Abstract
Fibroblasts are a major stromal cell type present in human connective tissue maintaining the structural integrity in health. Depending on the situation, location and various conditions, fibroblasts exhibit considerable variation in morphology, size and shape that suggest the existence of discrete cellular subsets. The purpose of this short communication is to provide information regarding the heterogenecity of fibroblasts and its variability in physiological and pathological conditions.
Keywords: Fibroblasts, fibrocytes, giant cell fibroblasts, myofibroblasts
INTRODUCTION
Mesenchymal cells are precursors for a varied group of connective tissue cells including endothelial cells, smooth muscle cells, pericytes, chondroblasts, adipocytes, osteoblasts, odontoblasts, and fibroblasts. Fibroblasts are a heterogeneous population of cells found in numerous tissues. The interrelationships between cell lineages and differentiation potential contribute to wide range of synthetic products, rates of product synthesis, reaction to regulatory molecules, cellular turnover rates and morphological features leads to fibroblast diversity. The tissue distribution and relative proportion of fibroblast subpopulations have a considerable impact on the regulation of connective tissue function in health and disease.[1]
FIBROBLASTS
The fibroblasts are predominant stromal cell type seen in soft connective tissues. They appear as plump spindle shaped or stellate shaped cells (active fibroblasts) with centrally placed oval or round nucleus [Figure 1]. These cells and their extracellular matrix products i.e. fibers and amorphous ground substance play pivotal roles in maintaining the structural integrity of connective tissues in health, healing processes, and in pathological alterations. In addition to well-described structural and functional similarities (e.g. collagen and fibronectin synthesis, vimentin intermediate filaments [Figure 2], β-actin), fibroblasts exhibit considerable variation of cytoskeletal proteins (e.g. α-smooth muscle actin expression), surface markers and size, that suggests the existence of discrete cellular subsets. Fibroblast variants can be seen both within a localized site (e.g. gingiva) and also between sites from various locations. The central hypotheses proposed that fibroblasts are phenotypically stable but exhibit heterogeneous subpopulations, which regulate tissue form and function. Fibroblasts exhibit change in morphology and appear as elongated cells with thin flat/wavy nuclei (inactive fibroblasts) in the areas of dense collagen fibre formation [Figure 3]. Fibroblasts can also undertake other functions (e.g., as immune accessory cells), which extend their utility as “architects and caretakers of connective tissues”.[1] Fibrosarcoma [Figure 4], a malignant mesenchymal neoplasm of fibroblasts or fibrohistiocytic lesions derived from a peculiar type of fibroblastic mesenchymal cell that show histiocytic areas and many other neoplasms can develop from fibroblasts.
Figure 1.
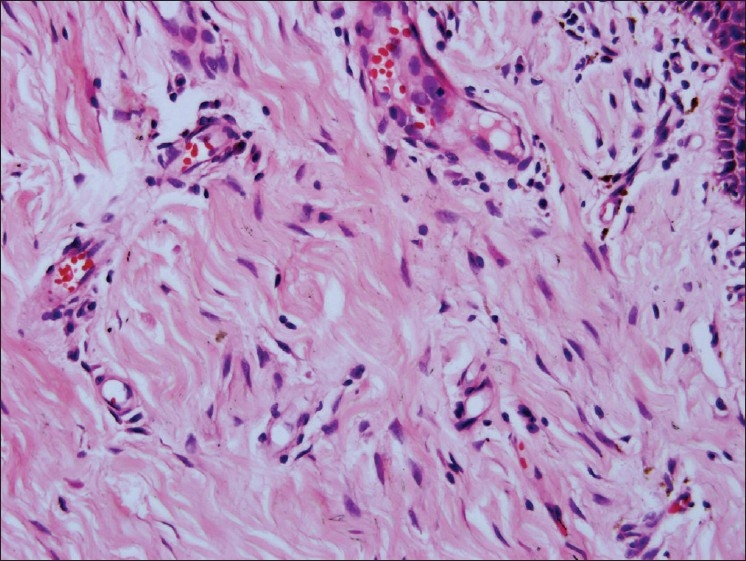
Active fibroblasts, 20×
Figure 2.
Vimentin positive, 20×
Figure 3.
