 33 cases of oral submucous fibrosis and 8 cases of normal tissue.
Results:
Histologically most of the collagen fibers were parallel to the epithelium, and there was a statistically significant difference in orientation between oral submucous fibrosis and control groups in both buccal mucosa and labial mucosa.
Conclusion:
The reason for unidirectional alignment of clinical fibrous bands could be due to chronic stimulation of oral mucosa by the irritants leading to change in the orientation of collagen fiber bundles, which might result in scar formation similar to that of wound healing, where the collagen fibers are oriented parallel to the epidermis.
Keywords: Buccal mucosa, collagen orientation, labial mucosa, oral submucous fibrosis
INTRODUCTION
Oral submucous fibrosis (OSF) is a chronic fibrotic disease of oral cavity and oropharynx associated with the chewing of areca quid.[1] This condition occurs predominantly among Indians and to a much lesser extent in other Asian population.[2] The condition affects approximately 0.5% (5 million) of the population of the Indian subcontinent.[3]
Being a chronic disease of oral mucosa, it is characterized by generalized fibrosis of the oral soft tissues resulting in marked rigidity and progressive inability to open the mouth. The most common initial symptoms of OSF are burning sensation of oral mucosa aggravated by spicy food, followed by either hypersalivation or dryness of the mouth.[4]
Associated features include pigmentation changes, vesicles, ulcerations, and petechiae. The most commonly affected locations are buccal mucosa where the fibrosis tends to present itself clinically from early to advanced stages in the form of palpable fibrous bands running vertically.[5]
Other areas being involved are the soft palate where the fibrous bands radiate from pterygomandibular raphae or anterior faucial pillar. Uvula is markedly involved in later stages. It may also involve labial mucosa and tongue. The disease exhibits regional variations in regard to the choice of site involvement, its extent, and the type of involvement.[6]
A large number of studies have been reported in the literature on changes in oral mucosa in OSF. However, the question why the fibrous bands run vertically and not horizontally clinically does not appear to have been addressed.
Hence, this study is aimed at evaluating the fibrous bands clinically and histologically, and to ascertain whether the direction in which collagen fibers are laid down, has any relationship with the movement of tissues. This study also intends to review the published literature on factors controlling collagen synthesis, deposition, and orientation in various tissue including experimental studies, to find an answer to the way in which the collagen fibers are oriented in OSF.
The objectives of this study were as follows:
Clinical grading of severity of fibrosis based on the degree of mouth opening.
To study the direction of palpable and visible fibrous bands clinically.
To evaluate the orientation of collagen fiber bundles histopathologically by special stain and co-relate with normal tissue as control.
To co-relate the orientation of collagen fibers laid down with closing and opening movement of the mouth.
MATERIALS AND METHODS
The sample size was estimated to be a minimum of 33 cases. For comparison, eight normal healthy tissue specimens were obtained from surgically excised tissues of individuals who were undergoing oral surgeries for other reasons. Informed written consent was taken from both patients and control group before taking the biopsy. Ethical committee clearance was obtained before conducting the study.
Detailed history including details of relevant habits and clinical data were obtained and recorded in a pro forma. A clinical confirmation of diagnosis was performed based on the presence of one or more of the following parameters:
Palpable fibrous bands.
Leathery feeling of the mucosae: texture of the mucosae was determined by palpation using the index finger.
Mobility of the tongue, assessed according to the ability to protrude.
Burning sensation on eating spicy food: only the presence or absence was determined and no attempt was made to quantify.
Examination of fibrous bands by observation, palpation, and resistance to stretching was carried out. Clinical grading of severity of fibrosis was carried out based on the study conducted by Lai DR, in which the oral submucous fibrosis population was divided based on the interincisal distance as:
Group A—mouth opening greater than 35 mm.
Group B—mouth opening between 30 and 35 mm.
Group C—mouth opening between 20 and 30 mm.
Group D—mouth opening less than 20 mm.[7]
Cases belonging to Groups B, C, and D,which presented additionally with palpable vertical fibrous bands in buccal mucosa and circumoral bands in labial mucosa, were included in this study.
The patients were divided randomly into two groups as follows:
Group I—patients from whom biopsy was taken from buccal mucosa.
Group II—patients from whom biopsy was taken from labial mucosa.
