Abstract
The pathway between the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST) is emerging as a critical mediator of stress-related affective processes. Evidence also indicates that exposure to drugs of abuse, like opioids, is associated with NMDA-type glutamate receptor-dependent plasticity in the CeA and BNST. However, there is little evidence that NMDA receptors are expressed in CeA neurons projecting to the BNST, or are required for opioid-induced BNST neural activation. Immunoelectron microscopy, tract tracing, and conditional gene deletion technology were used to investigate the synaptic organization of the NMDA receptor and the mu-opioid receptor (μOR) in the CeA-BNST pathway. By dual labeling electron microscopy, numerous CeA-BNST projection neurons expressed the NMDA-NR1 receptor subunit (NR1) or μOR. By triple labeling, it was also found that NR1 and μOR were co-expressed in some CeA-BNST projection neurons. Despite being colocalized in somato-dendritic compartments of CeA neurons, NR1 and μOR were rarely expressed in their axonal terminations in the BNST. Deleting the NR1 gene in CeA neurons resulted in a reduction of morphine-induced Fos protein labeling in the ventral BNST. In summary, NR1 and μOR are coexpressed in somatodendritic sites of CeA neurons, including those projecting to the BNST. In addition, expression of the NR1 gene in CeA neurons is required for morphine-induced BNST neural activation. Thus, postsynaptic NMDA receptors and μORs are positioned for the co-modulation of CeA projection neurons to the BNST, which may provide a synaptic substrate for stress-induced emotional processes critically involved in opioid addictive behaviors.
Keywords: Addiction, Cre recombinase, Glutamate, Morphine, Synaptic plasticity
Introduction
Glutamate-dependent neural plasticity has been implicated in the emergence of addiction to various psychoactive agents, including opioids (Reissner and Kalivas, 2010). The ionotropic N-methyl-d-aspartate (NMDA) type glutamate receptor may play a key role in linking glutamate signaling with neural and behavioral plasticity, events that are highly relevant to opioid addiction (Glass, 2010).
The NMDA receptor is implicated in the development and expression of several components of opioid addictive behaviors, including drug self-administration (Glick et al., 2001; Semenova et al., 1999), dependence (Trujillo and Akil, 1991), contextual learning to drug-reward (Tzschentke and Schmidt, 1995) and withdrawal-aversion (Higgins et al., 1992), as well as the extinction (Myers and Carlezon, 2010) and reinstatement (Ma et al., 2007) of learned behaviors implicated in relapse. Despite this evidence, the neural circuitry linking NMDA receptors to opioid addictive behaviors is not well understood.
The central nucleus of the amygdala (CeA) is an important coordinator of behavioral reactions to stressful events. It has numerous projections to limbic-related brain areas, including the bed nucleus of the stria terminalis (BNST), and plays a role in opioid addictive behaviors (Koob, 2009). Neurons in the CeA express the NMDA-NR1 (NR1) receptor subunit gene (Sato et al., 1995) and protein (Petralia et al., 1994), as well as NMDA ligand binding sites (Monaghan and Cotman, 1985). At the cellular level, functional CeA NMDA receptors play an important role in neural signaling (Samson and Paré, 2005) and plasticity (Pollandt et al., 2006). At the behavioral level, CeA NMDA receptors are implicated in the learned rewarding (Rezayof et al., 2007) and aversive (Glass et al., 2008) properties of opioid exposure and opioid withdrawal, respectively. The CeA also contains intrinsic neurons and axon terminals that contain opioid peptides and μ-opioid receptors (μOR) (Cassell and Gray, 1989; Fallon and Leslie, 1986; Poulin et al., 2006). Although it has been shown that NR1 and mu-opioid receptors are co-expressed in the CeA (Glass et al., 2009) where they co-modulate excitatory signaling (Zhu and Pan, 2005), it is not known if either of these proteins are expressed in CeA projection neurons.
The BNST is a major target of CeA projection neurons (Cassell et al., 1986; Roder and Ciriello, 1993; Weller and Smith, 1982; Zahm et al., 1999), and this pathway is implicated in stress-induced reinstatement of drug self-administration (Gastard et al., 2002). Along with the CeA, the BNST plays a critical role in coordinating the experience of stress with autonomic, endocrine, and behavioral processes (Ulrich-Lai and Herman, 2009). It contains neurons that are responsive to opioids, as measured by markers of neural activity and plasticity like the immediate early gene product Fos (Bot and Chahl, 1996). The BNST is also involved in opioid addictive behaviors (Delfs et al., 2000; Walker et al., 2000). Although electrophysiological evidence indicates that NMDA receptors are involved in excitatory postsynaptic signaling in the BNST (Egli and Winder, 2003), ultrastructural (Gracy and Pickel, 1995) and pharmacological (Forray et al., 1995) data suggest that this protein may also have an axonal expression in this brain region. With regard to μOR, stimulation of this receptor in the BNST has been shown to produce a complex set of electrophysiological responses, including primarily excitatory (Dalsass and Siegel, 1990), inhibitory (Sawada and Yamamoto, 1981), or mixed (Casada and Dafny, 1993) actions, consistent with fine structural reports that μOR is present in both pre- and postsynaptic sites in the BNST (Jaferi and Pickel, 2009).
Current evidence suggests competing circuit-level models of glutamate and opioid receptor distributions in the CeA and BNST, however the synaptic organization of NMDA receptors and μOR in this pathway is unknown. A combination of immunocytochemical electron microscopy (EM), tract tracing, and conditional gene deletion technology was used to characterize the synaptic organization of NMDA receptors and μOR in the pathway between the CeA and BNST.
Methods
Animals
Experimental protocols involving animals and their care were approved by the Institutional Animal Care and Use Committee at Weill Medical College of Cornell University and conformed to NIH guidelines. Adult (20–30 g) male C57BL/6 and floxed NR1 (fNR1) mice were used in these studies. As previously described (Glass et al., 2008), mice homozygous for the fNR1 gene have loxP site placed in the intron that lies between exons 10 and 11 and a second site downstream after exon 22, the last exon. The two loxP sequences flank a region of the NR1 gene that encodes the 4 membrane domains and the entire C-terminal sequence of the polypeptide chain. These mice are maintained on the C57BL/6 background.
Tracer and virus administration
Under deep isoflurane anesthesia, tracers or viral vectors were unilaterally administered into the respective brain sites of adult mice. To identify somatodendritic processes of CeA projection neurons to the BNST, fluorogold (FG [Fluorochrome, Denver, CO]; 1% in PB) was unilaterally injected in the BNST 0.3 mm anterior and 0.8 mm lateral to bregma, at a depth of 4.3 mm ventral to the skull surface according to the atlas of Hof et al. (2000). To identify the axon terminals of CeA neurons that project to the BNST, the tracer biotinylated dextran amine (BDA [Invitrogen, Carlsbad, CA]; 10% in phosphate buffer [PB]) was unilaterally delivered 1.2 mm posterior and 2.5 mm lateral to bregma, at a depth of 4.6 mm ventral to the surface of the skull. To unilaterally delete the NR1 gene, approximately 200–250 nl of rAAV-GFP-Cre or rAAV-GFP (5 × 106 viral particles per µl; generously provided by Dr. Charles E. Inturrisi) were injected into the CeA, or a control area 1 mm ventral to this target, in fNR1 mice. Microinjections were made by interfacing a picospritzer (Picospritzer II, General Valve Corp., Fairfield, NJ) to a glass pipette (WPI, Sarasota, FL), whose tip was pulled to a diameter of ~50 µm, via a pipette holder and plastic tubing. Injections were made over a 15-minute interval. In all cases, to prevent leakage of solution, the pipette was left in place for an additional 15 min. Bone wax was used to cover the bore-hole, and the mice were allowed to recover in their home cages. For tracer studies, animals were sacrificed at least 7 days post-injection (Aicher et al., 2000; Glass et al., 2002). For the spatial–temporal gene deletion study, at least 14 days were required for maximal gene deletion (Glass et al., 2008) and the beginning of experimental procedures.
