Abstract
Anticancer agents target various subcellular components and trigger apoptosis in chemosensitive cells. We have recently reported the tumor cell growth inhibitory properties of a mixture of triterpenoid saponins obtained from an Australian desert tree (Leguminosae) Acacia victoriae (Bentham). Here we report the purification of this mixture into two biologically pure components called avicins that contain an acacic acid core with two acyclic monoterpene units connected by a quinovose sugar. We demonstrate that the mixture of triterpenoid saponins and avicins induce apoptosis in the Jurkat human T cell line by affecting the mitochondrial function. Avicin G induced cytochrome c release within 30–120 min in whole cells and within a minute in the cell-free system. Caspase inhibitors DEVD or zVAD-fmk had no effect on cytochrome c release, suggesting the direct action of avicin G on the mitochondria. Activation of caspase-3 and total cleavage of poly(ADP-ribose) polymerase (PARP) occurred between 2 and 6 h posttreatment with avicins by zVAD-fmk. Interestingly, in the treated cells no significant changes in the membrane potential preceded or accompanied cytochrome c release. A small decrease in the generation of reactive oxygen species (ROS) was measured. The study of these evolutionarily ancient compounds may represent an interesting paradigm for the application of chemical ecology and chemical biology to human health.
Keywords: mitochondria, cytochrome c, caspases, ROS, membrane potential
Apoptotic cell death plays a critical role in normal cell development, tissue homeostasis, and the regulation of the immune system (1). Inadequate apoptosis is an integral part of cancer development (2). Many anticancer drugs have been shown to cause the death of sensitive cells through the induction of apoptosis. The death circuitry in mammalian cells has two major apoptotic pathways. One is a receptor-mediated pathway involving Fas and other members of the tumor necrosis factor (TNF) receptor family that activate caspase-8 (3), whereas the other involves cytochrome c, Apaf-1, and caspase-9 (4). Over the last 5 years, substantial evidence has been found to suggest that several mitochondrial events are essential for programmed cell death (5). One of the early crucial steps in the process of apoptosis is the release of cytochrome c through the outer mitochondrial membrane into the cytosol. In the cytosol, cytochrome c unleashes the activation of caspases, which are cysteine proteases with aspartate specificity (6). A large number of substrates for caspases have been reported, including poly(ADP-ribose) polymerase (PARP), a 116-kDa DNA repair enzyme that is cleaved during apoptosis (7).
Increasing evidence demonstrates that plants are a rich source of unique compounds that can induce apoptosis in premalignant or malignant human cells (8). These compounds target various cellular components, including tubulin (9) and topoisomerase (10). To identify plant compounds that may selectively induce apoptosis of cancer cells, we began to evaluate extracts of plants from the Leguminosae family. Plants from this family are known for their medicinal value (11) and several compounds have been isolated that inhibit the growth of tumor cells in vitro and in vivo (12). Bioassay-directed fractionation using cytotoxicity with human tumor cell lines as the monitor yielded an active mixture of triterpenoid saponins (F094) from the seedpods of the Australian desert tree Acacia victoriae (Bentham). In Jurkat cells this mixture was previously found to induce cell cycle arrest and inhibit phosphatidylinositol-3-kinase (PI3K) activity as well as Akt phosphorylation (46). In this study, we report the purification and structures of two active triterpenoid saponins from F094 that have been termed avicin D and avicin G (A. victoriae triterpenoid saponins). We further investigate the mechanism underlying tumor cell growth inhibition by these agents and find mitochondria to be one of the primary targets of the avicins' proapoptotic function.
Methods
Purification of Avicins.
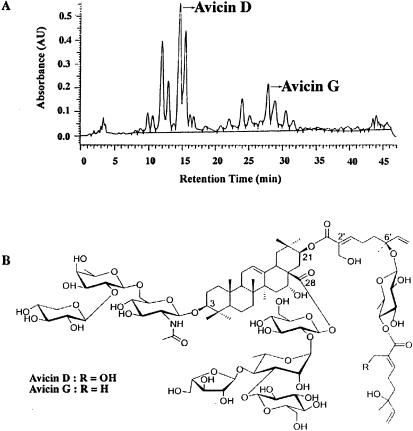
The ground seedpods of A. victoriae were extracted in 20% MeOH at 60°C. Solvent/solvent partitioning of the extract concentrated the bioactivity in a polar fraction (F094). The HPLC analysis of this fraction (Fig. 1A) on a C-18 reverse-phase MetaChem Intersil column (3 μ, 4.6 × 150 mm) using an acetonitrile and acidified water gradient elution program indicated a very complex mixture of compounds. Initial subfractionation of the extract on a C-18 reverse-phase semipreparative HPLC column and subsequent bioassay indicated that the regions around avicins D and G contained the most activity. The isolation of these compounds was achieved by a two-step preparative HPLC using a pentaflurophenyl column (50 × 250 mm, 10 μm; ES Industries, West Berlin, NJ) employing an aqueous methanol solvent system. The details of the isolation will be published elsewhere.
