Abstract
Background:
Histological grading is an important diagnostic tool to predict the clinical and biological behaviour of cancer. Cervical lymph node metastasis indicate poor prognosis of oral cancer. Considering economical status of a third world country, Anneroth's grading system is less expensive, more informative than TNM staging and Broder's grading system. Anneroth's grading system also shows co-relationship with lymph node metastasis. So we are trying to evaluate the Anneroth's grading as a standard one among these three system.
Objective:
To study the grading of histological malignancy according to Anneroth's classification of biopsy specimens in relation to metastasis in the cervical lymph nodes and comparing the Anneroth's with the other two recognized classifications.
Materials and Methods:
Fifty(50) patients with oral squamous cell carcinoma was included in the study. Specimen of 35 non-metastatic tumors were compared with 15 metastatic cases. All of the patients were graded to TNM, Broder's and Anneroth's system. TNM is clinical assessment and Broder's is based on only differentiation of cells. On the other hand, six parameters of Anneroth's gives a detail about the morphology of the tumor, invasion criteria in the host tissue and show its correlation with lymph node metastasis. Scoring system of Annearoth's grading indicates demarcation points of worseness of tumor and signifies the possibility of lymph node metastasis.
Results:
Both Anneroth's(P=0.002) and Broder's grading(P=0.012) have been significant but Anneroth's one is more significant then Broder's.
Conclusion:
Anneroth's classification can be taken as a standerd diagnostic factor and predictive factor of lymph node metastasis.
Keywords: Oral squamous cell carcinoma, malignancy grading system, metastasis
INTRODUCTION
Oral cancer is becoming a pressing problem in the world and the WHO predicts a continuing worldwide increase in the number of patients with oral cancer.[1] According to WHO report in 1983, oral cancer is the most common cancer in South East Asia.[2] In India, the incidence of oral cancer is 30–50% of whole body tumor.[3] In Sri Lanka, it forms 30% of all cancers in males[4] and in Pakistan oral cancer is responsible for one-third of all cancers (WHO, 1984), whereas in the UK and USA, oral cancer accounts for only 2% of all malignancies.[5] In Bangladesh, according to Shaheed and Molla, oral cancer accounts for 20% of the whole body malignancy.[6] 90% of oral cancers are squamous cell carcinoma.
Prognostic evaluation for oral squamous cell carcinoma (OSCC) is mainly based on clinical TNM classification, but this staging system is not sufficient for optimal prognostication and must be supplemented by other reliable methods.[7,8]
The biological activity of OSCC is evaluated and descriptively categorized as highly, moderately and poorly differentiated. Broder primarily developed this quantitative grading of cancer in 1920. Lack of correlation between Broders’ grades and the prognosis of OSCC has been explained by the fact that SCC's usually exhibit a heterogeneous cell population with probable differences in invasiveness and metastasis behavior.[7]
In 1973, Jakobsson et al. developed a multi-factorial malignancy grading system in order to obtain a more precise morphologic evaluation of the growth potential of SCC's in the head and neck region.[7,9] To make the morphologic criteria more precise, Anneroth and Hansen modified the grading system developed by Jakobsson et al. for application to SCCs in the tongue and the floor of the mouth.[10] Histological grading based on Broder's classification has been a traditional pathologic tool with documented prognostic value, but has not been incorporated into standard therapeutic planning strategies.[11] This is mainly due to the subjectivity of the current grading system and the lack of consensus regarding its prognostic value.[11,12] Extensive scoring methods have been designed in an attempt to increase the objectivity of these parameters.[13–17] Other ancillary parameters, such as mitotic index, DNA content, Ki-67, proliferation cell nuclear antigen, and bromodeoxyuridine labelling index, may be used in an attempt to identify new prognostic indicators.[18–25] But our limitation, is that more advanced parameters are not available in most of the Third World countries. We should select those methods that are available in the rural areas part of our country. We also should consider cost, benefit and effectiveness.
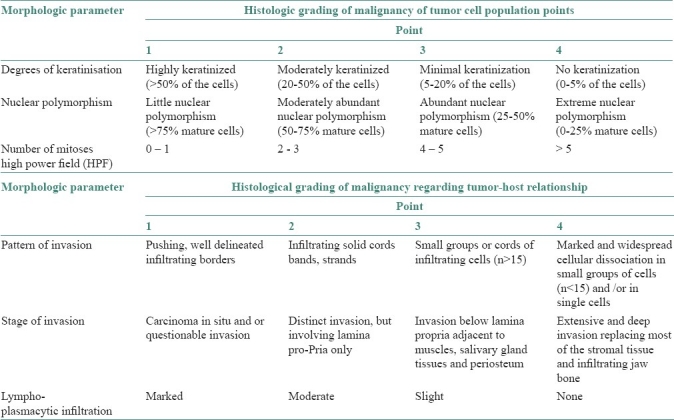
An attempt was made to evaluate the six parameters of Anneroth's classification [Table 1] because it is a standard method for OSCC, is easy to understand by a lab technician, gives more specific result, predicts the prognosis and guides the surgeon about the proper treatment plan. This system is constituted by six histological variables of equal value in the determination of the grade of malignancy, three connected with the tumor cellular population (differentiation and proliferation mitosis) and the other three connected with tumor-host relationship (pattern and stage of invasion; and cellular response).