Tissue preparation and procedures for light microscopic identification of tracer injection sites
Mice were anesthetized with pentobarbital (150 mg/kg, i.p.), and their brains were fixed by aortic arch perfusion sequentially with: (a) 15 ml of normal saline (0.9%) containing 1000 units/ml of heparin, (b) 40 ml of 3.75% acrolein in 2% paraformaldehyde (PFA) in 0.1 M PB (pH 7.4), and (c) 100 ml of 2% paraformaldehyde in PB, all delivered at a flow rate of 25 ml/min. The brains were removed and post-fixed for 90–120 min in 2% paraformaldehyde in PB. Coronal sections (40 µm) from the forebrain at the level of the BNST and CeA were cut with a vibrating microtome. Tissue sections were treated with 1.0% sodium borohydride in PB and then washed in PB. To enhance tissue permeability, sections were immersed in a cryoprotectant solution (20% sucrose and 8% glycerol in 0.05 MPB) at room temperature followed by 15 min in −80 °C. Sections processed for immunolabeling of FG were incubated for 30 min in 0.5% bovine serum albumin (BSA) to minimize nonspecific labeling, followed by overnight incubation in primary rabbit anti-FG antisera (1:1000). Then FG sections were washed in TBS, incubated in anti-rabbit IgG conjugated to biotin and rinsed in TBS. Subsequently, the brain sections from both the FG and BDA groups were incubated for 30 min in avidin–biotin–peroxidase complex (ABC; 1:100, Vectastain Elite Kit, Vector Laboratories) in TBS. The bound peroxidase was visualized by reaction for 6 min in 0.2% solution of 3, 3'-diaminobenzidine (DAB) and 0.003% hydrogen peroxide in TBS. Sections were mounted in 0.05 M PB, dehydrated, and coverslipped on glass slides. These sections were examined using a Nikon light microscope.
Dual labeling immunocytochemical procedures for EM
Tissue sections from nine mice (FG:6; BDA:3) with injections in their respective target region were processed for dual labeling immunocytochemistry as previously described (Chan et al., 1990; Leranth and Pickel, 1989). Tissue sections were treated with 1.0% sodium borohydride in PB for 30 min and then washed in PB, followed by immersion in cryoprotectant solution (20% sucrose and 8% glycerol in 0.05 MPB) at room temperature followed by 15 min in −80 °C. Sections were next rinsed in 0.1 M TBS, and then incubated for 30 min in 0.5% BSA to minimize nonspecific labeling. For FG labeling in the CeA, tissue sections were incubated in a cocktail of primary antisera containing rabbit anti-FG antisera (1:1000) and one of the following 1). mouse anti-NR1 (1:100), or 2). guinea pig anti-μOR (1:100) for 48 h. Sections from the CeA were processed for dual immunocytochemical labeling of NR1 by immunogold-silver and FG by the more sensitive immunoperoxidase method in order to maximize detection of the retrograde tracer. Two sets of tissue sections processed for BDA staining in the BNST were similarly treated except with primary antisera only for 1). NR1 or 2). μOR at the same dilutions as above. Following primary antisera incubation, sections were washed in TBS. Tissue sections processed for FG were incubated in anti-rabbit IgG conjugated to biotin (1:400, 30 min) and rinsed in TBS. Tissue sections processed for FG and BDA were then incubated for 30 min in ABC in TBS. The bound peroxidase was visualized by reaction for 6 min in 0.2% solution of DAB and 0.003% hydrogen peroxide in TBS. For immunogold labeling, sections were rinsed in 0.01 M phosphate buffered saline (PBS, pH 7.4), and blocked for 10min in 0.8% BSA and 0.1% gelatin in PBS to reduce non-specific binding of gold particles. Sections were then incubated for 2 h in anti-mouse (NR1) or antiguinea pig (μOR) IgG conjugated with 0.6 nm gold particles (1:50, AuroProbeOne, Amersham, Arlington Heights, IL), and then rinsed in 0.5% BSA and 0.1% gelatin in PBS, followed PBS. After gold conjugated secondary antisera incubation, sections were incubated for 10 min in 2% glutaraldehyde in PBS, and then rinsed in PBS. The bound gold particles were enlarged by a 5 min silver intensification using an IntenSE-M kit (Amersham, Arlington Heights, IL). For the FG study, in order to investigate possible antisera cross-reactivity, tissue was processed with omission of one or the other primary antisera followed by incubation with the secondary antisera corresponding to the alternate species.
Triple labeling immunocytochemical procedures for EM
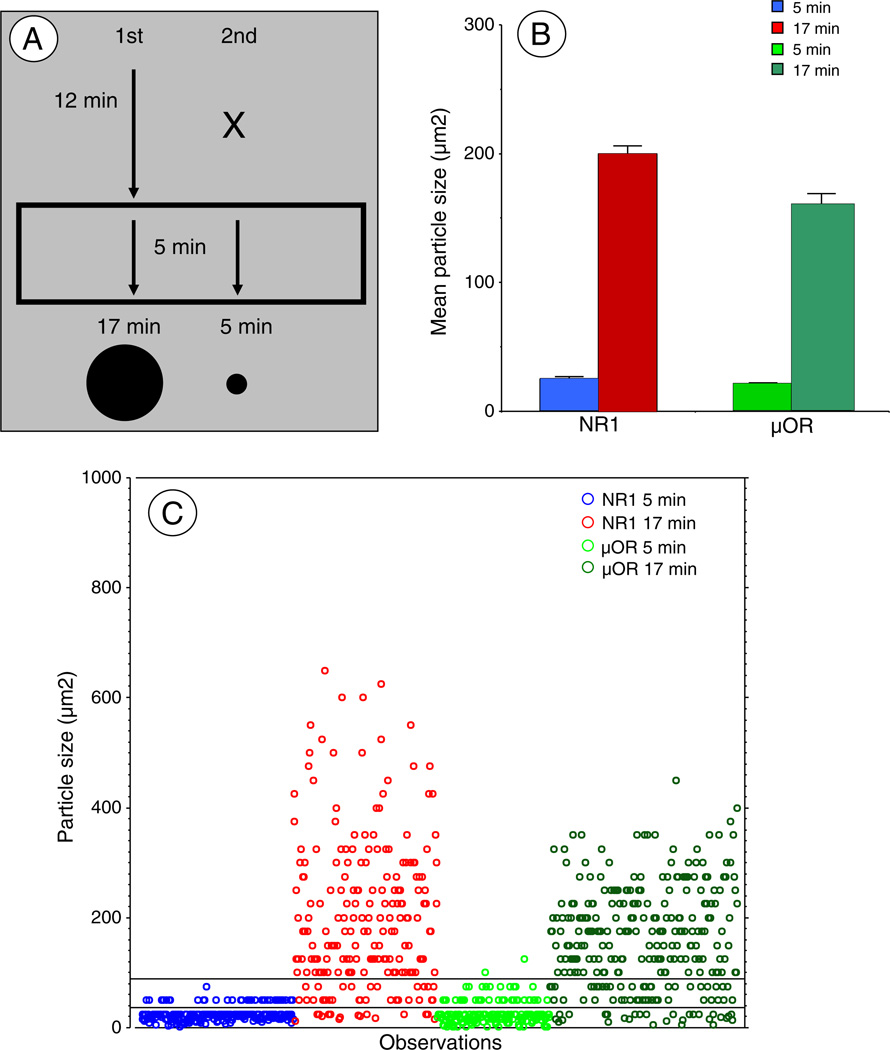
Forebrain tissue sections containing the CeA were processed for triple labeling of FG by immunoperoxidase, and NR1 and μOR by immunogold based on a previously published double labeling pre-embedding immunogold-silver enhancement procedure (Paspalas and Goldman-Rakic, 2004) as outlined in Fig. 4A. Briefly, sections were incubated in a primary antiserum cocktail including FG (peroxidase: 1:1000) along with NR1 (gold: 1:100) and μOR (gold: 1:100), and processed for peroxidase identification as described above. In preparation for immunogold labeling, sections were rinsed in 0.01 M PBS, and blocked for 10 min in 0.8% BSA and 0.1% gelatin in PBS to reduce non-specific binding of gold particles. Sections were then incubated for 2 h in either anti-mouse or anti-guinea pig IgG conjugated with 0.6 nm gold particles (1:50), then rinsed in 0.5% BSA and 0.1% gelatin in PBS, and then PBS. The bound gold particles were enlarged by incubation in a silver enhancement solution for 12 min. Then the brain sections were washed, incubated in the second gold-conjugated secondary antisera, and silver-enhanced for another 5 min to enlarge the second gold-conjugated secondary antisera. These times were chosen based on a single labeling time-course study (not shown) for each antisera showing that these two durations produced significantly different mean particle sizes (Fig. 4B). To control for non-specific labeling or other artifacts of tissue processing, in each labeling condition, tissue was processed with omission of one, two, or three primary antisera followed by incubation with the respective secondary antisera. These include the following combinations: 1). FG + NR1-(μOR); 2). FG-(NR1) + μOR; 3). NR1 + μOR-(FG); 4). NR1-(μOR)-(FG); 5). μOR-(NR1)-(FG); 6). FG-(NR1)-(μOR); 7). -(FG)-(NR1)-(μOR).