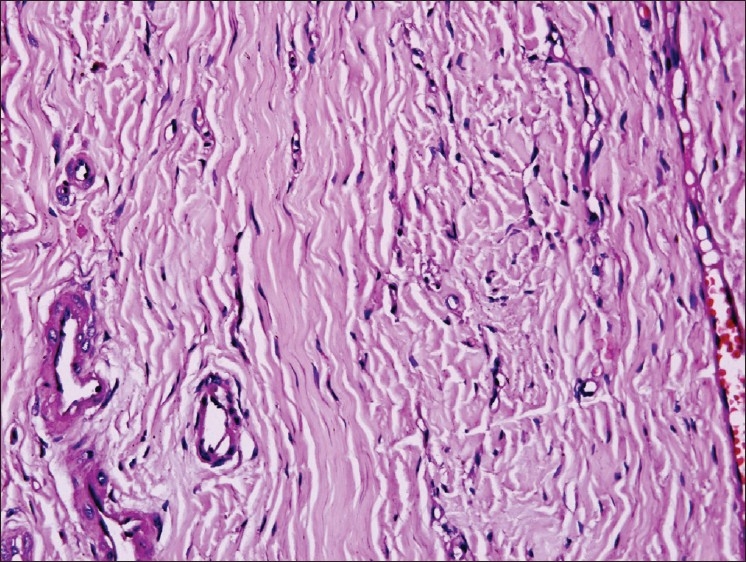
Inactive fibroblasts, 20×
Figure 4.
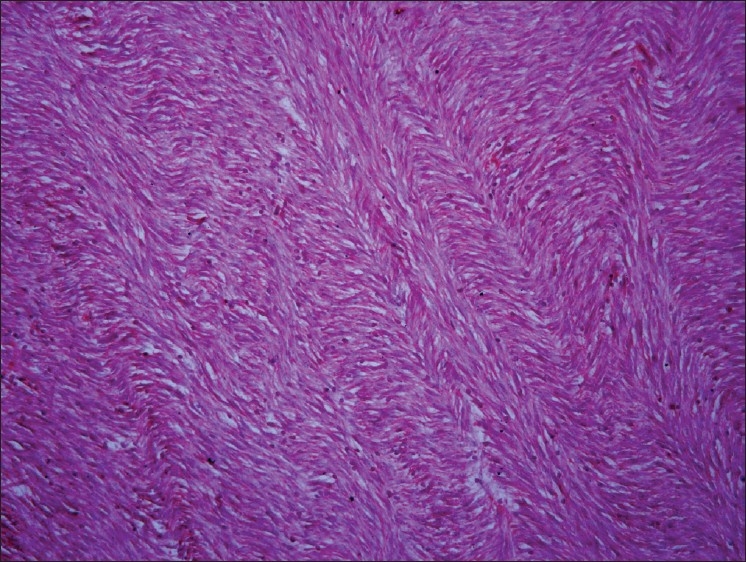
Fibrosarcoma, 20×
FIBROCYTES
Fibroblasts found in the wound are considered essential for the healing process. The concept that wound fibroblasts originating from peripheral blood cells goes back almost 100 years. Since then, numerous studies have reported the differentiation of peripheral mononuclear cells into fibroblast-like cells. In 1994, a distinct population of blood-borne fibroblast-like cells that rapidly enter sites of tissue injury was described and were termed fibrocytes.[2] These cells comprise 0.1 - 0.5% of non-erythrocytic cells in peripheral blood and show an adherent, spindle-shaped morphology. Fibrocytes express collagen (Col) I, Col III, and fibronectin, as well as the leukocyte common Ag (CD45RO), the pan-myeloid Ag (CD13), and the hemopoietic stem cell Ag (CD34). In addition, fibrocytes express MHC class II and costimulatory molecules (CD80 and CD86). Morphology, growth properties and cell surface markers of fibrocytes appear to be distinct from monocytes/macrophages, dendritic cells, and other antigen presenting cell types.[3]
Based on their presence in wounds and their secretion of proinflammatory cytokines, chemokines, and extracellular matrix proteins, fibrocytes have been postulated to play a role in connective tissue formation in wound healing.