Table 1.
Anneroth's multi-factorial grading system
In this study, TNM classification (in relation to metastasis in the cervical lymph nodes) and the degree of histological malignancy (according to Anneroth's classification of biopsy specimens) were evaluated clinically and histologically respectively in patients with OSCC encountered in 1 year period. Biopsy and histopathological confirmation of the lesion as cancer were mandatory.
Local recurrence occurs in up to half of patients with even microscopically negative surgical margins, and in these patients it is the leading cause of treatment failure. So, initial diagnosis with maximum accuracy is the key of successful treatment.
Patients of OSCC visit our hospital in advanced stage and many of them have also palpable regional lymph node. Histological grading quite often fails to indicate the actual types of lesion and its relationship with metastasis.
In developing countries, investigating with more advanced parameters is not possible. So, the method has to be selected considering the required pathological lab facility, skilled pathologists and lab technicians’ availability and patients’ accessibility. As such, it is of importance to reach to a consensus about a standard method of OSCC diagnosis which will be easy to use by the lab technicians, give more accurate results, easy to reproduce, help the surgeons better to predict patient's condition and decide proper management. The study was conducted with the following aims and objectives:
To study the grading of histological malignancy according to Anneroth's classification of biopsy specimens in relation to metastasis in the cervical lymph nodes.
To compare three recognized classifications.
MATERIALS AND METHODS
The archives of the Oral and Maxillofacial Department of Dental Faculty of Bangabandhu Sheikh Mujib Medical University (BSMMU) were used as the main source of data collection. All cases of OSCCs registered from 1 Nov 2003 to 31 to Dec 2004 were studied prospectively.
All pathologic and clinical records of the selected cases were reviewed. Only the patients for whom adequate histological material was available or could be obtained were included in the study. General information including age, sex, past medical history, duration of the disease, greatest tumor diameter, number and size of involved nodes were all registered. Age group of the study population was above 30 years. Patients of both the genders were randomly selected. Sample size of the study was 50.
Grading systems
For each case, the main slides containing the whole thickness of the tumor (including invasive margins) were used for histopathological grading and each case was graded according to the following classifications:
The American Joint Committee on Cancer (AJCC) designated staging by TNM Classification was used.
TNM clinical classification:

Border's sysetm (descriptive system):
Anneroth's classification (multifactor grading system)
According to the classification, three parameters reflecting tumor cell features including keratinization, polymorphism, and mitoses were evaluated in the whole thickness of the tumor and each was scored from 1 to 4. Mode of invasion and inflammatory infiltration representing tumor-host relationship were graded in the most invasive margins and scored from 1 to 4
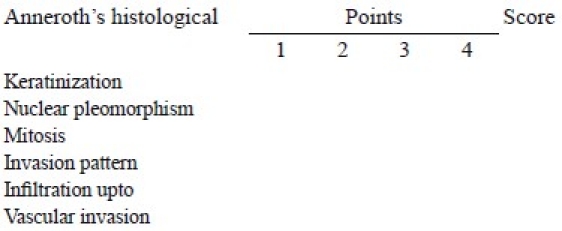
Each parameter was given a score according to its extent as points 1,2,3 and 4. Total points scored by each patient were calculated by adding the points of each parameters. The final grading was done as follows:

The results were compared in the metastasizing and non-metastasizing groups. The evaluation of all histological variables must be realized in the same microscopic field.
Data collection technique
A standardized structured data collection instrument was used to collect necessary information of the study subjects, which included the following:
-
a)
History of the patients: A relevant questionnaire was used which was history sheet of patient.
-
b)
Clinical examination and histological findings of lesion and lymph node were recorded in a checklist.
- Clinical examination: History sheets were filled by collecting history of each patient, conducting physical examination and documentation of those data for clinical evaluation. The information included age, gender, primary site of lesion, etiology, type, diameter and duration of lesion and evaluation of the regional metastasis if lymph node is palpable.
- Histological examination: Incisional biopsy of the primary lesion- A wedge-shaped tissue taken from the lesion including healthy tissue, marked any side of specimen as a side demarcation and keep in 10% formalin. After labeling, the specimen was subjected to histopathology studies according to Anneroth's and Broder's classification.
Fine needle aspiration cytology (FNAC) for suspected lymph node; A 21 gauge needle was selected for FNAC of the lymph node. After aspiration the specimen was examined for histological grading of metastasis.
Statistical analysis
Spreadsheet was prepared on the basis of parameters of TNM, Broder's and Anneroth's grading system. All parameters of each patient according to the clinical and histopathologic reports were compiled in the datasheet.