Fig. 4.
Different gold–silver enhancement times yield distinct particle sizes(A). Schematic outline of the dual gold–silver enhancement procedure. Sections were incubated with one of the pair of gold-conjugated antisera, which was then silver enhanced for 12 min. This was followed by incubation with the second gold-conjugated antisera, followed by a further 5-minute silver enhancement. This resulted in the first secondary antisera being incubated for 17 min, and the second antisera for 5 min, yielding two distinct but partially overlapping particle size distributions. Arrows represent time periods of silver enhancement, X denotes time period without secondary antisera exposure. The bottom of this figure illustrates representations of silver enhanced gold particle sizes for the two incubation times. Particles are not drawn to scale. (B). Mean particle sizes for each antisera at different silver-enhancement times. These values were obtained in single labeling pilot experiments designed to determine optimal enhancement times. Gold–silver particles in the 5 and 17 minute conditions yielded significantly different particle sizes that could be distinguished visually. (C). Distribution of particle sizes obtained in experiments represented in B above. Although there was some overlap in the particle sizes produced by different silver enhancement times, the vast majority fell into two distinct size ranges. Since most of the particles in the short incubation time were below 40 µm2 and most of the large particles were above 100 µm2, only particles lower or greater than these values were counted. These criteria minimized, but did not eliminate the possibility of selecting the low number of small particles produced in the long incubation time. Moreover, since there were only a minimal number of particles over 100 µm2 with the short incubation time, selecting only the largest particles insured that these came from the appropriate treatment.
Dual labeling EM immunocytochemical procedures for receptor protein labeling
To analyze the ultrastructural relationships between NMDA and mu-opioid receptors in the BNST, forebrain sections were processed for dual labeling of NR1 and μOR, with biotinylated and gold-conjugated antisera reversed in alternate sections. Tissue sections were incubated for 48 h in a primary antiserum cocktail including NR1 (peroxidase: 1:400; gold: 1:100) and μOR (peroxidase: 1:400; gold: 1:100), and processed for dual immunoperoxidase and immunogold labeling as described above in the procedures for dual labeling.
Electron Microscopy
For electron microscopic processing, the tissue was postfixed in 2% osmium tetroxide in PB for 1 h, and dehydrated in a series of alcohols, through propylene oxide, and flat embedded in EM BED 812 (EMS, Fort Washington, PA) between 2 sheets of Aclar plastic. Ultrathin sections (60–80 nm) from the surface of flat-embedded sections containing the CeA or BNST (Figs. 1 and 5, respectively) were cut with a diamond knife using an ultramicrotome (Ultratome, NOVA, LKB, Bromma, Sweden), and sections were collected on grids. Electron microscopic images of this tissue were obtained using a digital camera (Advanced Microscopy Techniques, Danvers, MA) interfaced with a transmission electron microscope (Technai 12 BioTwin, FEI, Hillsboro, OR). For preparation of figures, images were adjusted for contrast and brightness using Photoshop 11 software, and imported into PowerPoint X to add lettering.
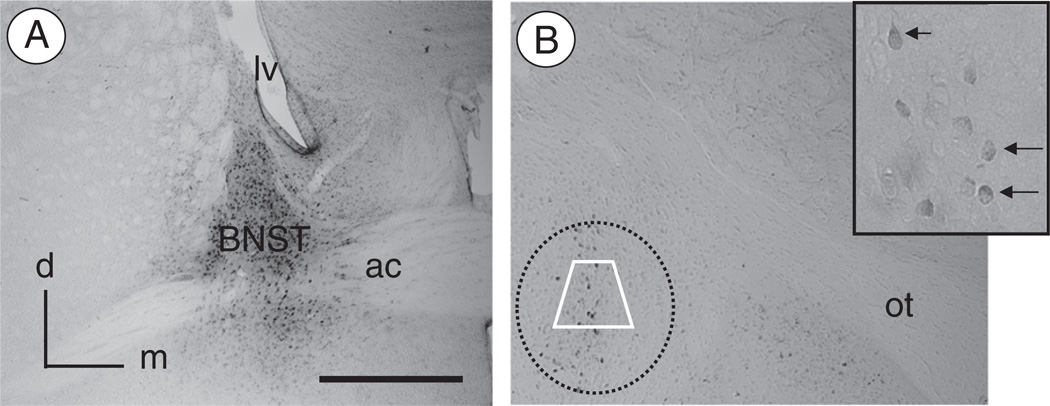
Fig. 1.
Injection of FG in the BNST results in retrograde labeling in CeA neurons(A). Light micrograph illustrating a representative example of FG immunolabeling in the BNST after local administration of the tracer. Immunoperoxidase reaction product for FG is present in the dorsal as well as ventral BNST surrounding the anterior commissure. There is little FG labeling in the regions adjacent to the BNST. (B). Retrogradely labeled neurons are present in the CeA. At high magnification (inset), immunoperoxidase reaction product for FG can be seen throughout the cytoplasm of neuronal cell bodies (arrows). Electron microscopic analysis of FG, NR1, and/or μOR labeling was performed in samples taken from the region of the CeA represented by the area bound by the trapezoid. ac: anterior commissure; d: dorsal; lv: lateral ventricle; m: medial; ot: optic tract. Scale Bar: 1 mm.
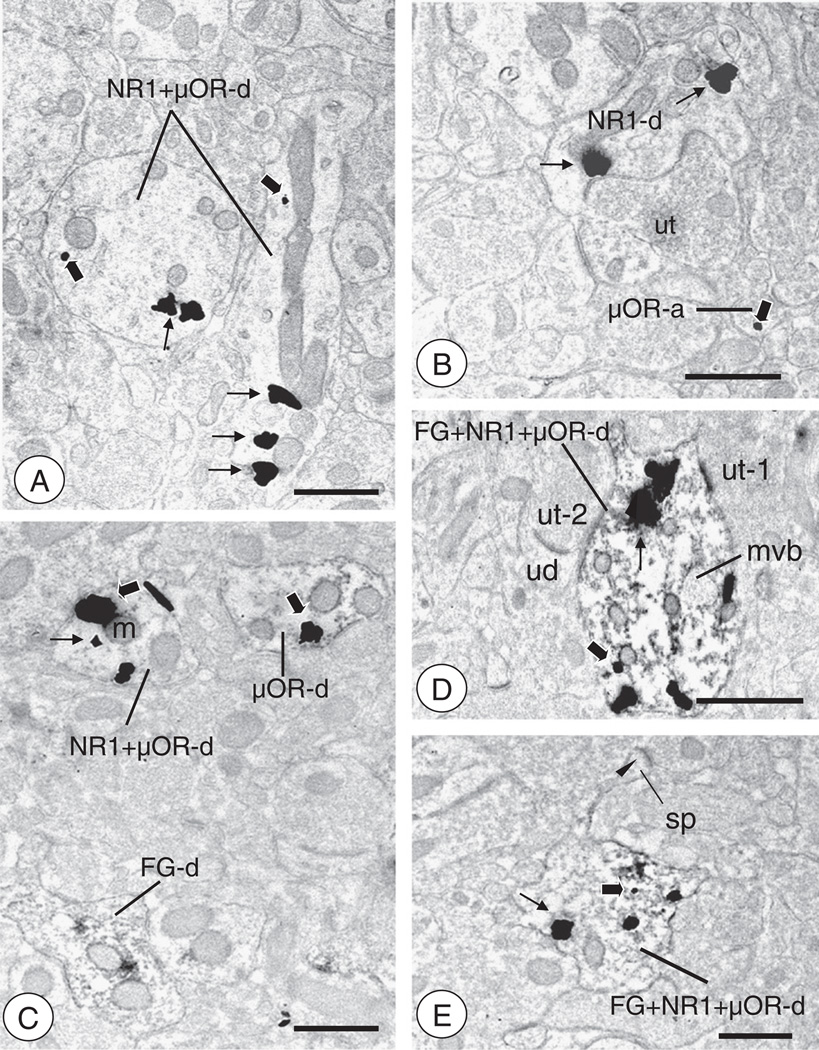
Fig. 5.
