Abstract
Background and Objectives:
Oral squamous cell carcinoma (OSCC) is one of the most common tobacco-related cancers affecting the Indian population. Various malignancy-grading systems based on different histopathological features have been proposed for OSCC. Due to inherent subjectivity, inter-observer variation and reproducibility of a grading system remains a problem. Grading systems based on nuclear morphometry have been proposed for laryngeal, renal and pharyngeal carcinomas. In this study, an attempt was made to grade oral OSCC based on computer-assisted microscopic evaluation of nuclear features. Our intention was also to evaluate the use of Feulgen stain for studying nuclear features.
Materials and Methods:
Sections made from buccal mucosa biopsies of normal mucosa as well as different grades OSCC were stained by Feulgen reaction. The nuclear features were evaluated by computer-assisted microscopic image analysis for nuclear area (NA), nuclear perimeter (NP) and nuclear form factor (NF) and correlated with histologic grading of OSCC. Nuclear shape, membrane outline, chromatin clumps, nucleoli, and abnormal mitoses were also evaluated.
Results:
NA and NP were observed to be significantly increased in OSCC (P < 0.001) when compared with the control group. These values increased in correlation with increasing grades of OSCC. However, NF was found to more in the control group (P < 0.001).
Conclusion:
It may be concluded from the results that computer-assisted nuclear morphometry is a reliable tool for grading OSCC. A new grading system based on nuclear features for OSCC has been proposed.
Keywords: Feulgen stain, nuclear area, nuclear form factor, nuclear grading, nuclear perimeter, oral squamous cell carcinoma
INTRODUCTION
Currently, prognostic evaluation and treatment planning for oral squamous cell carcinoma (OSCC) is mainly based on clinical staging and histological grading which are in turn based on subjective evaluation of parameters and are therefore often not sufficiently reproducible. Since nucleus is the main center controlling cell metabolism and function, nuclear analysis for predicting biological behavior of diseases has gained importance in the recent years. Computer-assisted morphometry reduces the problems of inter-observer variation and reproducibility of grading systems. Nuclear morphometry has been used for grading carcinomas of larynx, esophagus, kidney, bladder, breast, prostate and colon,[1] and to distinguish oral hyperplasias and dysplasias,[1] oral lichen planus and lichenoid lesions.[2]
Feulgen reaction along with micro-spectrophotometry was first employed for the evaluation of oral cancer by Doyle and Manfold in 1975 for predicting the transformation of oral leukoplakia to OSCC.[3] There are a good number of studies of oral cytosmears from dysplastic oral lesions and OSCC, stained with Feulgen reaction for DNA measurements. Relatively few studies have employed Feulgen staining for paraffin-embedded sections of oral dysplasia and OSCC. Hannen et al.[4] studied the nuclear morphology and chromatin pattern in formalin-fixed, paraffin-embedded, and Feulgen-stained tissue sections of surgical resection specimens by means of high-resolution computer-assisted image analysis in primary tongue carcinomas.
In this study, an attempt has been made to grade OSCC based on computer-assisted microscopic evaluation of nuclear features. Our objective was to observe nuclear features in normal buccal mucosa and compare them with those of different grades of OSCC carcinoma. Nuclear features of different grades of OSCC are also compared with each other. Our objective also included the evaluation of Feulgen reaction for studying the nuclear features in OSCC.