The numerical difference between each grade of these three grading system, numerical difference between palpable/nonpalpable lymph node and the numerical difference between FNAC positive or negative were calculated as a measure of overall bias. This along with the absolute percent difference were used to classify the tumors into three subgroups, representing FNAC +ve group, FNAC –ve group and nonpalpable lymph node group in each grade of the three grading systems.
A tabulation plan was prepared and necessary programs were developed using statistical software package SPSS/PC+. Chi-square test was performed to compute the P value. Correlation coefficient was used to find the degree of relationship among the variables. Mean test was done to compare between groups.
RESULTS
A total of 50 OSCC cases, reported during the study period between 1 Nov 2003 - 31 Dec 2004 and were reviewed and diagnosed histopathologically according to TNM system, Broder's and Anneroth's classification for final analysis.
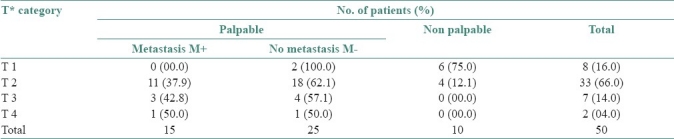
The relationship between T factors and the frequency of cervical lymph nodes metastasis is shown in Table 2. We found 16.0% cases were of T1 category, of which 25.0% were palpable and all those 25.0% were FNAC negative. Totally 66.0% cases were of T2 category, of which 87.9% were palpable and within that 37.9% were FNAC positive. 14.0% cases belonged to T3 category, the entire 14.0% were palpable and 42.8% of these cases were FNAC positive. Only 4.0% cases were in T4, of which the entire 4.0% were palpable and 50.0% of those palpable cases were FNAC positive. Statistical analysis found no relationship between T factors and lymph node metastasis.
Table 2.
Number of patients and the frequency of cervical lymph nodes metastasis according to TNM system (n=50)
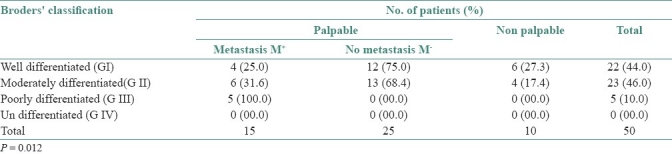
The relation between Broder's classification and lymph node metastasis is shown in Table 3. We can see that totally 44.0% cases were in G-I, of which 72.7% were palpable. Out of those palpable cases, 25.0% cases were metastasis positive. Likewise, 46.0% cases were in G-II of which 82.6% were palpable, within which 31.6% were FNAC positive. In G-III we found only 10.0% cases and these entire 10.0% were palpable and FNAC positive. Statistical analysis found the relationship (P=0.012) between Broder's grades and lymph node metastasis.
Table 3.
Number of patients and the frequency of cervical lymph nodes metastasis according to Broder's grading system (n=50)
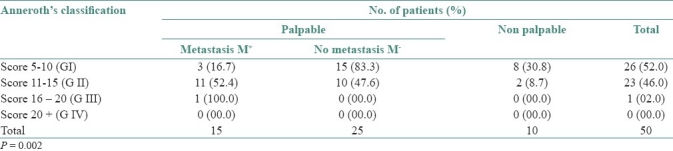
The relationship between differentiation based on Anneroth's classification and the frequency of cervical lymph node metastasis is given in Table 4. 52.0% cases were in G-I, of which 69.2% were palpable and out of these 16.7% were FNAC positive. 46.0% cases were of G-II, of which 91.3% were palpable within which 52.4% were FNAC positive. Only 2.0% cases were in G-III, of which the entire 2.0% are palpable and FNAC positive. Statistical analysis found the relationship between Anneroth's classification and lymph node metastasis (P=0.002).
Table 4.
Number of patients and the frequency of cervical lymph nodes metastasis according to Anneroth's system (n=50)
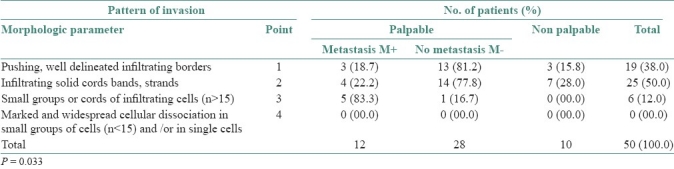
To understand the effects of different parameters of tumor-host relationship on Anneroth's classification, the relationship between pattern of invasion and the frequency of cervical lymph node metastasis was studied and is shown in Table 5. In Table 5, it can be seen that 38.0% cases were ranked as point-1, of which 84.2% were palpable within which 18.7% were FNAC positive. Total 50.0% cases were ranked as point-2, of which 72.0% were palpable and of these 22.2% were FNAC positive. 12.0% cases were ranked as point-3, of which the entire 12.0% were palpable and 83.3% were FNAC positive. According to statistical analysis these parameters of Anneroth's grades showed a relationship with lymph node metastasis (P=0.033).