Figure 1.
(A) HPLC profile of avicins. (B) Chemical structures of avicin D and avicin G.
Cell Culture.
Jurkat cells were grown in RPMI medium 1640 supplemented with 10% FBS, 2 mM glutamine, and 0.05% gentamicin. For all treatments, cells were taken at 1 × 106/ml.
Assay for Growth Inhibition.
The growth inhibitory activity of F094 and avicins was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] reduction assay (13). Cells (1 × 104/well) were cultured with varying concentrations of F094, avicin D, or avicin G in 96-well plates for 72 h at 37°C. The cells were stained with MTT for 2 h and then incubated with lysis buffer (20% SDS in 50% N,N-dimethylformamide) for another 6 h. Optical density at 570 nm was used as a measure of cell viability.
Annexin V-FITC Binding Assay.
Induction of apoptosis was studied by annexin V-FITC binding assay. Jurkat cells (1 × 106) were treated with 2 μg/ml of F094, avicin D, or avicin G at 37°C. After washing the cells in cold PBS, they were resuspended in binding buffer (10 mM Hepes/NaOH, 140 mM NaCl, 2 mM CaCl2). Annexin V-FITC conjugate (BioWhittaker) was added (1 μg/ml) and incubated for 15 min at room temperature in the dark. Cells were then stained with propidium iodide (5 μg/ml) and analyzed by flow cytometry (14).
Detection of Cytochrome c Release from Mitochondria.
Release of cytochrome c from mitochondria was detected by Western blot analysis. Jurkat cells (1 × 107) were treated with F094, avicin D, or avicin G (2 μg/ml) at 37°C. After washing the cell pellets in sucrose buffer (0.25 M sucrose, 30 mM Tris, pH 7.7, 1 mM EDTA), they were resuspended in 20 μl of sucrose buffer containing 1 μM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 μg/ml aprotinin. Cells were disrupted by douncing 120 times in a 0.3-ml Kontes douncer with a B pestle. Cellular protein (50 μg) was resolved on an SDS/15% polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. The membrane was probed with monoclonal anti-cytochrome c antibody (PharMingen) followed with anti-mouse antibody conjugated to horseradish peroxidase (HRP). Protein bands were detected by chemiluminescence (ECL, Amersham Pharmacia).
Isolation of the Submitochondrial Fraction.
Jurkat cells (1.5–2.0 × 107) were suspended in 1 ml sucrose buffer (250 mM sucrose in 30 mM Tris⋅HCl, pH 7.4) and transferred into an N2 cavitation chamber (PARR Instruments, Moline, IL). The cells were subjected to N2 cavitation (300 psi for 5 min) according to the manufacturer's instructions. Under these conditions most of the cell membrane was disrupted with no change in the mitochondrial respiratory activity. Next, DNA and the nuclear fraction were removed by centrifugation (1,500 × g for 30 sec). The supernatant was further centrifuged (16,000 × g for 10 min) and the pellet was used as the submitochondrial fraction.
Assay for Caspase-3 Protease.
Caspase-3 activity was measured as described (15) with some modifications. Briefly, Jurkat cells (1 × 106) were treated with F094, avicin D, or avicin G (2 μg/ml) at 37°C. Cytosolic extracts were prepared by repeated freeze thawing of the cells in 300 μl of an extraction buffer (12.5 mM Tris, pH 7.0, 1 mM DTT, 0.125 mM EDTA, 5% glycerol, 1 μM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 μg/ml aprotinin). Cell lysates were diluted 1:2 with an ICE buffer (50 mM Tris, pH 7.0, 0.5 mM EDTA, 4 mM DTT, and 20% glycerol) and incubated with 20 μM of a caspase-3 substrate (acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin; Calbiochem) at 37°C. Caspase-3 activity was monitored by the production of fluorescent aminomethylcoumarin that was measured at excitation 355 nm and emission 460 nM using Fluoroscan II (Labsystems, Helsinki, Finland).
Immunoblot Analysis of PARP Degradation.