MYOFIBROBLASTS
Myofibroblasts (MF) are modified fibroblasts with smooth muscle like features characterized by the presence of contractile apparatus. Myofibroblasts disclose several typical histologic traits characterized by large, spindle shaped stellate cells with long cytoplasmic extensions, amphophillic cytoplasm and indented nucleus with conspicuous nucleoli. These are extremely heterogenous and multifunctional cell population exhibiting a different phenotype and first discovered by electron microscopy in experimental granulation tissue. MF plays a key role in extracellular matrix synthesis, reorganization and tissue contraction during both physiologic process and pathologic process like wound healing and tumorigenesis.[4]
Ultrastructurally these cells disclose irregular, stellate cellular outlines with numerous long cytoplasmic connections connected by intermediate or adherens junctions and are connected to the extracellular matrix by cell-to-stroma attachment sites through fibronexus. They contain bundles of cytoplasmic filaments arranged parallel to the long axis of the cell, a well developed rough endoplasmic reticulum, golgi and indented nucleus with prominent nucleoli.[5]
Myofibroblasts discloses five cytoskeletal immuno phenotypes: Phenotype V, represented by cells expressing only vimentin; phenotype VA represented by cells expressing vimentin and alpha smooth muscle actin (α-SMA); phenotype VAD represented by cells expressing vimentin, α-SMA and desmin; phenotype VD represented by cells expressing vimentin and desmin; phenotype VAM represented by cells expressing vimentin, α-SMA and myosin. Most of the MF expresses alpha smooth muscle actin (α-SMA), an actin isoform found in vascular smooth muscle cells and regulated by TGF-β and is considered as main immunohistochemical marker of myofibroblastic differentiation.[6]
MYOFIBROBLASTS IN ORAL TISSUES
In the oral cavity these MF were reported in the human palatal mucosa and granulation tissue during wound healing. The process of wound healing is highly orchestrated sequence of events in which myofibroblasts appear to be key cells. Prostaglandins derived from myofibroblast are key factors in promoting healing by restitution and reconstitution of the epithelium. Another important event in wound healing is the contraction of the wound which is due to the myofibroblasts because of the presence of alpha smooth muscle actin filaments in the cytoplasm of these cells. The extra cellular matrix complex is a mixture of collagens, glycoproteins and proteoglycans that form a scaffold for tissue formation. Type I, III, IV and VIII collagens, fibronectin, tenascin are secreted by the myofibroblasts and tissue remodelling following injury which is mediated mainly by the matrix metalloproteinases, which are also secreted by the myofibroblasts.[7]
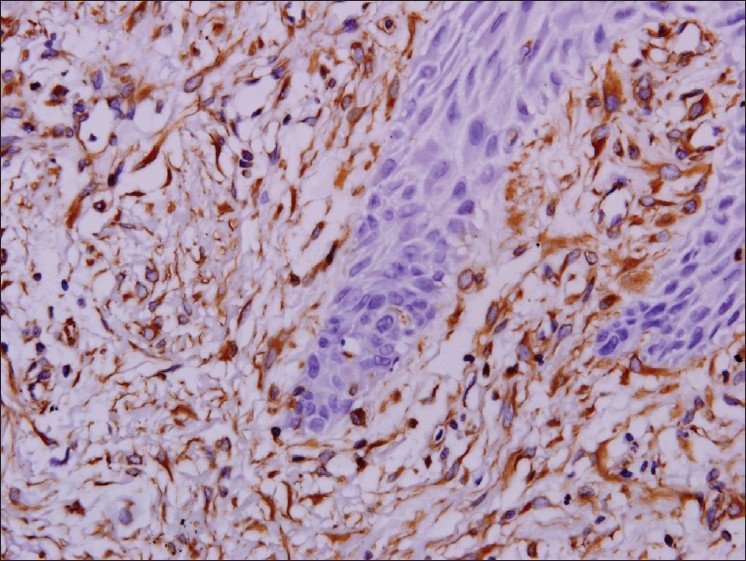
Myofibroblasts have been reported in many locally aggressive odontogenic lesions like odontogenic keratocyst [Figure 5] and solid ameloblastomas. In oral submucous fibrosis there was significant increase of myofibroblasts when compared to that of the normal control and could be used as markers for evaluating the severity of oral submucous fibrosis. The abundant myofibroblasts at the invasion front of oral squamous cell carcinomas [Figure 6] contribute in growth of the tumor cells by secreting angiogenic molecules, growth factors and invasion by secretion of matrix metalloproteinases and suppression of host immune response.[8]
Figure 5.