CeA-BNST projection neurons express NR1 and/or μOR(A). In tissue processed for triple labeling, some areas of the neuropil are populated by dendritic profiles expressing NR1 and/or μOR, but not FG. Two dendritic profiles (NR1 ± μOR-d) show labeling for NR1 (thin arrows, long enhancement time) and μOR (thick arrows, short enhancement time). (B). Regions of the CeA processed for triple labeling show neuronal profiles singly labeled for NR1 or μOR, but not FG. A dendritic profile (NR1-d) showing labeling for NR1 (thin arrow, long enhancement time) is contacted by an unlabeled axon terminal. A small presynaptic structure (μOR-a) shows a single particle for μOR (thick arrow, short enhancement time). (C). An area of the CeA processed for triple labeling contains single and dual labeled neuronal profiles. A single labeled dendritic profile (μOR-d) containing a gold–silver particle (thick arrow, long enhancement time) and an NR1 and μOR labeled dendritic profile (NR1 ± μOR-d) are present in the neuropil, which also contains a FG labeled dendrite (FG-d). (D). Triple labeled dendritic profile from a CeA neuron (FG ± NR1 ± μOR-d). Large gold particles for NR1 (thin arrows, long enhancement time) are present near the plasma membrane and clustered intracellularly. A small gold particle for μOR (thick arrow, short enhancement time) is present intracellularly. This profile contains a multivesicular body (mvb) and is contacted by unlabeled axon terminals (ut). (E). Triple labeled dendritic profile of a CeA neuron (FG ± NR1 ± μOR-d). A large silver–gold particle for NR1 (thin arrow, long incubation time) is present near the plasma membrane. A small silver–gold particle for μOR (large arrow, short incubation time) is present intracellularly beneath the shaft of the neck of a spiny process (sp) that receives an asymmetric synapse (arrow head). Scale Bars: 0.5 µm.
Ultrastructural analysis
In order to control for potential labeling artifacts due to penetration of cytological reagents, sampling was performed at the tissue surface as determined by proximity to the epon–tissue interface. This was achieved by collecting electron micrographs exclusively in the transition zone where one edge of the sampling area was in contact with epon in a field of at least three grid squares. Digital images were captured and analyzed to determine the number of single, dual, and triple labeled neuronal and glial profiles. The classification of labeled dendrites was based upon descriptions by Peters et al. (1991). Dendrites were identified by the presence of postsynaptic densities, as well as ribosomes and both rough and smooth endoplasmic reticulum. However, profiles were also considered dendritic whenever postsynaptic densities were observed, independent of endoplasmic reticulum. Somata were distinguished by the presence of a nucleus. Axon terminals were identified by size (at least 0.2 µm diameter) and the presence of synaptic vesicles. Astrocytes were identified by their irregular shape, the presence of filamentous membranes apposing dendrites or axons, or the presence of gap junctions. Synapses were defined as either symmetric or asymmetric, according to the presence of either thin or thick postsynaptic specializations, respectively. Appositions were distinguished by closely spaced plasma membranes that lacked recognizable specializations, or interposing astrocytic processes. Immunoperoxidase labeling is identified by a diffuse brown/black precipitate, while immunogold labeling is characterized by dense uniformly black granules. Both markers are readily distinguishable by visual inspection. With peroxidase and immunogold methods, non-specific labeling is usually along the membranes of damaged profiles. Background usually contributes 3% or less of silver-enhanced gold particles (Wang et al., 2003). Data were analyzed by one-or two-way factorial Analysis of Variance, and differences in means were analyzed by Fisher's Protected Least Significant Difference.
Antisera
Brain sections containing the CeA and BNST were processed for labeling of NR1 and μOR using monoclonal mouse (Penninsula, San Carlos, CA) and polyclonal guinea pig (Chemicon, Temecula, CA) antipeptide antisera, respectively. The specificity of these antisera has been described in recent publications (Glass et al., 2009; Jaferi and Pickel, 2009). A commercially available rabbit anti-FG antisera (Chemicon) was used to label the retrograde tracer (Glass et al., 2002). Previously characterized rabbit anti-GFP (Invitrogen, Carlsbad, CA), and rabbit anti-Fos (Santa Cruz, Santa Cruz CA) antisera were used to identify brain regions of viral mediated gene transfer and neural activation, respectively (Glass et al., 2004; South et al., 2003).
Viral vectors
The viral vector used in these studies to spatially and temporally delete the NR1 gene is a specially engineered recombinant adeno-associated virus (rAAV; ~4.7 kb) expressing Cre recombinase (Cre) and a GFP reporter, which will be referred to with the following abbreviation: rAAV-GFP-Cre. The vector also includes a CMV promoter/enhancer, a multiple cloning site for insertion of the GFP-Cre coding sequences, and poly A sequences (South et al., 2003). The control vector (rAVV-GFP) does not express Cre and thus does not result in gene knockout.
Tissue preparation for light microscopic immunohistochemistry and non-radioactive in situ hybridization
Mouse brains were prepared for light microscopy according to previously described methods (Glass et al., 2008). Briefly, mice were deeply anesthetized with sodium pentobarbital (150 mg/kg, i.p.) and their brains were fixed by aortic arch perfusion sequentially with: (a) 15 ml of normal saline (0.9%) containing 1000 units/ml of heparin and (b) 100 ml of 4% PFA in 0.1 M PB at a flow rate of 25 ml/min. The brains were removed and post-fixed overnight in 4% paraformaldehyde in PB, and then cryoprotected in 15% sucrose for 24 h, followed by 20% sucrose for another 1–3 days. Coronal forebrain sections 20 µm thick were cut with a cryostat. For immunoperoxidase labeling, brain sections were incubated for 24 h in rabbit anti-GFP (1:1000) or mouse anti-Fos antisera (1:1000) in 0.1% BSA. After incubation, sections were rinsed in 0.1 M TBS, incubated in anti-mouse or anti-rabbit IgG conjugated to biotin (1:400), rinsed in TBS, and then incubated for 30 min in ABC in TBS. The bound peroxidase was visualized by reaction for 8–12 min in 0.2% solution of DAB and 0.003% hydrogen peroxide in TBS. Following DAB incubation, sections were washed in 0.1% TBS, then 0.05 M PB, dehydrated through a series of alcohols and then coverslipped.
For in situ hybridization, NR1 gene expression was measured by use of a non-radioactive 2.2 kb antisense riboprobe whose DNA sequence was deleted by Cre-loxP recombination in fNR1 mice. Slide mounted cryostat-cut brain sections (20 µm) were incubated in 4% PFA (20 min), washed in 1× PBS, incubated in Proteinase K (2 min), and washed in 1× PBS. Sections were again incubated in 4% PFA (5 min), washed in 1× PBS, incubated in 0.25% acetic anhydride in 0.1 M Triethanolamine (10 min), and washed in 2× saline sodium citrate (SSC). Next, sections were incubated for 2 h in prehybridization solution (50% formamide, 0.3 M NaCl, 10 mM TBS, 1 mM EDTA, 500 µg/ml salmon sperm DNA, and 500 µg/ml yeast tRNA) at 65 °C in a chamber containing towels moistened with 4× SSC and 50% formamide. After incubation, the prehybridization solution was drained off the slide onto an RNAse free towel. Then, sections were hybridized with digoxigenin (DIG)-labeled antisense or sense probes for NR1 (1:1000) in hybridization solution (50% formamide, 0.3 M NaCl, 20 mM TBS, 5 mM EDTA, 10 mM sodium phosphate buffer, 10% dextran sulfate, 1× Denhardt's solution, and 500 µg/ml yeast tRNA), coverslipped, and placed overnight in an oven heated to 65 °C. After incubation, the coverslips were removed and the hybridized brain sections were washed in 5× SSC at 55 °C, followed by 2× SSC and 50% formamide at 65 °C (20 min). Next, sections were incubated in RNAse buffer at 37 °C, followed by 50 µg/ml RNAse A solution (30 min), then RNAse buffer. Sections were then washed sequentially in 2× SSC and 50% formamide at 65 °C (20 min), 2× SSC, washing buffer (10 min), and blocking solution (30 min). Sections were subsequently incubated in primary anti-DIG primary antisera conjugated to alkaline phosphatase (1:1000) for 1 h, followed by washing buffer. The sections were then incubated in detection buffer, followed by the chromogen NBT/BCIP (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate). After approximately 16 h, slides were rinsed in distilled water and dehydrated through a series of alcohols and xylene, then coverslipped in Permount.