Induction of apoptosis was also examined by proteolytic cleavage of PARP (7). Jurkat cells (3 × 106) were treated with 2 μg/ml of F094, avicin D, or avicin G at 37°C. Cell lysates were prepared in a buffer containing 20 mM Hepes, 250 mM NaCl, 2 mM EDTA, 0.1% Nonidet P-40, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 0.5 μg/ml benzamidine, 1 mM DTT, and 1 mM PMSF. Cellular proteins (60 μg/ml) were separated on a SDS/7.5% polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. The membrane was probed first with monoclonal anti-PARP antibody (PharMingen) and then with anti-mouse antibody conjugated to horseradish peroxidase (HRP). Protein bands were detected by chemiluminescence (ECL). The appearance of an 85-kDa cleavage product was used as a measure of apoptosis.
Measurement of Mitochondrial Membrane Potential (Δψm).
Mitochondrial Δψm was measured as described by Zamzami et al. (16). After treatment with F094, avicin D, or avicin G (2 μg/ml) at 37°C, Jurkat cells were incubated with 80 nM of DiOC6 for 15 min at room temperature. They were then analyzed on a cytofluorometer (FACSCalibar from Becton Dickinson; excitation, 488 nm; emission, 552 nm).
Assay for Generation of Reactive Oxygen Species (ROS).
The generation of ROS was estimated by oxidation-sensitive fluorescent dye 5,6-carboxy-2′,7′-dichlorofluorescin diacetate (DCFH-DA) (Molecular Probes) by the method described (17). Jurkat cells seeded in 96-well plates (5 × 104 cells per well) were treated with F094, avicin D, or avicin G (2 μg/ml) in a Krebs–Ringer buffer containing 20 mM Hepes, 10 mM D-glucose, 127 mM NaCl, 5.5 mM KCl, 1 mM CaCl2, and 2 mM MgSO4 (pH 7.4). DCFH-DA was added into the wells at 5 μg/ml. Untreated cells received DCFH-DA alone. The cells were excited at 485 nm, and fluorescence was measured every 2 min at 538 nm for up to 2.5 h in a Fluoroskan II ELISA plate reader equipped with temperature control (Labsystems, Helsinki, Finland). Fluorescence was measured in the linear range.
Results
Chemical Analysis Elucidates the Structures of Avicin D and Avicin G.
Avicin D, the major component in F094, was isolated as a colorless, amorphous solid. Its molecular weight from matrix-assisted laser desorption ionization (MALDI) mass spectrum was 2,104 atomic mass units (amu), which is the sodium adduct of 2,081. A high resolution FAB mass spectrum gave the molecular formula C98H155NO46, thereby confirming the molecular weight of 2,081. The analysis of the proton NMR of avicin D revealed that it is a saponin with a side chain containing two units of the acyclic monoterpene, trans-2-hydroxymethyl–6-methyl-6-hydroxy-2,7-octadienoic acid connected by a quinovose sugar and attached to acacic acid at carbon 21. It also has a trisaccharide at carbon 3 and a tetrasaccharide at carbon 28. With the aid of various two-dimensional NMR experiments and degradative studies, the structure of avicin D is depicted in Fig. 1B (R = OH). Avicin G, another active component in F094, is a saponin very similar to avicin D. Its MALDI mass spectrum suggested a molecular weight of 2,065. The proton NMR indicated a similar side chain as in avicin D, but with the outer monoterpene replaced by trans-2,6-dimethyl-6-hydroxy-2,7-octadienoic acid as indicated in Fig. 1B (R = H). The detailed structure elucidation of the compounds will be published elsewhere.
F094 and Avicins Inhibit Growth by Induction of Apoptosis.
F094 and avicins were found to inhibit the growth of Jurkat cells in culture. The inhibitory concentration 50 (IC50) of F094 was 0.331–0.407 μg/ml, whereas that of avicin D and avicin G was 0.320–0.326 μg/ml and 0.160–0.181 μg/ml, respectively. In contrast, when tested in the normal human fibroblast cells, F094 and avicins had 10–35 times higher IC50 values.
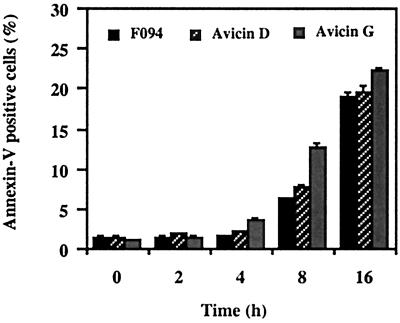
To understand the mechanism of growth inhibition, Jurkat cells treated with the F094 or avicins were analyzed for annexin V-FITC binding. Cells were simultaneously stained with propidium iodide to check for their viability. Treatment with all three preparations resulted in a time-dependent increase in viable annexin V positive cells (Fig. 2).