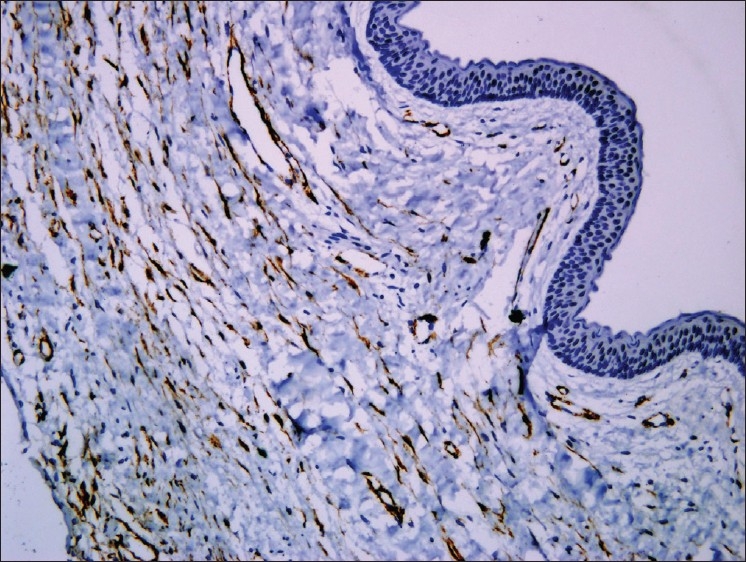
α SMA positive myofibroblasts in odontogenic keratocyst, 10×
Figure 6.
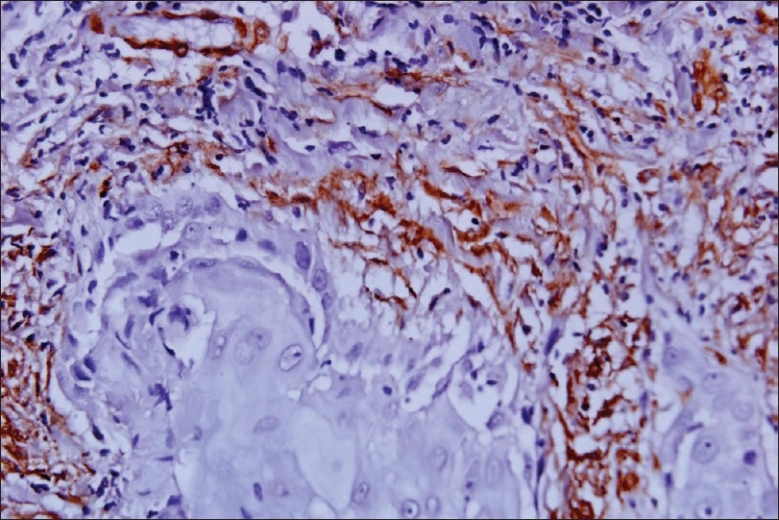
α SMA positive myofibroblasts in squamous cell carcinoma, 20×
GIANT CELL FIBROBLASTS
Giant cell fibroma (GF) is characterized by the presence of many large stellate mono or multinucleate giant cells in a fibrous tissue containing abundant collagen and the degree of fibrosis varies from area to area within the fibrous tissue [Figure 7]. In Giant Cell Fibroma, the giant cells are located beneath the epithelium. The cytoplasmic borders of these cells are usually distinct with some fading at the apex of angular extension. The cytoplasm is basophilic, granular, containing some vacuoles [Figure 8]. Some of these cells, especially those located beneath the epithelium may contain small brown granules with the staining characteristics of melanin. An artefactual space separating the giant cells from the collagen fibers is sometimes present. The overlying epithelium is hyperplastic with thin elongated retepegs and the inflammatory infiltrate is usually minimal. Common irritation hyperplasias like fibrous hyperplasia, fibroepithelial polyp, fibroma show similar histologic features but the presence of numerous stellate giant cells differentiates giant cell fibroma from other lesions.
Figure 7.