A wedge-shaped incisional biopsy, perpendicular to the orientation of clinically palpable fibrous bands, measuring about 1–1.5 cms and 0.6 cm in depth in relation to surface of normal mucosa was obtained from patients with (OSF) lesions after taking informed consent. The obtained tissue was cut into two equal halves.
For comparison, eight samples of normal healthy tissue from both buccal and labial mucosa were taken from surgically excised tissues of individuals who were undergoing oral surgeries for other reasons. Each excised tissue was cut into two equal halves similar to the study group.
All the specimens were fixed with 10% neutral buffered formalin for 24 hours. The tissues were processed and sections of 5 µm thickness were obtained using soft tissue microtome from paraffin-embedded blocks. As the obtained tissue was cut into two equal halves, one half of the tissue was sectioned along the clinically observed plane of fibrous bands and labeled as A and the other perpendicular to the previous section was labeled as B. The sections were then stained with hematoxylin and eosin stain and picrosirius red stain.
Picrosirius red special stain was used because the sirius red, a strong cationic dye, stains collagen by reacting via its sulfonic acid groups with basic groups present in the collagen molecules. The elongated dye molecules are attached to collagen fibers in such a way that their long axis is parallel. This parallel relationship between dye and collagen molecules results in enhanced birefringency. The role of picric acid is to prevent the indiscriminate staining of non-collagenous structures by sirius red.[8]
Sections stained with hematoxylin and eosin stain and picrosirius red stain were then observed under both conventional light and polarized light microscope by two different observers, to evaluate the orientation of collagen fibers both in the buccal mucosa and labial mucosa.
Use of polarized light microscope to identify collagen orientation in picrosirius stained material was for two reasons-
Its specificity for collagen and
The increasing sensitivity and resolution provided by it.[8]
Histopathological diagnosis of oral submucous fibrosis was confirmed in relation to clinical diagnosis using hematoxylin and eosin sections.
Orientation of collagen fiber bundles in relation to epithelium was evaluated using picrosirius red–stained sections. The collagen fiber bundles underneath any five rete ridges measuring more than 10 μm in thickness and about 30 μm in length were captured using 3 chip CCD camera (Proview, Media cybernetics Davangere, Karnataka) with a 60× objective. Fibers inserting into the basement membrane were excluded from the study.
Orientation of collagen fiber bundles in relation to epithelium was assessed based on the following grading criteria:
Parallel in relation to epithelium.
Non-parallel in relation to epithelium.
Same criteria were applied to evaluate the orientation of collagen fiber bundle in control group. Orientation of loose fibers if any and the orientation of fiber bundles near and between muscle bundles were observed in all the slides.
All the data were entered in master chart for analysis and comparison. Statistical analysis was performed to evaluate to collagen fiber orientation in both buccal mucosa and labial mucosa using the clinical and histopathological findings in patients affected by oral submucous fibrosis.
RESULTS
Clinical features
Clinically the presenting signs and symptoms included burning sensation in 29 cases, blanching of mucosa in 33 cases, depapillation and restricted tongue movements in 2 cases, and difficulty in swallowing in 29 cases.
Clinical staging depending on the severity of fibrosis was graded based on the degree of mouth opening; out of 33 cases, 6 cases showed moderate fibrosis, 19 cases with severe fibrosis, and 8 cases, very severe fibrosis [Table 1].
Table 1.
Clinical signs and symptoms
Clinical examination of the direction of the palpable and visible fibrous bands showed vertical fibrous bands in buccal mucosa in all the 33 cases; 30 cases showed circumoral fibrous bands in labial mucosa and 25 of them had fibrous bands running along the direction of the palatal muscles [Table 1].
When the orientation of fibrous bands was correlated with the direction of vertical movement of the mandible, it was concluded that in all the cases the orientation of the fibrous bands laid down was in the direction of vertical movement of the mandible.