Figure 2.
Annexin V-FITC binding in F094- or avicin-treated Jurkat cells. Jurkat cells (1 × 106/ml) were treated with 2 μg/ml of F094, avicin D, and avicin G for different time periods. Cells were stained and analyzed by flow cytometry as described in Methods.
F094 and Avicins Lead to Cytochrome c Release from Mitochondria.
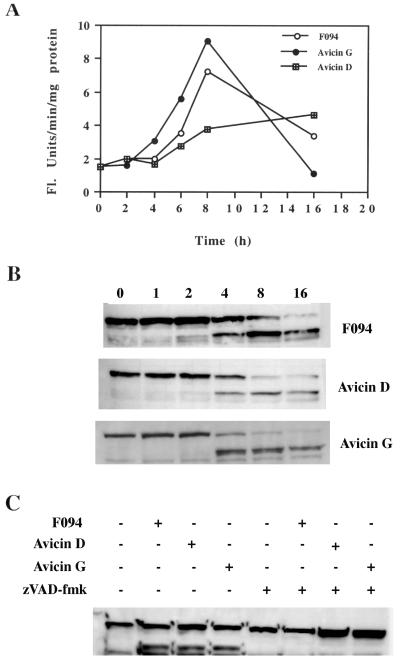
The release of cytochrome c from mitochondria into the cytosol appears to be one of the early events leading to apoptosis. F094-treated cells showed an increase in cytosolic levels of cytochrome c ≈4 h posttreatment (1.5-fold) (Fig. 3). Cytochrome c levels in the cytosol of avicin D-treated cells showed a more rapid increase. A 1.5-fold increase was seen within 30 min and a 3-fold increase within 4 h. Interestingly, in the cytosol of avicin G-treated cells, a dramatic increase (3.5-fold) in cytochrome c levels was seen as early as 30 min posttreatment. By 4 h, an 8.4-fold increase in the levels of cytochrome c was seen (Fig. 3).
Figure 3.
Effect of F094 or avicins on release of cytochrome c from mitochondria. Jurkat cells (1 × 107) were treated with F094, avicin D, or avicin G (all 2 μg/ml) at 37°C for the indicated time periods. Cells were homogenized, and lysates were assayed for cytochrome c levels by Western blot analysis as described in Methods.
To observe whether triterpenoid saponins directly affected mitochondria to induce apoptosis, experiments were carried out using avicin G in a cell-free system. Mitochondria were isolated from Jurkat cells by the N2 cavitation method. Treatment of this mitochondrial fraction with 2 μg/ml of avicin G resulted in a time-dependent release of cytochrome c, starting within 1 min of the treatment and reaching a peak between 5 and 10 min (Fig. 4A). A study of the dose–response revealed that most of the cytochrome c release was achieved with 0.5–2.0 μg/ml of avicin G incubated for 10 min (Fig. 4B). Pretreatment of the mitochondrial fraction with DEVD-CH2F, an irreversible caspase-3 inhibitor or z-Val-Ala-Asp-CH2F (zVAD-fmk), a broad cell-permeable irreversible caspase inhibitor of broad specificity, did not affect the release of cytochrome c, once again suggesting that these agents act directly on the mitochondria and that caspases upstream of cytochrome c are probably not involved.
Figure 4.
(A) Kinetics of cytochrome c release from the mitochondrial fraction of Jurkat cells in a cell-free system. Mitochondria were isolated as described in Methods. Avicin G (2 μg/ml) treatment was given for 0, 1, 2, 5, 10, and 20 min at 37°C. (B) Dose–response of avicin G-induced cytochrome c release. The isolated mitochondria were treated with different concentrations of avicin G for 10 min at 37°C. (C) Effect of caspase inhibitors on avicin G-induced cytochrome c release. The isolated mitochondria were pretreated with DEVD-CH2F (25 μM) or zVAD-fmk (25 μM) for 5 min at 37°C. This was followed by treatment with avicin G (2 μg/ml) for 10 min at 37°C. Release of cytochrome c was analyzed by Western blot as described in Methods.
F094 and Avicins Induce Activation of Caspases.