MATERIALS AND METHODS
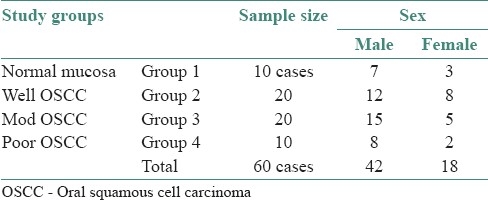
The different study groups and sample size are as shown in Table 1. The age range of subjects included in the study was between 20 and 86 years. Thick tissue sections (5 mm) were obtained from formalin-fixed, paraffin-embedded tissue blocks using soft tissue microtome (Leica RM 2165, Wetzlar, Germany). The sections were stained by employing Feulgen reaction for nuclear DNA.[5,6] All slides were coded to ensure that clinical diagnosis and histological grading were not known during the measurement. The stained sections were observed using a trinocular research microscope (Olympus BX51, Tokyo, Japan). All the images were captured under 20× apochromatic objective (NA 0.07) using a 3-chip CCD camera (Proview, Media cybernetics, USA). For each case, 10 microscopic fields were selected randomly. All the images captured were transferred onto a computer where they were analyzed using image analysis software (Image Pro Plus, Version 4.1.0.0 for Windows 95/NT/98, Media Cybernetics, Bethesda, USA).
Table 1.
Various study groups and sample size
For each case, 100 nuclei of tumor cells with clear, complete, non-overlapping outlines were selected randomly and were analyzed for eight nuclear variables: nuclear area (NA), nuclear perimeter (NP), nuclear form factor (NF), nuclear shape, nuclear membrane outline, nucleoli, chromatin clumps and abnormal mitotic figures per 10 high power microscopic fields (HPF). NF was calculated using the following formula:[7]

The outline of nuclei (where a complete outline could be clearly seen) was traced on the computer screen (monitor) using a cursor controlled by a mouse. The nuclear measurements were carried out using measurement tool of the software Image Pro Plus. The measurements were in microns (calibration: 1 μ = 3.260 pixels). All measurements were transferred to Microsoft Excel® for further statistical analysis.
Statistical methods
The mean, standard deviation and inter-quartile deviation were calculated for NA, NP and NF. These values were correlated with histological grading. The reliability of the method was ascertained by locating mode values. One-way analysis of variance (ANOVA) and Z-test were used to compare the quantitative parameters, while Chi-square test was used for comparing qualitative parameters between various study groups. Mann–Whitney U test was used for pair-wise group comparison.
RESULTS
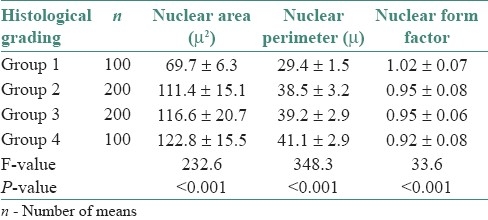
The mean NA and mean NP were found to be increased in carcinoma groups when compared to normal mucosa (P < 0.001) and these values increased gradually with increasing grades of carcinoma (P < 0.001) as shown in Table 2. The mean NF was observed to be more in the control group when compared to carcinoma group (P < 0.001) and these values decreased gradually with increasing grades of carcinoma. However, it could not be used to differentiate group 2 and group 3 [Table 2]. When histological grading and grouping based on NA, NP, and NF were correlated, a fair correlation was observed. However, some overlapping of values was observed between the study groups. Similar finding was observed when mode values were distributed on inter-quartile ranges in various groups.
Table 2.
Mean and standard deviation of nuclear area, nuclear perimeter and nuclear form factor in various study groups (Mean ± SD)
It was found that in normal mucosa, there were good numbers of both round and oval nuclei. In carcinoma group, majority of the nuclei were oval and few were non-definable. Nuclei of the normal mucosa had regular nuclear membrane outlines, while majority of the nuclei in the carcinoma group showed irregular outline. Membrane irregularity increased with increasing grades of carcinoma. Chromatin clumping increased with increasing grades of carcinoma, while the nuclei of normal mucosa showed no clumps.
Nucleoli increased in number, became prominent and irregular with increasing grades of carcinoma, while in normal mucosa, the nucleoli were inconspicuous. Poorly differentiated carcinoma showed frequent abnormal mitotic figures compared to other grades, while the control group had none.
