Abstract
A morphine-like spasmolytic action (not naloxone reversible; involving the inhibition of acetylcholine release) and also effects on the transmural transport of electrolytes (Na+ and K+) and water have been reported as possible modes of the antidiarrhoeal action of polar fractions of Psidium guajava leaf extractives. The objective for this study was to verify if the reported modes of the antidiarrhoeal action should be broadened to include direct antimicrobial actions on some of the more common bacteria known to cause toxin-induced acute diarrhoea. Serial dilutions of a water-soluble, freeze-dried methanolic extract were tested on 10 such organisms, grown separately on nutrient agar plates, to determine the minimum inhibitory concentration (MIC) for each of these bacteria. These included the causative agents for (i) enteric fever (Salmonella typhi, Salmonella paratyphi A, Salmonella paratyphi B and Salmonella paratyphi C), (ii) food poisoning (Salmonella typhimurium and Staphylococcus aureus), (iii) dysentery (Shigella dysenteriae, Shigella flexneri and Shigella sonnei), and (iv) cholera (Vibrio cholerae). The growth of all these organisms was inhibited at the MIC of 10mg/ml of the extract, which is equivalent to 2.5μg/ml of active extractable flavonoids. The most sensitive organisms (MIC = 1mg/ml) were Staphylococcus aureus, Vibrio cholerae and Shigella flexneri.
Keywords: antimicrobial effects, bacterial toxin-induced diarrhoea, Psidium guajava extract
Introduction
For the millions of rural populations in countries of the developing world, diarrhoeal diseases continue to be the major cause of morbidity and mortality, with an estimated 1 billion episodes of illness and some 5 million or more deaths in children under 5 years (5–8). In such populations, preparations from herbs and plants remain the most common forms of treatment for diarrhoeal disease. Decoctions of the leaf, root or stem of the guava plant, Psidium guajava Linn. (Myrtaceae), have been used as antidiarrhoeal therapy in many systems of traditional medicine in tropical countries.
Using the electrically driven isolated guinea-pig ileum and a rat experimental diarrhoea model, it has been shown that the polar fraction of extracts from the leaf of this plant may owe its antidiarrhoeal activity to a neuronal spasmolytic effect, an inhibition of the increased secretion of Na+ and K+, and a decrease in water transport across the wall of the gut (1,2). The flower and leaf of the plant have been reported to have antibiotic activities (9). It was, therefore, deemed logical to test the possibility of such an action on some of the more common bacteria that are known to cause toxin-induced acute diarrhoea. This would be one more possible mode of the antidiarrhoeal action of polar fractions of the leaf of Psidium guajava.
Materials and Methods
Preparation of extract
Fresh leaves of Psidium guajava collected from the vicinity of the campus of the Universiti Sains Malaysia, Kelantan State of Malaysia, were washed in water and air-dried at a room temperature of 22 °C. The material was then oven-dried at 60 °C for 3 days, machine-ground into a coarse powder, and extracted with methanol (75%) for a total of 48 hours. The extract was centrifuged for 15 min. at 2,000 rev/min, and the supernatant was dried by rotary evaporation. A stock solution of 200 mg/ml in sterile distilled and deionised water was prepared, and the pH was adjusted to 7.2 with 0.01N HCl.
Growth and maintenance of micro-organisms
The 10 types of micro-organisms were all obtained from stock culture of the Department of Medical Microbiology, School of Medical Sciences, Universiti Sains Malaysia, Kelantan, Malaysia. These included agents of enteric fever (Salmonella typhi, S. paratyphi A, S. paratyphi B and S. paratyphi C), agents that cause food poisoning (Salmonella typhimurium and Staphylococcus aureus), the agent of cholera (Vibrio cholerae), and those that cause dysentery (Shigella dysenteriae, S.sonnei and S. flexneri). The organisms were maintained on blood agar plates and tested periodically, using standard identification methods to check purity. For experimental purposes, the organisms were inoculated into nutrient broths and incubated at 37 °C on a rotary shaker for 18 h. At least, 3 transfers were made at a dilution of 1:100, before the organisms were used in the experiments. Only fresh 24-hour cultures were used in all experiments.