The release of cytochrome c from mitochondria into the cytosol triggers the activation of a cascade of caspases that are crucial downstream effectors in various cell death pathways. Therefore, we next studied the status of caspase-3 in treated Jurkat cells. F094- and avicin D-induced activation of caspase-3 was detectable at 4–6 h posttreatment and thereafter (Fig. 5A). However, with avicin G, an increase in caspase activity was observed 2–4 h posttreatment. By 16 h, caspase activity was down to the basal level (Fig. 5A).
Figure 5.
(A) Kinetics of caspase-3 activation induced by F094 or avicins. Jurkat cells (1 × 106) were treated with 2 μg/ml of F094, avicin D, or avicin G for different time periods. Caspase-3 activity in the cytosolic extracts of these cells was determined as described in Methods. (B) Cleavage of PARP by F094 or avicins. Jurkat cells (3 × 106) were treated with 2 μg/ml of the agents for the indicated time periods. Cell lysates were prepared and assayed for cleavage of PARP as described in Methods. (C) Effect of zVAD-fmk treatment on PARP cleavage induced by F094 or avicins. Cells (3 × 106) were cultured ± zVAD-fmk (100 μM) for 1 h at 37°C and then followed by treatment with 2 μg/ml of F094 or avicins for 4 h at 37°C. Cleavage of PARP was studied as described above.
One of the downstream targets of caspase-3 is PARP, which is proteolytically cleaved by caspase-3. F094 and avicins all induced cleavage of PARP starting at 4 h posttreatment (Fig. 5B). With avicin G, cleavage was almost complete by 4 h, whereas with the mixture and avicin D, complete cleavage took 8–16 h. Pretreatment of cells with zVAD-fmk totally blocked the cleavage of PARP (Fig. 5C).
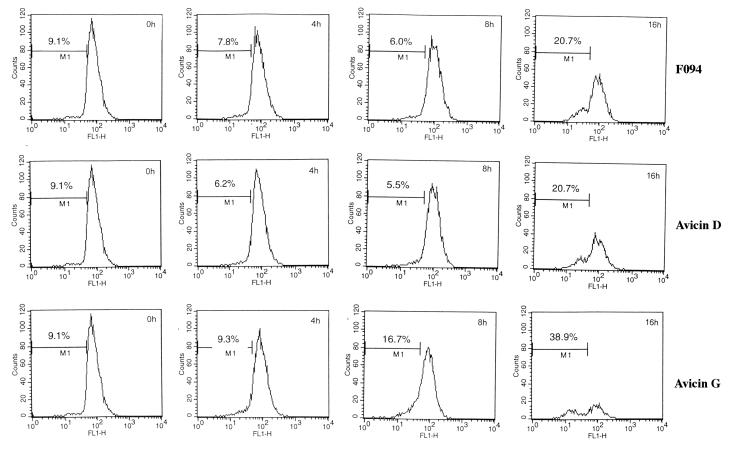
F094 and Avicins Do Not Affect the Mitochondrial Membrane Potential (Δψm).
Release of cytochrome c into the cytosol is usually preceded or accompanied by a drop in the mitochondrial Δψm. Treatment of Jurkat cells with F094, avicin D, or avicin G for up to 8 h did not produce any significant changes in the Δψm. However, longer treatments (16 h) induced a significant drop in the Δψm (Fig. 6).
Figure 6.
Effect of F094 or avicins on mitochondrial membrane potential. Jurkat cells (1 × 106/ml) were treated with 2 μg/ml of F094, avicin D, or avicin G for different time periods. Cells were stained with DiOC6 and analyzed by flow cytometry as described in Methods.
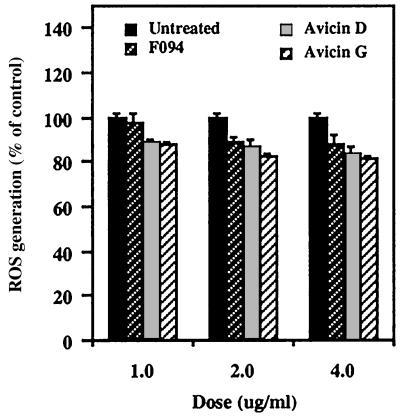
F094 and Avicins Decrease Generation of ROS.
Most apoptosis-inducing agents release ROS, which is considered one of the key mediators of apoptotic signaling. However, treatment of Jurkat cells with avicins for up to 2.5 h led to a decrease in the levels of ROS in a dose-dependent manner (Fig. 7). This decrease in ROS levels reached a plateau, and no further change could be seen upon longer exposures (up to 24 h) to the agents (data not shown).
Figure 7.