Light microscopic cell counting
Cell counting was performed using relative optical density measurements via Microcomputer Imaging Device software (MCID, Imaging Research Inc., Ontario, Canada), as previously described (Glass et al., 2008). Briefly, mounted sections were viewed with a Nikon Microphot-FX microscope (Nikon, Garden City, NY) equipped with a digital CoolSNAP camera (Photometrics, Huntington Beach, CA). The light microscopic images were acquired through an interface between the camera and a Macintosh computer. Pixel intensity thresholding procedures were performed as per manufacturer's guidelines. Electronic images were imported into MCID, which automatically calculates a relative threshold level for each image, then adjusted using an object enhancement filter that maximizes the contrast between large objects and background. Cell counts were made in three to five rostrocaudal bilateral sections containing brain regions of interest, averaged, and analyzed by ANOVA. Cell counts were also performed manually to verify the consistency of automated tallies. For Fos activation, treatment effects were determined by comparing the number of Fos labeled cells in NR1 deleted and control groups, calculated as the ratio of the number of Fos labeled cells in the injected hemisphere (i.e. ipsilateral) to the uninjected hemisphere (i.e. contralateral).
Morphine injections
In order to habituate mice to the injection procedure and reduce non-specific stress-induced Fos labeling, mice were handled and given saline injections for five days prior to their test injection. Morphine sulfate (10 mg/kg; NIDA) was prepared in 0.9% saline solution and given intraperitoneally on the test day. Control animals received an injection of saline. Two hours following injections, animals from each group were sacrificed and transcardially perfused in preparation for immunohistochemistry and in situ hybridization.
Statistical analyses
All data were presented as mean ± SEM. Data were analyzed by t-tests, or one-or two-way factorial ANOVA where applicable. For analysis of the data presented as a ratio, the data were converted by arcsine prior to analysis to allow for comparison of proportions. Differences in means were analyzed by Fisher's PLSD. The criteria for significance were set at p < 0.05.
Results
Central amygdala neurons projecting to the BNST express somatodendritic NR1 and/or μOR
Microinjection of the tracer FG in the BNST (Fig. 1A) resulted in retrogradely labeled neurons in the CeA (Fig. 1B). As seen by light microscopy, neurons labeled for FG were present throughout the extent of the CeA, but were preferentially found in the lateral CeA at intermediate and caudal levels as previously described in the rat (Roder and Ciriello, 1993).
In forebrain tissue sections processed for EM analysis of dual FG and NR1 labeling, a total of 515 labeled somata or dendritic profiles were counted (Table 1A) in an approximately 18,386 µm2 tissue sample. These profiles included those labeled for FG, NR1, or both. Of all labeled somata and dendritic profiles, approximately 37% expressed labeling for FG, 38% for NR1, and 25% were dually labeled. Central amygdala somata labeled for FG and NR1 were similar to those previously described as expressing NR1 labeling (Glass et al., 2009). These cell bodies were medium in size (Fig. 2A), expressed small or large nuclei, some with prominent invaginations of the nuclear membrane, indicative of active gene expression (Chan et al., 2000). Immunoperoxidase labeling was diffusely expressed throughout the cytoplasma, but was also frequently associated with large endosomal and lysosomal organelles (Wessendorf, 1991). Immunogold-silver labeling for NR1 was seen throughout the cytoplasma, particularly near small round and tubulovesicular structures. In addition to somata, dual labeled dendritic profiles were also observed. These included large or intermediate-size dendritic profiles with numerous mitochondria and vesicular organelles (Fig. 2B).Many smaller distal dendrites showed diffuse immunoperoxidase labeling for FG. Labeling for FG was seen in the shaft of small caliber dendritic processes with or without spiny appendages. Immunoreactivity for NR1 was observed in these small dendritic profiles (Fig. 2C), where immunogold particles were affiliated with intracellular vesicular structures or the plasma membrane. These dually labeled dendrites were apposed by axon terminals that made non-synaptic contacts or formed postsynaptic synapses that were typically asymmetric excitatory-type specializations.
Table 1.
| A FG, NR1, and FG + NR1 labeled somata and dendrites as a percentage of all labeled somata and dendrites in the CeA. Raw values in parentheses. | ||
|---|---|---|
| Profile | Somata | Dendrites |
| FG | 13% (17) | 45% (171) |
| NR1 | 20% (27) | 44% (168) |
| FG + NR1 | 67% (89) | 11% (43) |
| Total | 133 | 382 |
| B FG, μOR, and FG + μOR labeled somata and dendrites as a percentage of all labeled somata and dendrites in the CeA. Raw values in parentheses. | ||
|---|---|---|
| Profile | Somata | Dendrites |
| FG | 8% (6) | 42% (186) |
| μOR | 23% (18) | 39% (174) |
| FG + μOR | 69% (54) | 18% (81) |
| Total | 78 | 441 |
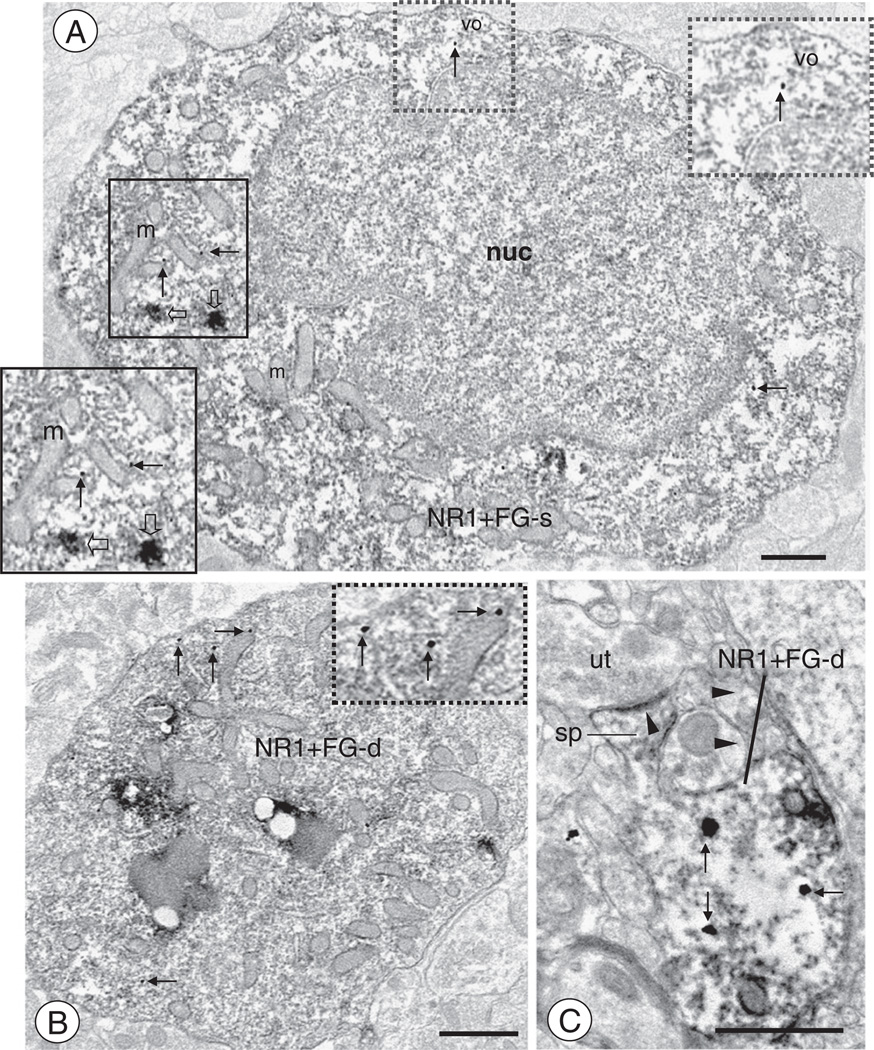
Fig. 2.