DISCUSSION
Nuclear DNA content is one parameter that can be reproduced repeatedly and known to correlate well with the malignant potential of a tumor. Feulgen reaction is a reliable and specific histochemical method for nuclear DNA.[6] Doyle and Manfold in 1975 did not find much use for Feulgen reaction in predicting the transformation of oral leukoplakia to OSCC.[3] However, in this study, we found that Feulgen reaction performed under carefully controlled conditions gives a reasonably good staining and accurate quantitative histochemical estimation of nuclear DNA. Similar observations were made by Abdel-Salam et al.[1,7] and Kasten.[8]
Computer-assisted image analysis of Feulgen-stained tissue sections of oral hyperplasia, dysplasia and OSCC for nuclear morphology and chromatin pattern have been studied by Abdel-Salem et al. (1986)[1] and Hannen et al. (1998).[4] The latter assessed nuclear morphology (size and shape) and chromatin pattern to develop a discriminant factor that can predict the occurrence of metastasis. In this study, we have studied the NF in addition to NA and NP.
Increased NA and NP observed could be due to rapid and abnormal growth of neoplastic cells. Nuclei in carcinoma group showed increased values of mean NA and NP when compared to the nuclei in normal mucosa. It has been shown in various studies that NA and NP increase gradually with increasing grades of carcinomas.[9–11] In this study, few nuclei of carcinoma groups 2 and 3 had values similar to that of the control group. This suggests that few nuclei are in a normal phase and not all nuclei in carcinoma group show features of malignancy.
A perfect circle has a form factor of 1.0, and elliptical structures deviate from unity toward zero as their degree of circularity becomes less perfect.[12,13] NF was found to be less in carcinoma groups than normal mucosa, suggesting that irregular or elliptical nuclei predominate in the carcinoma groups. Though NF reduced as grades of carcinoma increased, the difference was not statistically significant between carcinoma groups 2 and 3.
Variation in nuclear shape is a distinctive feature of malignancy. Lowest levels of malignancy are characterized by spherical nuclei of approximately equal size, and highest grade tumors are characterized by profound anisonucleosis.[14] Artificial nuclear shape changes can result from poor cellular fixation, trauma, degeneration, cautery or hormonal changes. These also have other degenerative features. A tangential section may result in elongated nuclear shape. By itself, changes in nuclear shape, if not extreme, are not sufficient for diagnosis in most tumors.
Irregularity of nuclear membrane was seen in neoplastic nuclei, while in normal cells nuclear membrane was found to be regular [Figures 1 and 2]. Irregularity of membrane is often the result of adhesion of abnormal chromatin clumps to the inner surface. Irregularity can result from cellular degeneration, but contours in neoplastic nuclei are sharp and well defined. Irregularity has been shown to increase with increasing gradations of carcinoma[15] which was also noticed in this study.
Figure 1.
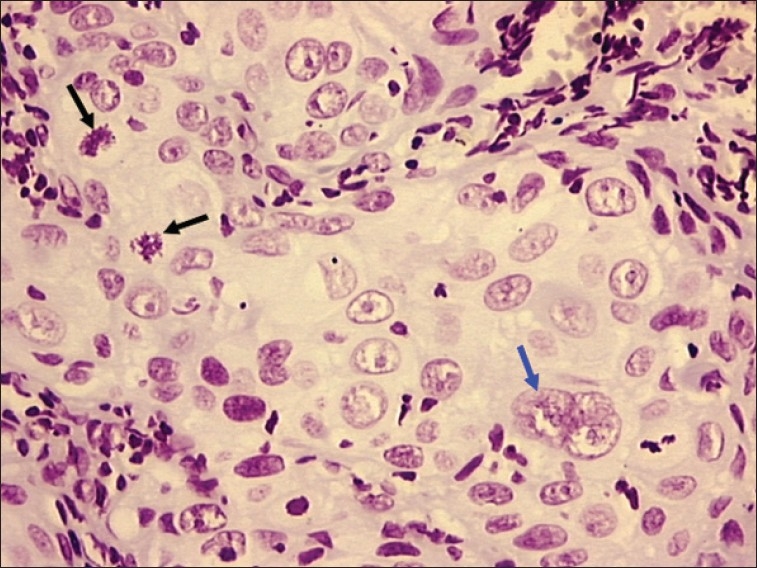
Photomicrograph of poorly differentiated OSCC showing bizarre mitotic figure (black arrow) and multiple nuclei with prominent nucleoli (blue arrow). Objective 20×, Feulgen stain
Figure 2.