Effect of F094 or avicins on the generation of reactive oxygen species. Jurkat cells (5 × 104/well) were treated with 1, 2, and 4 μg/ml each of F094, avicin D, or avicin G. Generation of ROS was determined as described in Methods.
Discussion
Homeostasis in eukaryotic cells depends on the delicate balance between survival and death signals from the extracellular environment (18). Any aberration in either of these signaling pathways can be detrimental to cell physiology. In cancer, dividing cells fail to initiate apoptosis after sustaining DNA damage (2). Several pathways that lead to apoptosis have been identified (19–21), including pathways within the mitochondria (5). One of the goals of cancer chemotherapy and prevention is the identification of novel agents that can induce apoptosis selectively in tumor cells by affecting one or more of these proapoptotic pathways.
We have recently reported a mixture of triterpenoid saponins isolated from a desert tree A. victoriae that inhibits the growth of a variety of human tumor cell lines. A study of the underlying mechanism revealed an inhibition of the phosphatidylinositol-3-kinase (PI3K) signaling pathway in Jurkat cells (46). However, these effects required long treatments (16 h) with the triterpenoid saponins, a period too delayed to explain the early onset of apoptosis in Jurkat cells. These findings led us to investigate the role of mitochondria, not only with the mixture of triterpenoid saponins, but also with two purified molecules. The mixture of triterpenoid saponins (F094) was purified by preparative HPLC into several components. Using cytotoxicity with Jurkat cells as the monitor, two molecules, avicin D and avicin G, were selected for biological characterization.
Cells beginning to undergo apoptosis reorient phosphatidylserine from the inner side of the plasma membrane to its outer leaflet. In this exposed condition they can bind to annexin V (22), and this property has been used as a marker for apoptosis. Jurkat cells treated with F094 or the avicins showed a time-dependent increase in annexin V-positive cells starting at 4 h posttreatment, indicating the induction of apoptosis in these cells.
One of the early events that initiates apoptosis is the release of cytochrome c from the mitochondria into the cytosol (23, 24). In the cytosol of treated Jurkat cells, cytochrome c was detected within 30 min (with avicin D and avicin G) to 4 h (with F094). Once in the cytosol, cytochrome c binds with APAF-1 and procaspase-9 in the presence of dATP to form the apoptosome (25). This complex activates caspase-9, which in turn cleaves and thereby activates caspase-3. In the treated cells, release of cytochrome c from the mitochondria was followed by activation of caspase-3. This event was closely followed by the cleavage of PARP, one of the substrates of caspase-3 that is a 116-kDa DNA repair enzyme. The cleavage of PARP inactivates the enzyme, thereby making DNA repair impossible.
The release of cytochrome c may be the initiating event in apoptosis, or it may be downstream of caspase activation, such as occurs in the CD95 (Fas) system. The latter depends on the release of caspase-8 (26). Upon pretreatment of the cells with zVAD-fmk, a broad caspase inhibitor, we could totally block the cleavage of PARP without affecting the release of cytochrome c (data not shown). This event suggests that cytochrome c release could be the result of a direct action of these agents on the mitochondria and is independent of caspase activation. To confirm these results, we used purified mitochondria in a cell-free system. In this system avicin G induced cytochrome c release in a dose- and time-dependent manner and was not affected by pretreatment with caspase inhibitors DEVD-CH2F and zVAD-fmk.
How cytochrome c, which resides in the space between the outer and inner membrane of mitochondria, translocates into the cytosol is still unclear. Some of the proposed theories include induction of mitochondrial permeability transition (PT) (27) and formation of specific channels in the outer mitochondrial membrane (28). More recently, it has been proposed that cytochrome c can be released without loss of the transmembrane potential, but in this case hyperpolarization of the inner membrane occurs (29). In the F094- or avicin-treated cells, cytochrome c release was not preceded or accompanied by changes in the inner mitochondrial membrane potential. However, at 16 h posttreatment we demonstrated a significant depolarization of the membrane that is compatible with reports suggesting activated caspases can directly induce PT (30). To further understand the mechanism of cytochrome c release, experiments looking at ATP/ADP exchange and the effect on the F0F1-ATPase proton pump and other mechanisms are being evaluated.