CeA neurons retrogradely labeled after FG administration in the BNST express NR1(A). A soma (NR1+FG-s) expressing diffuse immunoperoxidase reaction product for FG also shows discrete labeling for NR1 (arrows). This cell body contains a large irregularly shaped nucleus (nuc), as well as numerous tubulovesicular organelles characteristic of smooth endoplasmic reticula (vo) and mitochondria (m). Large aggregates of immunoperoxidase reaction product for FG (open arrows) are seen in the cytoplasma. Gold-silver enhanced particles for NR1 (thin arrows) are seen near vesicular organelles, including those associated with mitochondria. Instances of aggregated FG immunoreactivity or NR1 labeling are magnified in the insets. (B). A large dendritic profile (NR1 + FG-d) shows dual immunoreactivity for FG and NR1. Immunoreaction product for FG is widely diffused throughout the intracellular compartment. Immunogold-silver enhanced particles are present near vesicular organelles. (C). A FG labeled dendritic profile also shows immunoreactivity for NR1 (NR1 + FG-d). Immunogold particles for NR1 (thin arrows) are present in intracellular sites of the dendritic shaft, beneath the neck and head of a dendritic spine (sp) that receives an asymmetric synapse (arrow head) from an unlabeled axon terminal (ut). Scale bars: 1 µm (A–B), 0.5 µm (C).
To determine the relationship between opioid receptors and CeA-BNST projection neurons, forebrain tissue sections containing the CeA were co-processed for dual immunoperoxidase and immunogold-silver labeling of FG and μOR. From this tissue, a total of 519 somatodendritic profiles were counted in an approximately 16,068 µm2 sample (Table 1B). Of all labeled somata and dendrites, approximately 37% showed exclusive labeling for FG, 37% for μOR, and 26% were labeled for both. Many central amygdala neurons projecting to the BNST expressed immunoreactivity for μOR (Fig. 3A). In dually labeled somata, immunogold-silver deposits for μOR were present throughout the cytoplasm, particularly near small tubulovesicular organelles. Immunolabeling for μOR was also seen in large proximal and small dendritic profiles also labeled for the retrograde tracer (Figs. 3B). Immunolabeling for μOR was found in intracellular membranous organelles, as well as non-synaptic areas of the plasmalemma.
Fig. 3.
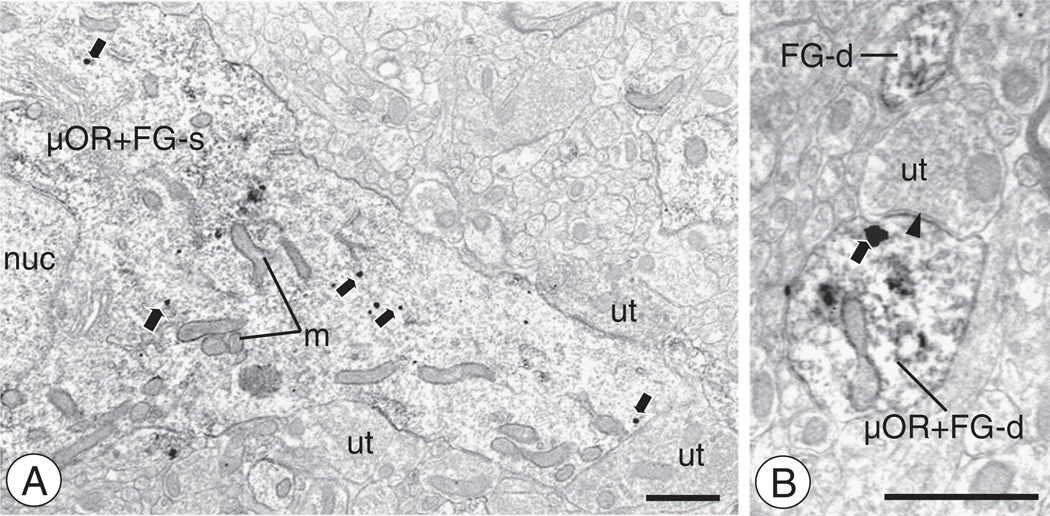
CeA neurons retrogradely labeled after FG administration in the BNST express μOR(A). A somatodendritic profile (μOR + FG-s) is labeled for FG and μOR. A nucleus (nuc) is seen in the cell body, along with multiple mitochondria (m) that also extend into the proximal dendrite. Multiple immunogold-silver particles for NR1 (arrows) are present in intracellular areas of the proximal dendritic process. This profile is also contacted by unlabeled axon terminals (ut).(B). A small dendritic profile (μOR + FG-d) expresses labeling for μOR and FG. A gold–silver particle (arrow) is present near the plasma membrane beneath the post-synaptic density of an asymmetric type synaptic junction (filled arrow head) formed with an unlabeled axon terminal (ut). Scale bars: 1 µm (A), 0.5 µm (B).
To determine if CeA-BNST projection neurons expressed both NMDA and opioid receptors, tissue was processed for triple labeling of FG, NR1, and μOR. Peroxidase was used to label FG, and NR1 and μOR were identified by the gold–silver method whereby each antisera was distinguished by distinct gold–silver particle incubation times (Paspalas and Goldman-Rakic, 2004) that yielded significantly different particle size distributions (Figs. 4A–C). Approximately 90% of particles had areas below 40 µm2 in the 5-minute enhancement group, and 90% over 100 µm2 in the 17-minute enhancement group. Only particles in the respective lower and upper particle size ranges were counted to eliminate most of the particles in the overlapping portions of each distribution. In CeA sections processed for triple labeling of FG, NR1, and μOR, profiles labeled for one, two, or all of the antigens were observed. Numerous dendritic profiles showing dual labeling for NR1 and μOR were seen in the CeA (Fig. 5A). These were frequently small to intermediate in size, where labeling for each protein was affiliated with non-overlapping intracellular compartments. Dendritic profiles showing exclusive labeling for NR1, μOR, or FG were also observed (Figs. 5B–C). Dendritic profiles expressing FG were also shown to express labeling for NR1 and μOR, typically in non-overlapping areas of the cytoplasm (Figs. 5D–E). Some of these triple labeled dendrites exhibited spiny protrusions that received asymmetric synapses (Fig. 5E). In control experiments, tissue was processed for triple labeling with omission of one, two, or all of the primary antisera. As expected, eliminating the primary antisera resulted in the absence labeling for the respective antigen (Figs. 6A–B). A semi-quantitative analysis of 10,506–16,763 µm2 of sampled CeA tissue processed by intensifying NR1 for 5 min/μOR 17 min, or the reverse (NR1 for 17 min/μOR 5 min), showed that 6–10% of labeled somata and dendrites were labeled for the retrograde transporter, NR1, and μOR (Table 2).
Fig. 6.