Histolopathological features
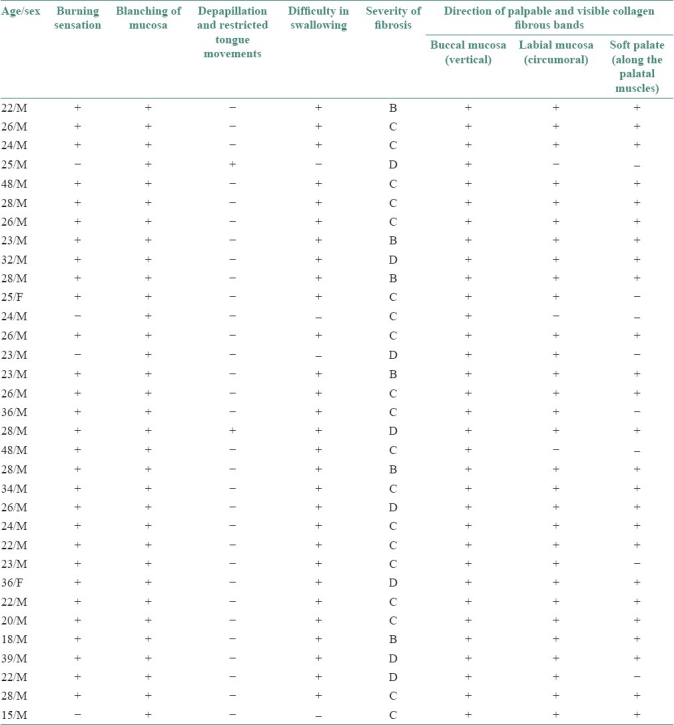
Histopathological examination of collagen fiber bundle orientation in buccal mucosa in relation to epithelium showed that 68% of fiber bundles were parallel to the epithelium [Figure 1], showing a statistically significant difference (P < 0.01) in the orientation of collagen fibers with control group [Figure 2, Table 2].
Figure 1.
Photomicrograph of thickened collagen fiber bundles parallel to epithelium in buccal mucosa sections (picrosirius stain, 40×)
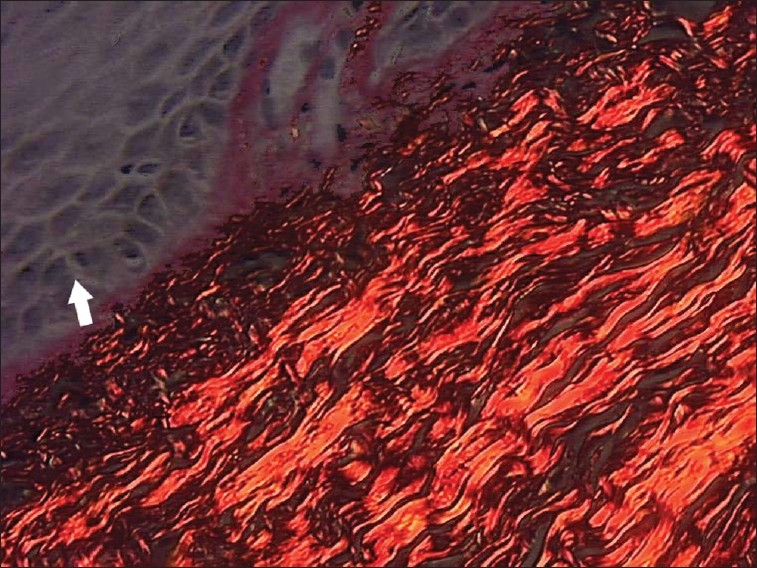
Figure 2.
Photomicrograph of haphazardly arranged thin collagen fibers in relation to epithelium in normal buccal mucosa sections (picrosirius stain, 40×)
Table 2.
Comparison of collagen fiber bundle orientation in relation to epithelium in buccal mucosa with reference to control

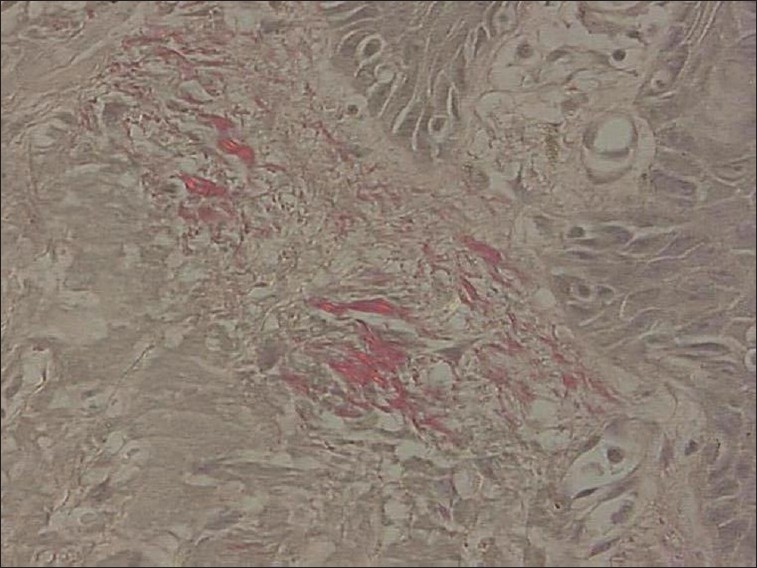
In the labial mucosa specimens of oral submucous fibrosis patients, it was observed that 78% of fibers were parallel to the epithelium [Figure 3]. And when compared with the normal [Figure 4] there was a highly statistically significant difference (P < 0.001) in the orientation between the study and control groups [Table 3].