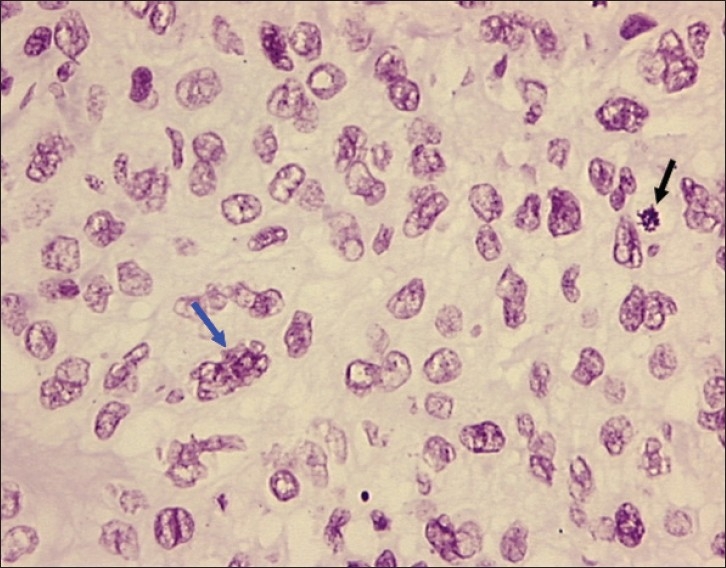
Photomicrograph of poorly differentiated OSCC showing tumor nucleus with multiple nucleoli and irregular nuclear membrane (blue arrow) and bizarre mitosis (black arrow). Objective 20×, Feulgen stain
In normal cell, chromatin is very finely granular and almost invisible, while in a neoplastic cell, chromatin forms well-defined irregular clumps with variable size, shapes and sharp pointed projections.[14] In this study, lower grades of OSCC showed delicate chromatin strands and homogenous chromatin pattern while higher grades exhibited coarse clumped chromatin and heterogeneous chromatin pattern [Figure 3].
Figure 3.
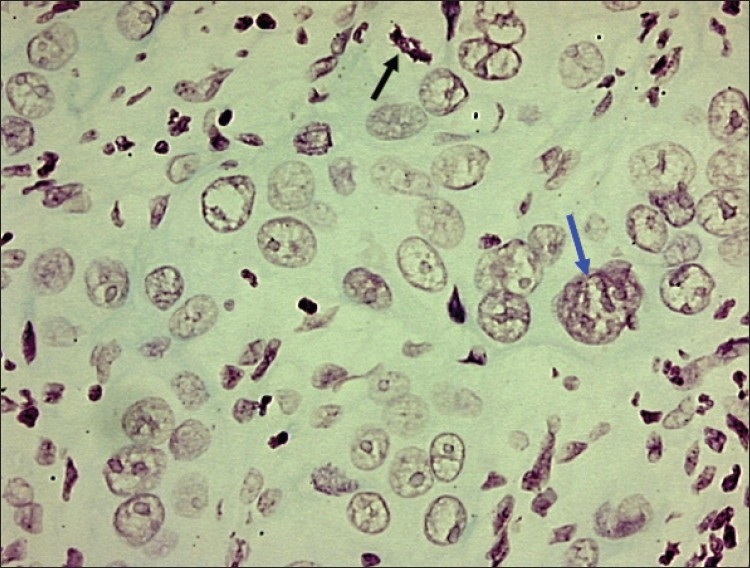
Photomicrograph of moderately well differentiated OSCC showing large tumor nucleus with multiple prominent nucleoli (blue arrow) and abnormal mitotic figure (black arrow). Objective 20×, Feulgen stain
Nucleoli are usually inconspicuous in normal cells. Nucleoli are prominent, more in number, enlarged and irregular with occasional sharp pointed projections in malignant cells. This is due to increased protein synthesis.[14] Similar features were witnessed in this study, where it was found that number and irregularity of nucleoli increased with increasing grades of carcinoma [Figures 1, 4 and 5]. Generally, higher grades of carcinoma show frequent abnormal mitotic figures than lower grades of carcinoma[14] and this study was no exception. This has been illustrated in Figures 1, 2, 5, and 6.