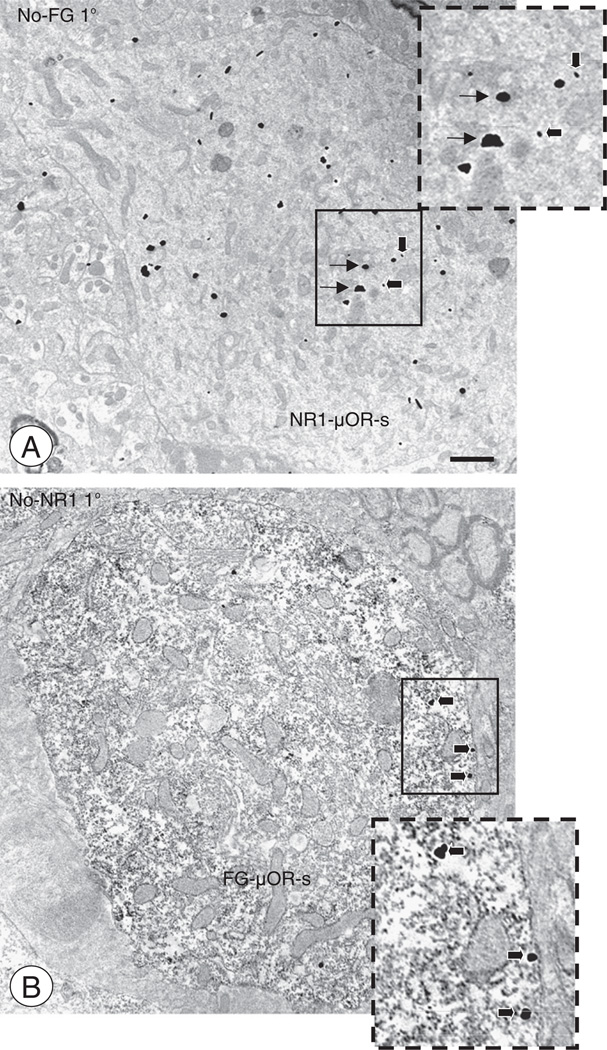
Omission of primary antisera in triple labeling experiments(A). A soma from the CeA is co-labeled for NR1 and μOR (NR1 ± μOR-s). This profile was observed in a control experiment where tissue was processed for triple labeling of NR1, μOR, and FG with omission of the FG primary antisera (No-FG 1°). Despite the lack of immunoperoxidase labeling, large and small immunogold-silver particles for NR1 (thin arrows, long incubation time) and μOR (thick arrows, short incubation time) are visually distinguishable (inset). (B). A cell body from the CeA shows co-labeling for FG and μOR (FG ± μOR-s). This profile was observed when sampling from tissue processed for a control experiment, where sections were prepared for triple labeling of NR1, μOR, and FG with omission of the NR1 primary antisera (No-NR 1°). Diffuse immunoperoxidase FG labeling and large particles for μOR (thick arrows, long incubation time) are seen in this soma (inset). Scale Bar: 1 µm.
Table 2.
Single, dual, and triple labeled profiles as a percentage of all labeled profiles in the CeA. Raw values in parentheses.
| Profile | NR1 (5 min)/μOR (17 min) | NR1 (17 min)/μOR (5 min) |
|---|---|---|
| FG | 10% (36) | 4% (26) |
| NR1 | 27% (96) | 45% (317) |
| μOR | 28% (101) | 20% (140) |
| FG + NR1 | 3% (12) | 3% (24) |
| FG + μOR | 6% (22) | 2% (13) |
| NR1 + μOR | 15% (54) | 19% (132) |
| FG + NR1 + μOR | 10% (35) | 6% (45) |
| Total | 356 | 697 |
Axons and axon terminals of CeA-BNST projection neurons are largely devoid of NR1 or μOR labeling
Microinjection of BDA in the CeA (Fig. 7A) resulted in anterogradely labeled processes in the BNST (Fig. 7B). By light microscopy, anterogradely labeled processes and boutons were present throughout the BNST in dorsal (dBNST) and ventral (vBNST) regions. Tissue sections containing the BNST were processed for dual immunocytochemical detection of BDA and NR1. By electron microscopy, presynaptic structures showing immunoperoxidase reaction product were present throughout the neuropil of the BNST. These included large myelinated axons, but small unmyelinated axons more commonly contained peroxidase reaction product (not shown). In addition, there were numerous instances of BDA labeled axon terminals. These axon terminals were typically small in size, contained numerous small synaptic vesicles, and, in some cases one or more large dense core vesicle. Anterogradely labeled axon boutons contacted somata (Fig. 7C), but more frequently apposed dendritic profiles (Figs. 7D–E). These dendritic profiles were usually small to intermediate in size. Anterogradely labeled axon terminals typically made non-synaptic appositions with unlabeled axonal or dendritic profiles. When synaptic contacts were made with the latter, these were most commonly symmetric inhibitory-type specializations (Figs. 7D–E). In addition to somatodendritic contacts, BDA labeled axon terminals were also apposed to unlabeled small unmyelinated axons and axon terminals (Figs. 7D–E).
Fig. 7.
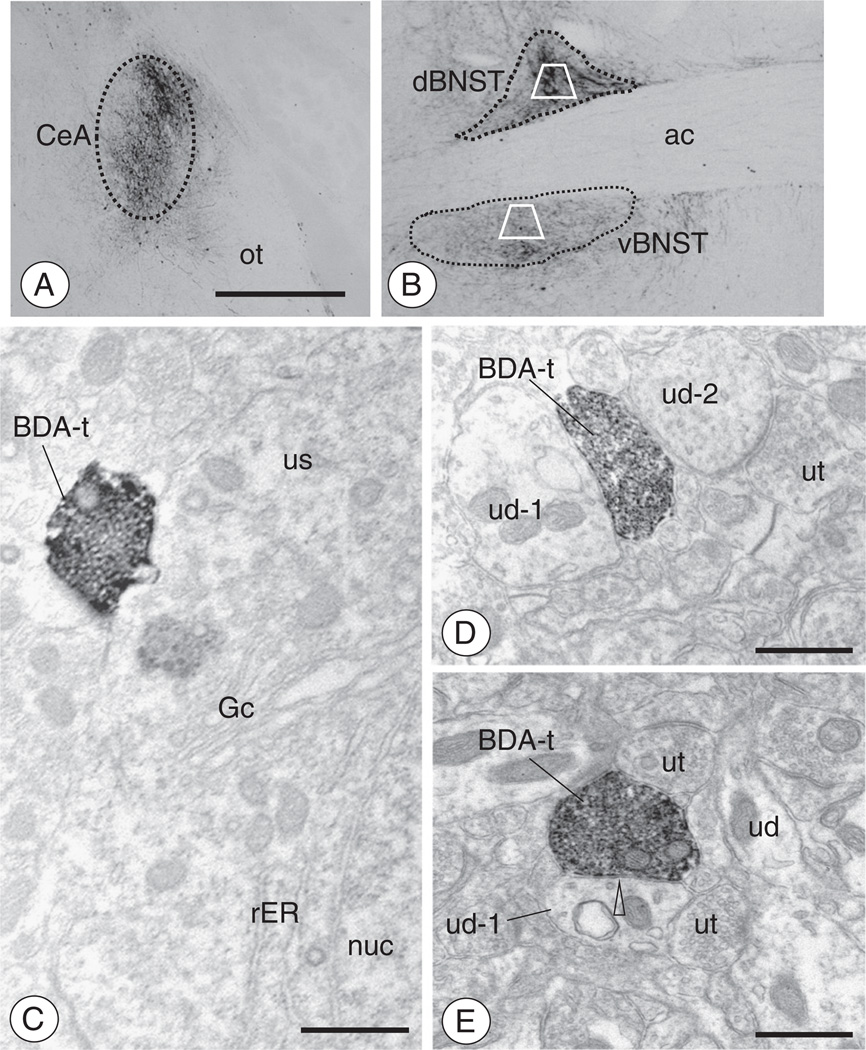
Administration of BDA in the CeA results in anterogradely labeled axonal processes and boutons in the BNST(A–B). As shown by light microscopy, administration of BDA into the CeA resulted in anterograde transport in the BNST. Fibers and punctate granules resembling boutons were present throughout the ventral and dorsal BNST. Areas bound by trapezoids represent regions of the dorsal and ventral BNST sampled by electron microscopy. (C). Electron micrograph showing a BDA labeled axon terminal (BDA-t) contacting the cell body of a dBNST neuron (us). Both Golgi (Gc) and rough endoplasmic reticula (rER) are seen near the nucleus (nuc) of this unlabeled soma. (D). An anterogradely labeled axon terminal (BDA-t) contacts a dendritic profile (ud-1). An unlabeled axon terminal (ut) and dendritic profile (ud-2) are also seen in the adjacent neuropil. (E). A BDA labeled axon terminal (BDA-t) is present in the neuropil with unlabeled dendrites (ud) and axon terminals (ut). The BDA labeled terminal forms an apparent symmetric inhibitory-type type synapse (open arrow head) with one of the dendritic profiles (ud-1). Scale Bars: (Top) 1 mm; (Bottom) 0.5 µm.