Table 5.
Relationship between pattern of invasion (Anneroth's classification) and the frequency of cervical lymph nodes metastases. (n =50)
In Table 6, we compared the differentiation on the basis of Broder's and Anneroth's classifications in relation to frequency of cervical lymph node metastasis. 30.0% of total cases as metastasis were plotted in the left table and 70.0% of total cases as negative metastasis were plotted in the right table. Then, each case was considered against the corresponding Anneroth's and Broder's grade and plotted according to the percentage of that group (metastasis or non-metastasis). Color code was used to denote agreement/disagreement of each case's grading between the two classifications. Disagreement in two extreme grades (G-I and G-IV) and disagreement in grades G-II and G-III (most concentrated of metastasis cases) were colored as red and named major disagreements. Likewise disagreement in lower grades (G-I and G-II) was colored as light pink and named as minor disagreement/agreement.
Table 6.
Comparison between Broder's and Anneroth's differentiation in relation to lymph node metastasis
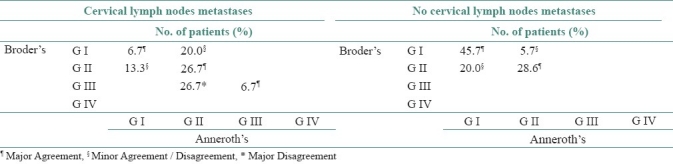
We found that 70.0% cases were palpable cervical lymph nodes with negative metastasis. Of this 70.0%, in 74.3% cases both Broder's and Anneroth's classifications agreed about the grading and in 25.7% cases these two classifications slightly disagreed.
In the case of palpable cervical lymph node with positive metastasis (30.0% of total patients) Broder's and Anneroth's classifications agreed in 40.1% cases, slightly disagreed in 33.3% cases and strongly disagreed in 26.7% cases in different grades.
It is evident that on grading, these two classifications agreed more in cervical lymph node with negative metastasis and disagreed more in cervical lymph node positive metastasis. In minor disagreement, in comparison to Broder's, Anneroth's classification graded more cases (20.0%) in lower grades (G-I) when it was negative metastasis and more cases (20.0%) in higher grade (G-II) when it was positive metastasis. However, such a trend reverses in cervical lymph node with positive metastasis in case of higher grading. When 26.7% samples were graded as G-II by Anneroth's classification, Broder's classification disagreed with those samples by grading them as G-III, a higher grade. According to statistical analysis, minimum score of metastasis is 7.0 (Anneroth's classification). So, Anneroth's G-II is highly susceptible for lymph node metastasis but in Broder's grading system the same samples were classified as G-I, II and III. In this point, Anneroth's grading system is more specific than Broder's grading system.
In the Table 7, it can be seen that 52.0% samples were concentrated in score 5–10 (G-I), 46% samples in score 11–15 (G-II) and only 2% in score 16–20 (G-III). In G-I, at higher scores, the number of samples with negative metastasis increases. Such a trend is evident in G-II at lower scores. In both G-I and G-II both, at higher scores, the number of samples with positive metastasis also increases but at a lower rate in comparison to negative metastasis. At higher scores or grade (G-III and G-IV), gradually the number of samples reduces but if any that comes as positive metastasis. Statistically minimum score of metastasis is 7 (Anneroth's).
Table 7.
The relationship between total histological malignancy score and frequency of cervical lymph nodes metastasis
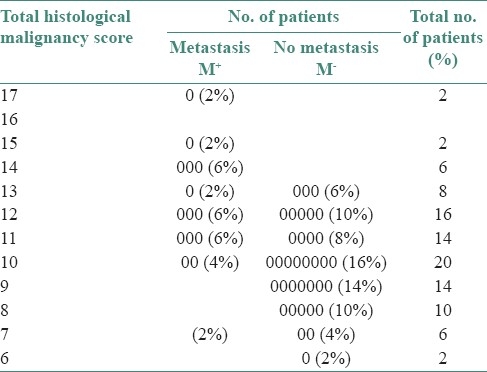
Figure 1, represents the Broder's and Anneroth's classification with clinical and radiological finding of the same patient. Histological slide of this patient is shown in Figures 2–4.
Figure 1.
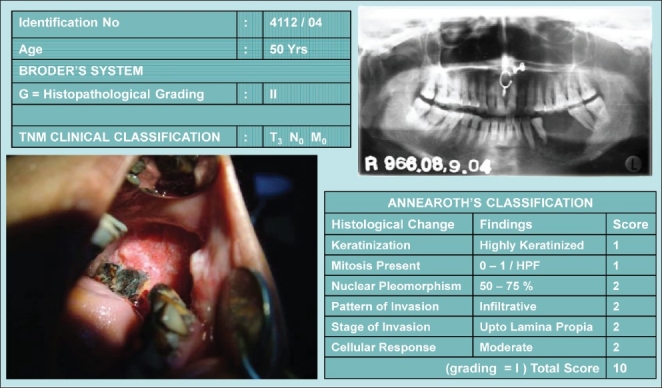
Broder's vs. Anneroth's classification in a same patient
Figure 2.