Figure 4.
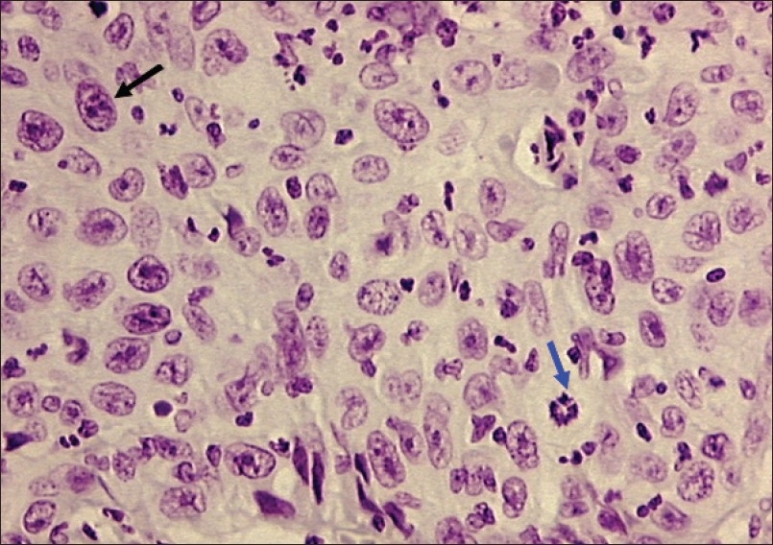
Photomicrograph of well differentiated OSCC showing bizarre mitotic figure (blue arrow) and tumor nucleus with multiple prominent nucleoli (black arrow).Objective 20×, Feulgen stain
Figure 5.
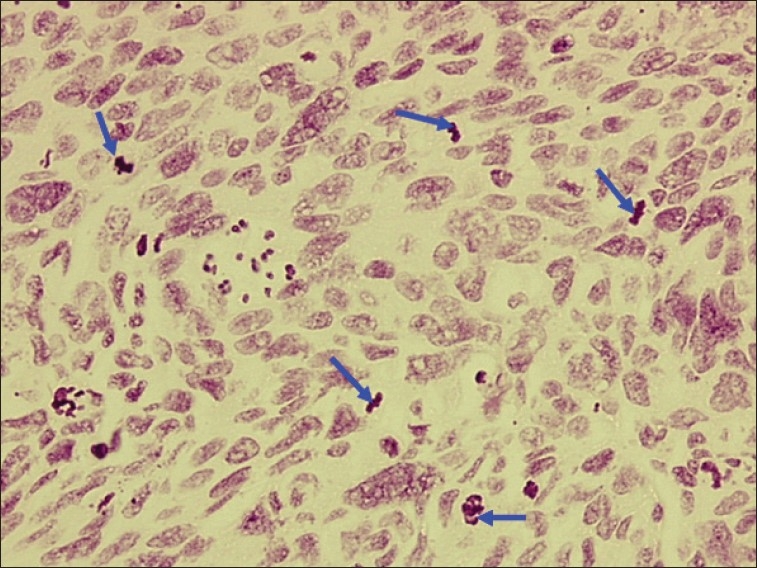
Photomicrograph of poorly differentiated OSCC showing anisonucleosis and numerous abnormal mitotic figures (blue arrows). Objective 20×, Feulgen stain
Figure 6.