Figure 3.
Photomicrograph of thickened collagen fiber bundles parallel to epithelium in labial mucosa sections (picrosirius stain, 40×)
Figure 4.
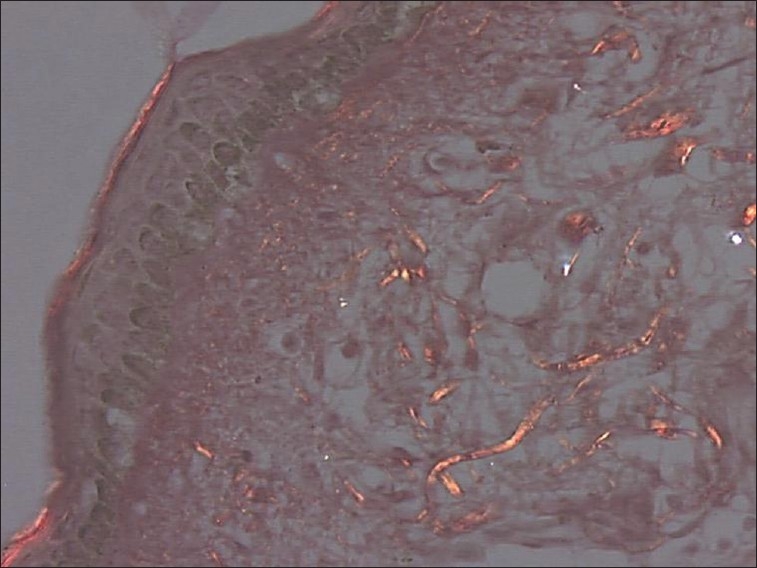
Photomicrograph of haphazardly arranged thin collagen fibers in relation to epithelium in normal labial mucosa sections (picrosirius stain, 40×)
Table 3.
Comparison of collagen fiber bundle orientation in relation to epithelium in labial mucosa with reference to control

When the orientation of collagen fibers was compared in sections A and B of buccal and labial mucosa, it was noticed that there was no statistically significant difference (P > 0.01 and P > 0.6) in both buccal and labial mucosa [Figure 5].
Figure 5.
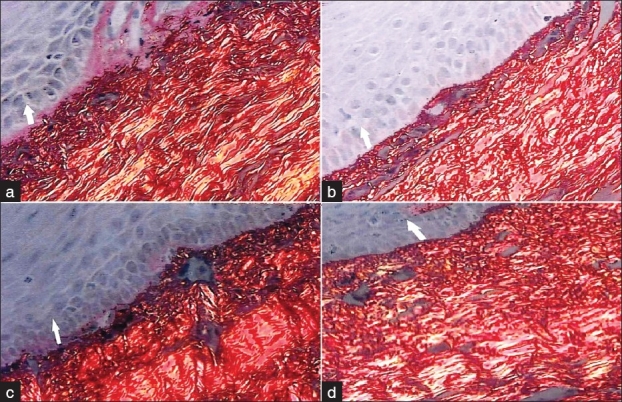
Photomicrograph showing no difference in collagen fiber bundle orientation in relation to epithelium between sections A and B in both buccal mucosa (a and b) and labial mucosa sections (c and d) (picrosirius stain, 40×)
DISCUSSION
The main pathology of OSF lies in two behavioral patterns: first is its effect on the epithelium leading to an increase in malignant potential and second is the progressive and continuous fibrosis leading to restricted mouth opening and overall loss of mobility of the mucosa.
The fibrosis tends to present itself clinically from early to advanced stages in the form of palpable fibrous bands running vertically in the buccal mucosa. Similar bands to a lower extent are seen affecting the soft palate from uvula to fauces. The question why the fibrous bands run vertically and not horizontally clinically does not appear to have been addressed in the existing scientific literature, and there is no data available on the orientation of fibers.