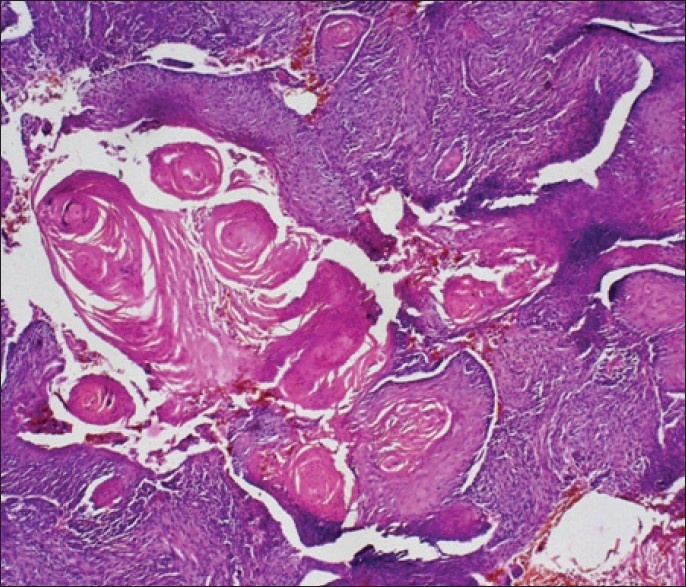
Keratinization of the malignant tissue (H and E stain, 10×)
Figure 4.
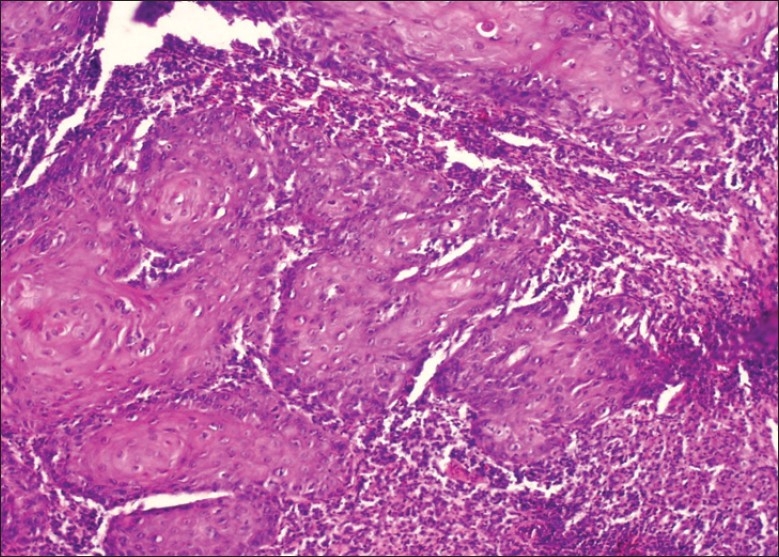
Tissue response (H and E stain, 10×)
Figure 3.
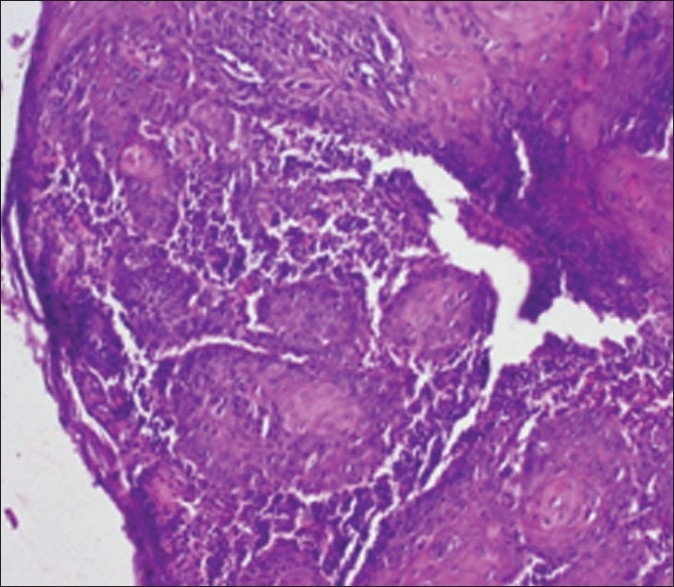
Pattern of invasion (Infiltration) (H and E stain, 10×)
DISCUSSION
Many studies on SCC's, correlating histological malignancy grading with different clinical parameters such as clinical staging, recurrence and prognosis have been published, and a close relationship between the degree of histological differentiation and the incidence of lymph node metastasis has been reported by several investigators.[7,26–31] Broder initiated the quantitative grading of cancer in 1920 and his classification system has been used for many years. A lack of correlation between Broder's’ grading system and prognosis has been mentioned. One of the main reasons is that, SCC's usually exhibit a heterogenous cell population with differences in the degree of differentiation.[7,8,28] In 1973, Jakobsson et al. developed a multifactorial malignancy grading system in order to obtain a more precise morphologic evaluation of the growth potential of SCC's in the head and neck region.[7,9] This malignancy grading system has been used during the last few years in both its original form and modified versions, especially for a retrospective study of SCC's.