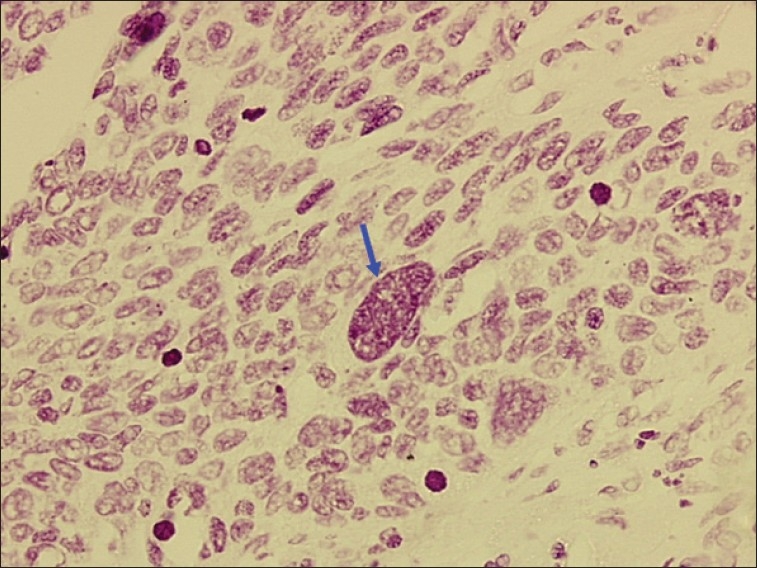
Photomicrograph of poorly differentiated OSCC showing large tumor nucleus with chromatin clumps (blue arrow). Objective 20×, Feulgen stain
The results of this study shed new light on nuclear changes occurring in OSCC. Nuclear grading systems for cancer have been proposed for renal cell carcinoma (Fuhrman et al., 1982)[15] and renal adenocarcinoma (Syrjänen and Hjelt, 1978).[16] Based on various nuclear variables in this study, we have proposed a nuclear grading system for OSCC which is presented in Table 3.
Table 3.
Proposed nuclear grading system for OSCC
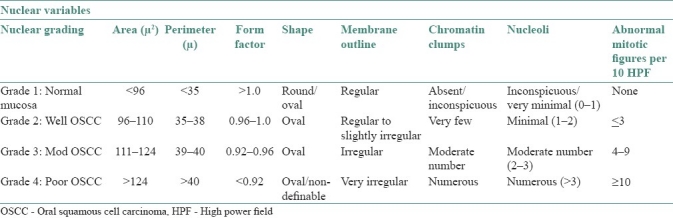
There was overlapping of few nuclear values between various groups when nuclear grading was correlated with histological grading, especially between well-differentiated OSCC and moderately differentiated OSCC as well as moderately differentiated OSCC and poorly differentiated OSCC. This finding supports the concept that well, moderate and poorly differentiated varieties of OSCC form a continuum.
It is highly possible that what was histologically diagnosed as moderately differentiated carcinoma was in fact is a poorly differentiated carcinoma or vice versa. This also raises the question of reliability of the present standardization of histological grading and clinical diagnosis of OSCC.