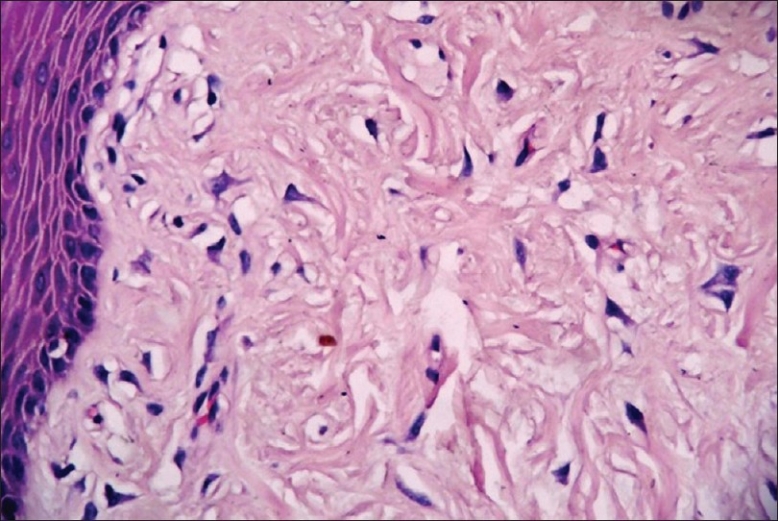
Giant cell fibroblasts in subepithelial region, 20×
Figure 8.
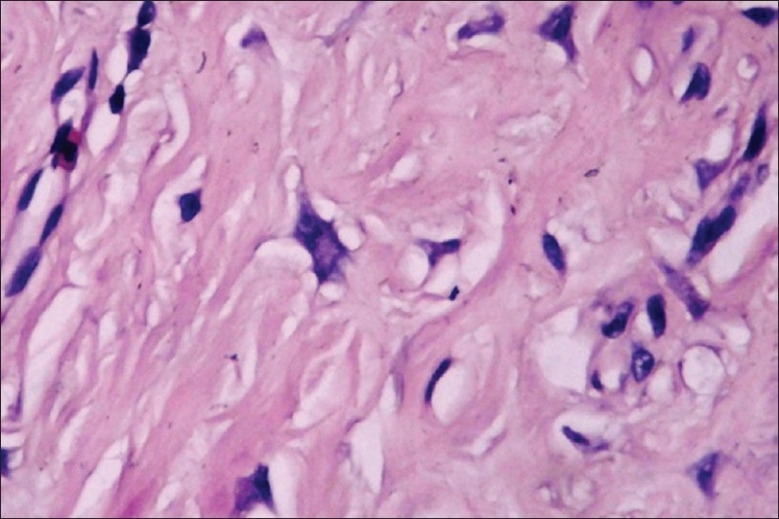
Giant cell fibroblasts, 40×
Ultra structurally, giant fibroblasts appear as stellate cells with a large hyperchromatic nucleus, while the cytoplasm is well demarcated and frequently the cells show dendritic-like processes; multinucleated giant cells in giant cell fibroma are unusual fibroblasts.[9]
Immunohistochemical studies on giant fibroblasts showed positivity only for Vimentin and negative reactivity for cytokeratin, neurofilament, HHF, CD68, HLA DR, tryptase, leukocyte common antigen, S100 protein.[10]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lekic PC, Pender N, McCulloch CA. Is fibroblast heterogeneity relevant to the health, diseases and treatments of periodontal tissues? Crit Rev Oral Biol Med. 1997;8:253–68. doi: 10.1177/10454411970080030201. [DOI] [PubMed] [Google Scholar]
- 2.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 3.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 4.Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173:548–50. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- 5.Gabbiani G, Chaponnier C, Huttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978;76:561–8. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurch W, Seemayer TA, Gabbiani G. Myofibroblast. In: Sternberg SS, editor. Histology for Pathologists. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 129–65. [Google Scholar]
- 7.Powell DW. Myofibroblasts: Paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000;111:271–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in the stroma of odontogenic cysts and tumors can contribute to variation in the biologic behavior of lesions. Oral Oncol. 2005;41:1028–33. doi: 10.1016/j.oraloncology.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Takeda Y, Kaneko R, Suzuki A, Niitsu J. Giant cell fibroma of the oral mucosa.Report of a case with ultrastructural study. Acta Pathol Jpn. 1986;36:1571–6. doi: 10.1111/j.1440-1827.1986.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 10.Reibel J. Oral fibrous hyperplasias containing stellate and multinucleated cells. Scand J Dent Res. 1982;90:217–26. doi: 10.1111/j.1600-0722.1982.tb00730.x. [DOI] [PubMed] [Google Scholar]