To make morphologic criteria more precise, Anneroth and Hansen modified the grading system developed by Jakobsson et al. for application to SCC's.[10] We used the Anneroth et al.'s classification along with Broder's grading and TNM system. Statistical analysis found the relationship between Anneroth's classification and palpable lymph nodes (0.002). This method is more reliable and gives more specific results. The preference of multifactorial grading system over Broder's and TNM system in the whole thickness of the tumor, with respect to the palpable lymph node was revealed.
Anneroth's classification included three parameters indicating histological feature of tumor and three parameters indicating tumor-host relationship. It projected a decreasing trend of case's concentration and increasing trend of existence of palpable lymph node metastasis along with incremental grading. Despite it showing maximum concentration of cases in G-II, the ratio between metastasis and the number of cases was in a reverse order excluding G-IV where there was no patient in this study. A wide range of scoring (5 to 20+) along with six parameters enables Anneroth's classification to provide us a detailed analysis, grade the cases according to scoring of each parameter and may help us to find out any relation with palpable lymph node. Thus, it is more informative then other two grading systems.
In this study, statistical analysis of Anneroth's grading system showed a relation with palpable lymph nodes (P=0.002). Broder's grading system also showed a relation with palpable lymph nodes (P=0.012). TNM system did not show any relation with palpable lymph nodes. Among these two systems (Anneroth's and Broder's grading system), Anneroth's grading system is more significant according to statistical analysis.
Statistically minimum score of metastasis was 7 (Anneroth's grading system). 30.0% cases were metastasis positive, 50.0% cases were metastasis negative and 20.0% cases were non-palpable. In G-I, total cases were 52.0% and 6.0% were with metastasis. 46.0% cases were in G-II and 22.0% were with metastasis. A total of 2.0% cases were in G-III, and all of them were with metastasis. So, we can consider that in G-I, metastasis may be positive or negative. In G-II,I maximum cases were metastasis positive, but in G-II probability of metastasis was higher. So, for the cases in this grade, along with malignancy, we must investigate the metastasis of lymph nodes.
The same number of patients in Broder's grading system was distributed in the first three grades. In G-I, total cases were 44.0% and 8.0% were with metastasis. 46.0% cases were in G-II and 12.0% were with metastasis. In G-III, all the 10% cases were with metastasis. In such, no grade could be specified that showed any close relationship with lymph node metastasis though this system as a whole showed relation with lymph node metastasis. This system does not have any scoring system. So, detailed histological analysis is not possible. In G-IV grade of both Anneroth's and Broder's grading systems, there was no sample size.
The rate of metastasis in the cervical lymph nodes were reported to be 35.3-60.0% in patients with OSCC.[32,33] However, in this study it was 30.0%. In cases of oral squamous cell carcinoma, metastasis in the cervical lymph nodes may occur even in T1 or T2 cases of primary tumor.[34] but in this study we had no cases in T1 category. It may be noted that we got 82.0% cases in T1 and T2 categories as against only 18.0% in T3 and T4 categories. Though the rate of metastasis in the cervical lymph nodes was as high as about 73.3% in T1 and T2 cases as compare to about 26.7% in T3 and T4 cases, no significant difference was noted between them, suggesting misprediction of metastatic cervical lymph nodes by means of T classification alone. Thus, it seemed necessary to assess systemically including other factors. The therapeutic regimen should be judged not only by means of T classification but also evaluating the degree of histological malignancy.
As TNM system is based on tumor size, it failed to signify any information related to the actual histological condition of lesion. According to Broder's grading,on the basis of only one parameter, i.e. nuclear polymorphism, 44.0% cases were found to be in G-1, 46.0% were in G-II and 10.0% cases were in G-III. With six morphological parameters (tumor cell population and tumor-host relationship) Anneroth's classification graded 52.0% cases as G-I, 46.0% as G-II and 2.0% cases as G-III. It maybe noted that all three systems graded same samples.
To predict the future prognostic value, Anneroth's classification has three parameters under tumor cell population along with three parameters under tumor- host relationship. Total scoring of these six parameters may give us an idea about the extension, involvement and aggressiveness of tumor. This grading system also revealed significant relation with palpable lymph nodes. Though Broder's grading system as a whole, has significant relation with palpable lymph nodes, its prognostic tool is only one parameter (degree of differentiation of cell). TNM system had no significant relation with palpable lymph nodes and its histological analysis was not possible, it was mainly based on tumor size and palpable lymph nodes.