Mitochondria are a rich source of ROS that are toxic byproducts of aerobic existence and play significant roles in various signal transduction pathways, including those leading to apoptosis (31). A study of the effect of avicin G up to 16 h on the levels of ROS showed a decrease in ROS generation, albeit minimal. Levels of ROS could also be directly or indirectly influenced by (i) increase in the level of antioxidants such as reduced glutathione or superoxide dismutase, (ii) aberrations in ATP/ADP exchange (29), (iii) oxidation of superoxide to oxygen by cytochrome c (32), or (iv) proteolysis of D4-GDP-dissociation inhibitor by caspases (33). By retaining some of the cytochrome c within the mitochondria or by virtue of the cells being glycolytic, the levels of cellular ATP could be maintained even after the onset of apoptosis, thereby delaying or inhibiting the production of ROS.
Studies indicate an increasing interest in the discovery of compounds that directly affect mitochondria (34). All of these agents induce apoptosis by either disrupting the membrane potential or releasing ROS, suggesting that the inner mitochondrial membrane is the primary target. Betulinic acid, a pentacyclic triterpene, was reported to induce apoptosis in neuroectodermal tumors (35) by acting directly on mitochondria in a CD95- and p53-independent fashion (36). Betulinic acid is a simple triterpene compound with a very restricted cell-type specificity. Avicins, on the other hand, which show broader cell specificity, contain a hydrophobic acacic acid as the core triterpene and have two acyclic monoterpene units connected by a quinovose sugar. The hydrophobic acacic acid core in the avicins probably allows it to traverse the membrane and affect the mitochondria, but characteristics of the remainder of the molecule may explain its biochemical effects and its differences from betulinic acid. We are currently truncating portions of avicin D and avicin G, including the side chains and the individual sugars, to determine which component is critical for the proapoptotic function. Avicins structurally appear very similar to elliptosides isolated previously from Archidendron ellipticum (37). However, recent NMR evaluation as well as chirality studies on the monoterpenes (unpublished results) suggest that there are subtle but significant differences between the saponins reported herein and those previously described as having antitumor activity.
Avicins are a class of triterpenoid saponins that induce apoptosis in Jurkat cells by affecting mitochondrial function independently of the membrane-bound death receptors. Of interest is the selectivity of apoptosis in Jurkat cells as compared with normal fibroblasts. Evan (38) has proposed that oncoproteins sensitize cells to apoptotic signals that are resisted by normal cells. The later effects of avicins appear to amplify the early signaling events leading to apoptosis. Phosphorylation of caspase-9 by Akt has been shown to inhibit its activity (39). By inhibiting the phosphorylation of Akt, avicins could be amplifying the proapoptotic effects seen after cytochrome c release. Recently, it has been demonstrated that APAF and caspase-9 are downstream effectors of p53-induced genes (40). Inhibition of Akt phosphorylation could also enhance the proapoptotic function of Bad by increasing its heterodimerization with Bcl-xL (41). Because of their direct effect on the mitochondria, avicins may be able to overcome resistance to apoptosis because of mutations in the p53 gene. The Jurkat cells used in this study lack p53. Therefore, avicins and other similar compounds may prove to be effective in the treatment of resistant cancers because of their ability to replace the function of lost or mutated suppressor genes.
The triterpenoid structure evolved more than a billion years ago as a critical membrane component of some prokaryotes (42). The saponins probably evolved later in plants and some marine organisms as secondary metabolites for defensive ecological purposes, and, as a result, may have potent biological activity in the mammalian system. Therefore, it is interesting that these compounds target the mitochondria, which are derived from ancient prokaryotes that have evolved in a symbiotic relationship within eukaryotes (43). Thus, findings reported in this paper suggest an interesting intersection between chemical ecology (i.e., signal transduction; 44) as well as chemical biology (45). Further studies may provide important leads for potential application to human health.
Acknowledgments
We thank Sarah Lock-Lim for excellent technical assistance and Ruth La Pushin and Rita J. Proske for helping with the flow cytometry. Research was conducted in whole or in part by the Clayton Foundation for Research. The work was also conducted by the Foundation for Research and the Biomedical Research Foundation.