Determination of MICs
The minimum inhibitory concentration (MIC) for each of the organisms studied was determined by using Mueller Hinton agar (MHA) in duplicate (10). Overnight grown cultures, with optical density equivalence of 0.5 McFarland, were used to lawn each quarter of the plate. In the centre of each quadrant, 4 holes were bored with the butt end of a sterile Pasteur pipette, and a sterile needle was used to remove the agar plugs. Four dose levels of the extract (1.0, 0.5, 0.05 and 0.005 mg) in aliquots of 50 μl, were introduced into the 4 wells, each marked for the concentration of extract used. The plates, quadruplicated for each test organism, were then incubated right-side-up at 37 °C overnight. The average diameter of the zone of inhibition produced by each concentration of the extract was determined, using callipers. Control culture plates for each organism were run in parallel with the test plates, using 50 μl of sterile distilled deionised water in the well.
Results
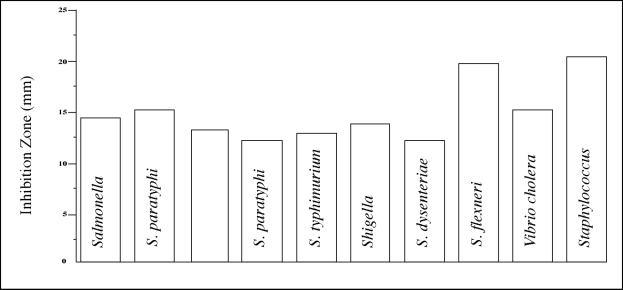
At a concentration of 20 mg/ml (1 mg/50 μl well), the growth of all 10 strains of bacteria was inhibited (Fig. 1).
Fig. 1:
Mean growth inhibitions (diameters of inhibition zones) in mm produced by 20 mg/ml (equivalent to 5μg/ml of the active compound) of Psidium guajava extract on 10 common diarrhoea-causing bacteria
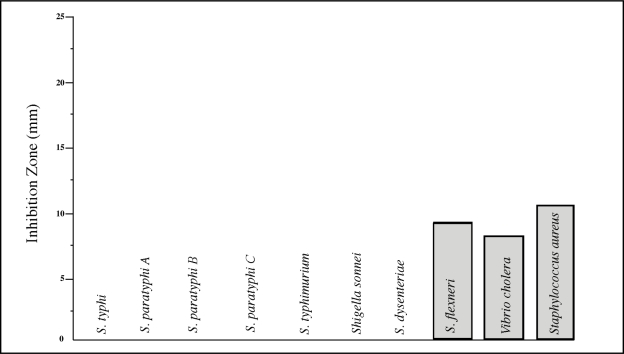
An MIC of 10mg/ml was obtained for 7 of the organisms, and the most susceptible, Shigella flexneri, Vibrio cholerae and Staphylococcus aureus, showed an MIC value of 1 mg/ml. The greatest sensitivity (11 mm zone of inhibition) was shown by Staphylococcus aureus (Fig. 2).
Fig. 2:
Mean growth inhibitions (diameters of inhibition zones) in mm produced by 1 mg/ml extract (MIC) of Psidium guajava on the 3 most sensitive of the 10 common diarrhoea-causing bacteria. The MIC for the other 7 organisms was 10 mg/ml (equivalent to 2.5 μg/ml of the active compound) of Psidium guajava extract
Discussion
An ideal antidiarrhoeal agent should possess the ability to reverse (i) the increased luminal osmolarity of osmotic diarrhoea, (ii) the increased electrolyte secretion of secretory diarrhoea, (iii) the decreased electrolyte absorption and (iv) the deranged intestinal motility that causes decrease in transit time (11). Previous reports have shown that the polar fraction from the leaf of Psidium guajava was efficacious in all these conditions (1,2). It has also been shown to have antinociceptive effects in gastroenteritis (12). The added antimicrobial activity, as shown by the present study, together with the effects on the pathophysiology in water and electrolyte transport, give scientific basis for the claimed efficacy in the use of extracts of the leaf of Psidium guajava in the treatment of acute diarrhoea in traditional medicine. The inhibition of growth of all the types of bacteria that commonly cause gastroenteritis is a very assuring addition to reported possible modes of action of this natural product. It is interesting to note that the extract could be used against the Salmonellae. Both the food-poisoning variety and the more virulent Salmonellae that cause typhoid and paratyphoid fevers were equally susceptible to the inhibitory effects of the extract. Organisms causing dysentery were also inhibited by the extract. Since acute diarrhoeal diseases have different modes of pathogenesis, it is very useful to have demonstrated, through the present and previous reports, that the antidiarrhoeal action is multifaceted, just like the pathophysiology of these diseases. The study also showed that Vibrio cholerae is highly susceptible to the effects of the extract. Such an inhibitory property would prevent the organism from producing the vibrio toxin, and this would imply that, when administered to cholera patients, it should significantly lower the morbidity and mortality, especially in children in remote places without hospital facilities. Use of this natural product might prevent death due to the dehydration caused by continuous diarrhoea in these children. Unfortunately, knowledge of the antidiarrhoeal effects of this natural product is rather limited to only a few practitioners of traditional medicine, even in countries where the plant grows abundantly in the wild or is planted in commercial orchards (4).