When the direction of the palpable and visible collagen fibrous bands was studied, all the 33 cases showed vertical fibrous bands in buccal mucosa, 30 cases showed circumoral fibrous bands in labial mucosa, and 25 of them had fibrous bands running along the direction of the palatal muscles. In the literature it has been said that in advanced cases of oral submucous fibrosis, the mucosa becomes leathery, with numerous vertical fibrous bands in buccal mucosa and circumoral bands in labial mucosa, similar to the findings of our study.
When the orientation of collagen fibers laid down was correlated with the vertical movement of the mandible, it was concluded that in all the cases the orientation of the collagen fibers laid down was in the direction of opening and closing movement of the mouth.
Histologically when the collagen fiber bundle orientation was evaluated, 68% of collagen fibers in buccal mucosa specimens and 78% of fibers in labial mucosa were parallel to the epithelium, and most of the collagen fiber bundle were about 20–30 μm thick. In no sections of normal tissue the collagen fibers were so thick that they could be measured and the fibers showed a haphazard arrangement. There was a statistically significant difference in collagen fiber orientation between the OSF and control groups.
In the literature, the normal oral mucosa especially of the lining mucosa is considered as loose areolar connective tissue that consists of a meshwork of thin collagen and elastin fibers interlacing in all directions to give a measure of elasticity and tensile strength.[9]
Till date there are no data available on orientation of collagen fiber in OSF. However, OSF is similar in many aspects to scleroderma, where continuous and uncontrolled fibrosis accompanies a chronic inflammatory reaction.[10] In scleroderma, sclerosis is histologically characterized by an inflammatory cellular infiltrate composed of lymphocytes, macrophages, mast cells, eosinophils, and plasma cells, and the collagen bundles are oriented parallel to the epidermis. Fibrosis is the pathologic hallmark of scleroderma which leads to induration of the skin and fixation of the epidermis to deeper subcutaneous tissue. Fibrosis in oral cavity causes induration and stiffness of mucosa which ultimately leads to inability to open and close the mouth.[11] Similar changes occur in OSF where increased thickened collagen deposition leads to restricted mouth opening.
The extracellular matrix is a very complex structure that could easily alter the effective diffusive ability of a chemical, via the chemical binding more readily in some regions of the matrix. Fibroblast orientation/polarity is important in determining how the cell orients these extracytoplasmic components and how the matrix is assembled and oriented.[12] Extracellular matrix remodeling has been extensively studied in OSF, and the matrix remodeling changes are regarded as an altered version of wound healing as the recent findings show the expression of various extracellular molecules is similar to those seen in maturation of a granulation tissue.[13]
However, in contrast to normal maturation of granulation tissue in oral submucous fibrosis, as there will be continuous irritation by the chemicals to the oral mucosa; normal wound healing does not occur instead result in scar formation.
Keloids and hypertrophic scars are characterized by excess deposition of collagen in the dermis and the subcutaneous tissues. Histologically, the collagen bundles appear stretched and aligned in the same plane as the epidermis. These collagen bundles are thicker and more abundant and are composed of aggregates of fibroblasts and small blood vessels throughout the dermis.[14] Results of this study are in agreement with this finding as most of the fibers are parallel to epithelium and clinically appear as palpable fibrous bands.
This unidirectional tight bands in buccal mucosa are believed to cause restricted movement in oral submucous fibrosis patients.[15]
The cause for this unidirectional orientation of collagen fibers has been explained by previous studies conducted on fibroblast culture. The collagen fibril assembly and collagen fiber bundle formation take place, outside the cell, in inlets formed between fibroblast cell processes in a precise configuration.[16] The alignment of cells and collagen fibers is a result of the forces generated by the cell-mediated gel contraction.[12] And in scars, the unidirectional alignment of collagen fibers occurs in as a sequence of mechanical stress with the fibers getting oriented in the direction of force exerted on the fibers.[17]
Unidirectional orientation of fibers seen in OSF might also be explained by the fact that the direction of force exerted on the fibers is in a vertical direction. But the response of this unidirectional alignment of fibers to unidirectional vertical movement is possibly different due to difference in the thickness of collagen fibers in contrast to the response of normal oral mucosa to vertical movement where the fibers are thin and haphazardly arranged. These thick and dense collagen fibers in OSF function as regular connective tissue similar to a tendon where the direction of collagen fiber alignment occurs along the direction of stress.