Abbreviations
- PARP
poly(ADP-ribose) polymerase
- ROS
reactive oxygen species
References
- 1.Jacobson M D, Weil M, Raff M C. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 2.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 5.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson D W, Thornberry N A. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 7.Tewari M, Quan L T, O'Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 8.Pezzuto J M. Biochem Pharmacol. 1997;53:121–133. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 9.Vyas D M, Kadow J F. Prog Med Chem. 1995;32:289–337. doi: 10.1016/s0079-6468(08)70456-9. [DOI] [PubMed] [Google Scholar]
- 10.Pommier Y, Kohlhagen G, Kohn K W, Leteurtre F, Wani M C, Wall M E. Proc Natl Acad Sci USA. 1995;92:8861–8865. doi: 10.1073/pnas.92.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen O N, Allen E K. The Leguminosae: A Sourcebook of Characteristics, Uses and Nodulation. Madison, WI: Univ. of Wisconsin Press; 1981. [Google Scholar]
- 12.Ali M, Heaton A, Leach D. J Nat Prod. 1997;60:1150–1151. [Google Scholar]
- 13.Hansen M B, Nielsen S E, Berg K. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 14.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, La Face D M, Green D R. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enari M, Hug H, Nagata S. Nature (London) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 16.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin S A, Petit P X, Mignotte B, Kroemer G. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oridate N, Suzuki S, Higuchi M, Mitchell M F, Hong W K, Lotan R. J Natl Cancer Inst. 1997;89:1191–1198. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- 18.Raff M C. Nature (London) 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 19.Polyak K, Xia Y, Zweier J L, Kinzler K W, Vogelstein B. Nature (London) 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Bayoumy S, Zhang Y, Lozano J, Kolesnick R. J Biol Chem. 1998;273:30419–30426. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- 21.Friesen C, Herr I, Krammer P H, Debatin K-M. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 22.Schutte B, Nuydens R, Geerts H, Ramaekers F. J Neurosci Methods. 1998;86:63–69. doi: 10.1016/s0165-0270(98)00147-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 24.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 25.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 26.Kuwana T, Smith J J, Muzio M, Dixit V M, Newmeyer D, Kornbluth S. J Biol Chem. 1988;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 27.Petit P X, Susin S-A, Zamzami N, Mignotte B, Kroemer G. FEBS Lett. 1996;396:7–13. doi: 10.1016/0014-5793(96)00988-x. [DOI] [PubMed] [Google Scholar]
- 28.Manon S, Chaudhari B, Guerin M. FEBS Lett. 1997;415:29–32. doi: 10.1016/s0014-5793(97)01087-9. [DOI] [PubMed] [Google Scholar]
- 29.Vander Heiden M G, Chandel N, Schumacker P T, Thompson C B. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- 30.Marzo I, Brenner C, Zamzami N, Susin S A, Beutner G, Brdiczka D, Xie Z-H, Reed J C, Kroemer G. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korsmeyer S J. Trends Genet. 1995;11:101–105. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 32.Skulachev V P. FEBS Lett. 1998;423:275–280. doi: 10.1016/s0014-5793(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 33.Na S, Chuang T-H, Cunningham A, Turi T G, Hanke J H, Bokoch G M, Danley D E. J Biol Chem. 1996;271:11209–11213. doi: 10.1074/jbc.271.19.11209. [DOI] [PubMed] [Google Scholar]
- 34.Costantini P, Jacotot E, Decaudin D, Kroemer G. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 35.Pisha E, Chai H, Lee I S, Chagwedera T E, Farnsworth N R, Cordell G A, Beecher C W, Fong H H, Kinghorn A D, Brown D M, et al. Nat Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 36.Fulda S, Friesen C, Los M, Scaffidi C, Benendict M, Nunez G, Krammer P H, Peter M E, Debatin K-M. Cancer Res. 1997;57:4956–4964. [PubMed] [Google Scholar]
- 37.Beutler J A, Kashman Y, Pannell L K, Cardellina J H, Alexander R A, Balaschak M S, Prather T R, Shoemaker R H, Boyd M R. Bioorg Med Chem. 1997;5:1509–1517. doi: 10.1016/s0968-0896(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 38.Harrington E A, Fanidi A, Evan G I. Curr Opin Genet Dev. 1994;4:120–129. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 39.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Frisch S, Reed J C. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 40.Soengas M S, Alarcon R M, Yoshida H, Giaccia A J, Hakem R, Mak T W, Lowe S W. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 41.Gross A, McDonnell J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 42.Ourisson G, Nakatani Y. Chem Biol. 1994;1:11–23. doi: 10.1016/1074-5521(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 43.Gray M W, Burger G, Lang B F. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 44.Clardy J. Proc Natl Acad Sci USA. 1995;92:56–61. doi: 10.1073/pnas.92.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrieber S L. Bioorg Med Chem. 1998;6:1127–1152. doi: 10.1016/s0968-0896(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 46.Mujoo, K., Haridas, V., Hoffman, J. J., Wächter, G. A., Hutter, L. K., Yilling, L., Blake, M. E., Jayatilake, G. S., Bailey, D., Mills, G. B., et al. (2001) Cancer Res., in press. [PubMed]