CONCLUSION
Squamous cell carcinoma is one of the challenges for oral and maxillofacial surgeons. Further studies including more sophisticated statistical methods and more comprehensive and homogeneous materials might clarify whether the grading of OSCC is of any greater significance in reflecting the growth capacity and malignancy of the tumor and in predicting the outcome of the disease at an early stage. It can be concluded that the different histological features outlined in the definitions of the six morphologic variables, including an assessment of the both tumor cell population and tumor-host relation, were appropriate for the classification of malignancy in oral squamous cell carcinoma.
A limitation of this study is that biopsies are not necessarily representative of the whole tumor content, but the biopsy is the only tissue sample available for histological evaluation. So, the pieces of biopsy should be sufficient to represent the parameters of malignancy and metastasis.
In conclusion, we consider that multifactorial grading system of OSCC according to Anneroth's classification could be taken as a valuable diagnostic factor and predictive factor of lymph node metastasis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sciubba, James J. Oral cancer. The importance of early diagnosis and Treatment. Am-J-Clin-Dermato. 2001;2:239–51. doi: 10.2165/00128071-200102040-00005. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization, Control of oral cancer in developing Countries. A WHO meeting. Bull World health Organ. 1984;62:817–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Henk JM. Malignant Tumors of The Oral Cavity (Management of Malignant Diseases.Series) In: Langdon JD, editor. London: Edward Arnold; 1986. pp. 2–8. [Google Scholar]
- 4.Chiba I, Muthumala M, Yamazaki Y, Uz Zaman A, Iizuka T, Amemiya A, et al. Characteristics of P53 gene of oral squamous cell carcinomas associated with betel quid chewing in Sri Lanka. Int J Cancer. 1998;77:839–42. doi: 10.1002/(sici)1097-0215(19980911)77:6<839::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Cawson RA, Odel EW. 6th ed. Philadelphia: Churchill Livingstone; 1998. Essentials of Oral Pathology and Oral Medicine; p. 128. [Google Scholar]
- 6.Shaheed I, Hossain A, Molla MR. Ogaka City. Japan: Proceedings of the 4th International Congress on Oral Cancer; 1995. Histological and Causative Factor of Oral Cancer in Bangladesh; pp. 27–30. [Google Scholar]
- 7.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 8.Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx (a pilot study) Acta Radiol Ther Phy Biol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- 10.Annearoth G, Hansen LS. A methodologic study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dent Res. 1984;92:448–68. doi: 10.1111/j.1600-0722.1984.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 11.Roland NJ, Caslin AW, Nash J, Stell PM. Value of grading squamous cell carcinoma of the head and neck. Head Neck. 1992;14:224–9. doi: 10.1002/hed.2880140310. [DOI] [PubMed] [Google Scholar]
- 12.Ensley JF, Crissman J, Kish J, Jacobs J, Weaver A, Kinzie J, et al. The impact of conventional morphologic analysis on response rates and survival in patients with advanced head and neck cancers treated initially with cisplatin-containing combination chemotherapy. Cancer. 1986;57:711–7. doi: 10.1002/1097-0142(19860215)57:4<711::aid-cncr2820570405>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsson PA. Histological grading of malignancy and prognosis in glottic carcinoma of the larynx. Workshop No: 14. In: Alberti PW, Bryce DP, editors. Centennial conference of laryngeal cancer. New York: Century Crofts; 1976. pp. 847–54. [Google Scholar]
- 14.Crissman JD, Lin WY, Gluckman JL, Cummings G. Prognostic value and histopathologic parameters in squamous cell carcinoma of the oropharynx. Cancer. 1984;54:2995–3001. doi: 10.1002/1097-0142(19841215)54:12<2995::aid-cncr2820541230>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Anneroth G, Hansen LS, Silverman S. Malignancy grading is oral squamous cell carcinoma: I, Squamous cell carcinoma of the tongue and floor of the mouth: Histologic grading in the clinical evaluation. J Oral Pathol. 1986;15:162–8. doi: 10.1111/j.1600-0714.1986.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 16.Zatterstrom UK, Wennerberg J, Ewers SB, Willen R, Attewell R. Prognostic factors in head and neck Cancer: Histologic grading, DNA ploidy, and nodal status. Head Neck. 1991;13:477–87. doi: 10.1002/hed.2880130603. [DOI] [PubMed] [Google Scholar]