Thus, it can be concluded that the reason for a particular direction of orientation could be due to chronic stimulation of oral mucosa by the irritants, leading to change in the orientation of collagen fiber bundles, which might result in scar formation similar to that of wound healing, where the collagen fibers are oriented parallel to the epidermis. As the collagen fibers tend to orient themselves along the direction of stress, the collagen fibers in oral submucous fibrosis also might orient in a vertical direction in buccal mucosa and in a circumoral fashion in labial mucosa.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Caniff JP, Harvey W, Harris M. Oral submucous fibrosis: Its pathogenesis and management. Br Dent J. 1986;160:429–34. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- 2.Akbar M. Oral submucous fibrosis – A clinical study. J Indian Dent Acad. 1976;48:365–73. [Google Scholar]
- 3.Chiu CJ, Chang ML, Chiang CP, Chen CJ, Hahn LJ, Hsieh LL. Interaction of collagen related genes and susceptibility to betel quid induced oral submucous fibrosis. Cancer Epidemiol Biomarkers Prev. 2002;11:646–53. [PubMed] [Google Scholar]
- 4.Rajendran R. Oral submucous fibrosis: Etiology, pathogenesis, and future research. Bull World Health Organ. 1994;72:985–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta FS, Hamner JE. Tobacco related oral mucosal lesions and conditions in India. Bombay: Basic Research Dental Unit; 1993. Tobacco related oral mucosal lesions and conditions in India; p. 60. [Google Scholar]
- 6.Nair SM, Rajendran R. Silver-binding nucleolar organizer region proteins as a possible prognostic indicator in oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1992;74:481–6. doi: 10.1016/0030-4220(92)90300-f. [DOI] [PubMed] [Google Scholar]
- 7.Mishra G, Ranganathan K. An overview of classification schemes for oral submucous fibrosis. J Oral Maxillofac Pathol. 2006;10:55–8. [Google Scholar]
- 8.Bignolas G, Brentani RR, Junqueira LC. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:455–7. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 9.Gray HW. Cells and Tissues. In: Grays A, Standring S, editors. 39th ed. Edinburgh: Churchill Livingstone; 2005. pp. 36–40. [Google Scholar]
- 10.Torneck CD. Oral Mucosa. In oral histology. In: Tencate AR, editor. Tencate's A.R Oral Histology. 5th ed. Singapore: Harcourt Asia Pte Ltd; 1999. p. 378. [Google Scholar]
- 11.de Vries HJ, Enomoto DN, van Marle J, van Zuijlen PP, Mekkes JR, Bos JD. Dermal organization in scleroderma: The Fast fourier transform and the laser scatter method objectify fibrosis in non lesional as well as lesional skin. Lab Invest. 2000;80:1281–9. doi: 10.1038/labinvest.3780136. [DOI] [PubMed] [Google Scholar]
- 12.Ginsel LA, Jansen JA, Walboomers XF. Early spreading events of fibroblasts on microgrooved substrates. J Biomed Mater Res. 2000;51:529–34. doi: 10.1002/1097-4636(20000905)51:3<529::aid-jbm30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Utsunomiya H, Tilakaratne WM, Oshiro K, Maruyama S, Suzuki M, Ida-Yonemochi H, et al. Extracellular cell remodeling in oral submucous fibrosis: Its stage specific modes revealed by Immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005;34:498–507. doi: 10.1111/j.1600-0714.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 14.Babu M, Jayaraman V, Meenakshi J, Ramakrishnan KM. Keloids and hypertrophic scars: A review. Indian Journal of Plastic Surgery. 2005;38:175–9. [Google Scholar]
- 15.Bhonsle RB, Daftary DK, Gupta PC, Murthi PR, Mehta FS, Pindborg JJ. Oral precancerous lesions and conditions of tropical interest. In: Prabhu SR, Wilson DF, Daftary DK, Johnson NW, editors. Oral diseases in the tropics. Oxford: Oxford University Press; 1992. pp. 417–22. [Google Scholar]
- 16.Cornelis JF, Jonker A, Maril L, Melis P, Van Noorlander CJ. A quantitative method to determine the orientation of collagen fibers in the dermis. J Histochem Cytochem. 2002;50:1469–74. doi: 10.1177/002215540205001106. [DOI] [PubMed] [Google Scholar]
- 17.Eun HC, Lee HG. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. Dermatol Sci. 1999;21:176–82. doi: 10.1016/s0923-1811(99)00037-7. [DOI] [PubMed] [Google Scholar]