CONCLUSION
Nucleus reflects cell's biological potential and general activity. A combination of several nuclear variables provides a more accurate indication of tumor aggressiveness and behavior rather than any single parameter. Nuclear morphometry was found to be a reliable tool for grading OSCC in this study. Large series of patients and truly prospective studies are required to determine the practical use and validity of this nuclear grading system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Abdel-Salam, Mayall BH, Hansen LS, Chew KL, Greenspan JS. Nuclear DNA analysis of oral hyperplasia and dysplasia using image cytometry. J Oral Pathol. 1987;15:431–5. doi: 10.1111/j.1600-0714.1987.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 2.Khoo SP, Primasari A, Saub R. Nuclear and cellular volumetric alterations in oral lichen planus and lichenoid lesions: A histomorphometric study. J Oral Sci. 2001;43:151–7. doi: 10.2334/josnusd.43.151. [DOI] [PubMed] [Google Scholar]
- 3.Doyle JL, Manhold JH. Feulgen microspectrophotometry of oral cancer and leukoplakia. J Dent Res. 1975;56:1196–9. doi: 10.1177/00220345750540061601. [DOI] [PubMed] [Google Scholar]
- 4.Hannen EJ, Van der Laak JA, Manni JJ, Pahlplatz MM, Freihofer HP, Slootweg PJ, et al. An image analysis study on nuclear morphology in metastasized and non-metastasized squamous cell carcinomas of the tongue. J Pathol. 1998;185:175–83. doi: 10.1002/(SICI)1096-9896(199806)185:2<175::AID-PATH69>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft JD. Proteins and nucleic acids. In: Bancroft JD, Stevens A, editors. Theory and practice of histological techniques. 4th ed. London: Churchill Livingstone; 1996. pp. 237–8. [Google Scholar]
- 6.Drury RA, Wallington EA. 4th ed. London: Oxford University Press; 1967. Carleton's histological technique. [Google Scholar]
- 7.Abdel-Salam M, Mayall BH, Chew K, Silverman S, Greenspan JS. Prediction of malignant transformation in oral epithelial lesions by image cytometry. Cancer. 1988;62:1981–7. doi: 10.1002/1097-0142(19881101)62:9<1981::aid-cncr2820620918>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Kasten FH. The Feulgen-deoxyribonucleic acid absorption curve in normal and tumorous tissues. J Histochem Cytochem. 1957;5:398–9. doi: 10.1177/5.4.398. [DOI] [PubMed] [Google Scholar]
- 9.Friedell GH, Bell JR, Burney SW, Soto EA, Tiltman AJ. Histopathology and classification of urinary bladder carcinoma. Urol Clin North Am. 1976;3:53–70. as quoted in. [PubMed] [Google Scholar]
- 10.Truelson JM, Fisher SG, Beals TE, McClatchey KD, Wolf GT. DNA content and histologic growth pattern correlate with prognosis in patients with advanced squamous cell carcinoma of the larynx. Cancer. 1992;70:56–62. doi: 10.1002/1097-0142(19920701)70:1<56::aid-cncr2820700110>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Welkoborsky HJ, Gluckman JL, Mann WJ, Freije JE. Comparison of quantitative DNA measurements and cytomorphology in squamous cell carcinomas of the upper aerodigestive tract with and without lymph node metastases. Ann Otol Rhinol Laryngol. 1993;102:52–7. doi: 10.1177/000348949310200110. [DOI] [PubMed] [Google Scholar]
- 12.Partin AW, Steinberg GD, Pitcock RV, Wu L, Piantadosi S, Coffet DS, et al. Use of nuclear morphometry, Gleason histologic scoring, clinical stage and age to predict disease free survival among patients with prostate cancer. Cancer. 1992;70:161–8. doi: 10.1002/1097-0142(19920701)70:1<161::aid-cncr2820700126>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Van der Wal N, Baak JPA, Schipper NW, Van der Waal I. Morphometric study of pseudoepitheliomatous hyperplasia in granular cell tumors of the tongue. J Oral Pathol Med. 1989;18:8–10. doi: 10.1111/j.1600-0714.1989.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 14.Francois C, Decaestecker C, Petein M, Van ham P, Peltier A, Pastees JL, et al. Classification strategies for the grading of renal cell carcinomas, based on nuclear morphometry and densitometry. J Pathol. 1997;83:141–50. doi: 10.1002/(SICI)1096-9896(199710)183:2<141::AID-PATH916>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Syrjänen K, Hjelt L. Grading of human renal adenocarcinoma. Scand J Urol Nephrol. 1978;12:49–55. [PubMed] [Google Scholar]