The results also showed S. aureus, a common food-poisoning organism, to be the most susceptible of all the bacteria studied. Since S. aureus is also commonly implicated in pus-causing wounds, it would be interesting to hazard a guess that the extract could be used as an alternative in the treatment of wounds infected with this bacteria or with the multi-resistant S. aureus (MRSA).
There is evidence that the antidiarrhoeal effects might be due to the presence of flavonoids in the extract (13), and the MIC of 10 mg/ml of the polar fraction used in this study is equivalent in potency to 2.5 μg/ml of these extractable flavonoids (3).
Acknowledgments
We wish to thank the School of Medical Sciences for the short-term grant. Our gratitude is also to Messrs Jamaluddin Ramli and Lokman Ali of the Department of Pharmacology and also Tuan Afifah Tuan Ibrahim of the Department of Medical Microbiology and Parasitology, School of Medical Sciences, Universiti Sains Malaysia, for their technical assistance. We are equally grateful to Roslindawati Mohd. Nasir for typing the manuscript.
References
- 1.Lutterodt GD. Inhibition of gastrointestinal release of acetylcholine by quercetin as a possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhoeal disease. Journal of Ethnopharmacology. 1989;25:235–47. doi: 10.1016/0378-8741(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 2.Lutterodt GD. Effect on electrolyte and water transport by Psidium guajava extract in a rat secretory diarrhoea model. Asia Pacific Journal of Pharmacology. 1994;9:189–93. [Google Scholar]
- 3.Lutterodt GD, Maleque A. Effects on mice locomotor activity of a narcotic-like principle from Psidium guajava leaves. Journal of Ethnopharmacology. 1988;24:219–31. doi: 10.1016/0378-8741(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 4.Lutterodt GD. Inhibition of Microlax-induced experimental diarrhoea with narcotic-like extract from Psidium guajava leaf in rats. In: Capasso, Mascolo N, editors. Natural Drugs and the Digestive Tract - Proceedings of the 1st International Symposium on Natural Drugs and the Digestive Tract; Napoli, Italy. 1992. pp. 129–40. [Google Scholar]
- 5.Synder JD, Merson MH. The magnitude of the global problem of acute diarrhoeal disease review of active surveillance data. Bulletin of World Health Organization. 1982;60(4):605–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Baltazar J, Briscoe J, Mesola V, Moe C, Solomon F, Vanderslice J, Young B. Can the case control method be used to assess the impact of water supply and sanitation on diarrhoea? A study in Phillipines. Bulletin of World Health Organization. 1988;66(5):62–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail A. An update of diarrhoel disease in Malaysia. Southeast Asian J Tropical Med. Pub. Health. 1988;19:397–400. [PubMed] [Google Scholar]
- 8.World Health Organization (2 ed.) The treatment and prevention of acute diarhoea, Practical Guidelines. WHO; Geneva: 1994. pp. 1–4. [Google Scholar]
- 9.Watt JM, Breyer-Brandwijk MG. Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. Edinburgh and London: E and S Livingstone Ltd; 1962. pp. 1457–8. [Google Scholar]
- 10.Collins CH, Lyne PM, Grange JM, editors. Microbiology Methods. 6 ed. London: Butterworth and Co. (Publishers) Ltd.; 1982. pp. 261–80. [Google Scholar]
- 11.Jinich H, Hersh T. Physicians’ Guide to The Etiology of Diarhoea. Medical Economics Company Inc.; Oradell, NJ: 1982. [Google Scholar]
- 12.Lutterodt GD. Analgesic efficacy of Psidium guajava extractive in mouse experimental pain models. Asia Pacific Journal of Pharmacology. 1993;8:83–7. [Google Scholar]