- 17.Barona de Guzman R, Martorell NA, Basterra J, Armengot M, Alvarez-Valdes R, Garin L. Prognostic value of Histopathological parameter in 51 supraglottic squamous cell carcinomas. Laryngoscope. 1993;103:538–40. doi: 10.1288/00005537-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Klijanienko J, Janot F, De Braud F, Carlu C, Micheau C, Armand JP, et al. Third International Head and Neck Research Conference, Las Vegas. Ann Arbor, MI: The University of Michigan Medical Centre; 1990. Correlation of tumor vascularization , mitotic index and Ki-67 expression in fresh biopsies from squamous cell carcinoma of the head and neck: New evaluation techniques and application of classic markers; pp. 26–8. [Google Scholar]
- 19.TUBIANA M. Kinetics of tumor cell proliferation. In: Cvitkovic E, Droz JP, Armand JP, Khoury S, editors. Handbook of Chemotherapy in Clinical Oncology. 2nd ed. Paris: Scientific Communication International; 1993. pp. 23–35. [Google Scholar]
- 20.Begg AC, Hofland L, Noonen H, Bartelink H, Schraub S, Bontemps P, et al. The predictive value cell kinetic measurement in a European trial of accelerated fractionation in advanced head and neck tumors: An interim report. Int J Radiat Oncol Biol Phys. 1990;19:1449–53. doi: 10.1016/0360-3016(90)90357-p. [DOI] [PubMed] [Google Scholar]
- 21.Kearslay JH, Furlong KL, Cooke RA, Waters MJ. An immunohistochemical assessment of cellular proliferation markers in head and neck squamous cell cancers. Br J Cancer. 1990;61:821–7. doi: 10.1038/bjc.1990.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tytor M, Frazen G, Olafsson J. DNA ploidy in oral cavity carcinomas with special reference to prognosis. Head neck. 1989;11:257–63. doi: 10.1002/hed.2880110312. [DOI] [PubMed] [Google Scholar]
- 23.Poleksic S, Kalwalc HJ. Prognostic value of vascular invasion in squamous cell carcinoma of the head and neck. Plast Reconstr Surg. 1978;61:234–40. doi: 10.1097/00006534-197802000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Micheau C, Sancho H, Gerard – Marchant R, Saravane D. Prognosticdes adenopathies cervicales metastatiques en fonction des facteurs anatomo-pathologiques (etude histologique et statistique de 884 evidements cervicaux - Italian) N Arch Ital Oncol. 1978;6:5–14. [Google Scholar]
- 25.Shingaki S, Suzuki I, Nakajima T, Kawasaki T. Evaluation of histopathologic parameters in predicting cervical lymph node metastasis of oral oand oropharyngeal carcinomas. Oral Surg Oral Med Oral Pathol. 1988;66:683–8. doi: 10.1016/0030-4220(88)90318-0. [DOI] [PubMed] [Google Scholar]
- 26.Bryne M. Prognostic value of various molecular and cellular features in oral squamous cell carcinomas: A review. J Oral Pathol Med. 1991;20:413–20. doi: 10.1111/j.1600-0714.1991.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 27.Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol. 1986;17:346–54. doi: 10.1016/s0046-8177(86)80457-9. [DOI] [PubMed] [Google Scholar]
- 28.Klijanienko J, EL-Naggar AK, DeBraud F, Rodriguez-Peralto JL, Rodriguez R, Itzhaki M, et al. Tumor vascularization, mitotic index, histopathologic grade and DNA ploidy in the assessment of 114 head and neck squamous cell carcinomas. Cancer. 1995;75:1649–56. doi: 10.1002/1097-0142(19950401)75:7<1649::aid-cncr2820750715>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Odell EW, Jani P, Sherriff M, Ahluwalia SM, Hibbert J, Levison DA, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. Cancer. 1994;74:789–94. doi: 10.1002/1097-0142(19940801)74:3<789::aid-cncr2820740302>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Platz H, Fries R, Hudec M, Min Tjoa A, Wagner RR. The prognostic relevance of various factors at the time of the first admission of the patient.Retrospective DOSAK study on carcinoma of the oral cavity. J Maxillofac Surg. 1983;11:3–12. doi: 10.1016/s0301-0503(83)80005-8. [DOI] [PubMed] [Google Scholar]
- 31.Shingaki S, Suzuki I, Nakajima T, Kawasaki T. Evaluation of histopathologic parameters in predicting cervical lymph node metastasis of oral and oropharyngeal carcinomas. Oral Surg Oral Med Oral Pathol. 1988;6:683–8. doi: 10.1016/0030-4220(88)90318-0. [DOI] [PubMed] [Google Scholar]
- 32.Yajima M, Minimura T, Nebasih S, Sunada O, Tamura M, Kurashina K, et al. Clinicostatistical study on the cases for radical neck dissection in of squamous cell carcinomas of oral cavity. Jpn J Oral Maxillofac Surg. 1989;35:647–54. [Google Scholar]
- 33.Nakayama A, Ogawa A, Fukuta Y, Kudo K. Relation between lymphatic vessel diameter and clinicopathologic parameters in squamous cell carcinomas of oral region. Cancer. 1999;15:200–6. doi: 10.1002/(sici)1097-0142(19990715)86:2<200::aid-cncr3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Okada Y, Mataga I, Katagiri M, Ishii K. An analysis of cercal lymph nodes metastasis in oral squamous cell carcinoma, Relationship between grade of histopathological malignancy and lymph nodes metastasis. Int J Oral Maxillofac Surg. 2003;32:284–8. doi: 10.1054/ijom.2002.0303. [DOI] [PubMed] [Google Scholar]