Axon terminals showing BDA staining rarely expressed labeling for NR1, however these axons were often present in the neuropil containing NR1 labeled somatodendritic profiles. Sometimes these BDA labeled axon terminals were located in proximity to NR1 labeled dendritic profiles with a single intervening glial (Fig. 8A) or neuronal process. In addition, BDA labeled axon terminals also formed non-synaptic appositions, or direct symmetric inhibitory-type synapses (Fig. 8B) with NR1 labeled dendritic processes. These dendritic profiles were small to intermediate in size and showed immunogold labeling for NR1 in intracellular and surface sites.
Fig. 8.
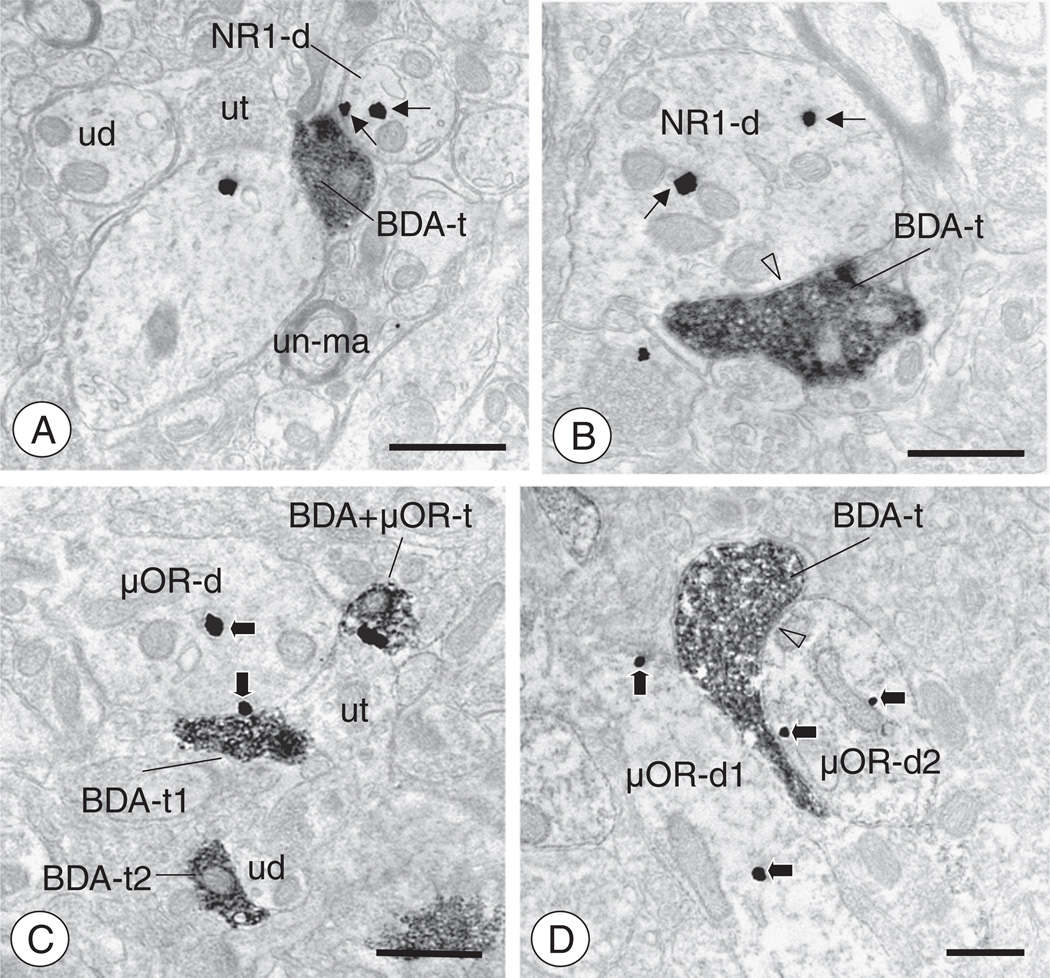
Axon terminals of CeA-BNST projection neurons contact neuronal profiles expressing NR1 or μOR(A). A BDA labeled axon terminal (BDA-t) is present in the dBNST neuropil, which also contains unlabeled dendritic (ud), unmyelinated axonal (un-ma), and axon terminal (ut) profiles. In addition, a dendrite expressing NR1 labeling (NR1-d) is present in close proximity to the anterogradely labeled axon terminal. This dendritic profiles expresses immunogold labeling for NR1 (thin arrows) in intracellular and surface sites. (B). An anterogradely labeled axon terminal (BDA-t) forms a symmetric inhibitory-type synapse (open arrow head) with an NR1 containing dendritic profile (NR1-d) in the dBNST. Immunogold particles for NR1 (thin arrows) are present in intracellular sites of this dendritic profile. (C). In the vBNST, small BDA labeled axon terminals are present in the neuropil with unlabeled or μOR labeled neuronal profiles. A μOR labeled dendritic profile (μOR-d) is contacted by axon terminal profiles expressing BDA (BDA-t1) or BDA and μOR (BDA + μOR-t), as well as a profile devoid of labeling (ut). Immunogold labeling for μOR (thick arrows) is found on the plasma membrane directly adjacent to the BDA labeled terminal, or present near intracellular membranous structures. Also present in the neuropil, a small BDA labeled axon terminal (BDA-t2) contacts an unlabeled (ud) dendritic profile. (D). In the vBNST, a BDA labeled axon terminal profile (BDA-t) contacts two distinct μOR labeled dendritic profiles (μOR-d). It forms a non-synaptic apposition with one dendrite (μOR-d1) and also forms a symmetric inhibitory-type synapse (open arrow head) with the other (μOR-d2). Immunogold labeling for μOR (thick arrows) is present in intracellular sites in both dendrites. Scale Bars: 0.5 µm.
From 9270 µm2 of tissue sampled from the vBNST, a total of 196 single or dual labeled axons were counted. Among these, 67% showed labeling exclusively for BDA, 33% for NR1, and <1% were dually labeled (Table 3A). In the 10,837 µm2 of tissue sampled from the dBNST, a total of 219 BDA labeled presynaptic structures were counted. In this sample, 76% were labeled exclusively for BDA, 22% for NR1, while 2% expressed labeling for BDA and NR1. A total of 29 synapses were made by BDA labeled axon terminals in the ventral and dorsal BNST, all of which were symmetric inhibitory-type specializations.
Table 3.
| A BDA, NR1, and BDA + NR1 labeled axons as a percentage of all labeled axons in the ventral and dorsal BNST. Raw values in parentheses. Number of appositions between BDA labeled axons and NR1 containing profiles are presented below. | ||
|---|---|---|
| Profile | vBNST | dBNST |
| BDA axons | 67% (131) | 76% (166) |
| NR1 axons | 33% (64) | 22% (49) |
| BDA + NR1 axons | <1% (1) | 2% (4) |
| Total labeled axons | 196 | 219 |
| BDA axons contacting NR1 profiles | 8 | 10 |
| B BDA, μOR, and BDA + μOR labeled axons as a percentage of all labeled axons in the ventral and dorsal BNST. Raw values in parentheses. Number of appositions between BDA labeled axons and μOR containing profiles are presented below. | ||
|---|---|---|
| Profile | vBNST | dBNST |
| BDA axons | 71% (158) | 71% (135) |
| μOR axons | 27% (61) | 25% (47) |
| BDA + μOR axons | 1% (3) | 4% (9) |
| Total labeled axons | 222 | 191 |
| BDA axons contacting μOR profiles | 7 | 9 |
In forebrain tissue containing the BNST processed for dual labeling for BDA and μOR, there were few instances of dual labeled presynaptic structures. However, axons and axon terminals of CeA-BNST projection neurons were located in the vicinity of μOR labeled profiles. These BDA labeled axon terminals were small to intermediate in size and formed non-synaptic appositions (Fig. 8C), or symmetric inhibitory-type synapses (Fig. 8D) with μOR labeled profiles. Immunogold μOR labeling in these dendrites was affiliated with intracellular vesicular organelles, as well as the plasma membrane.
In 8652 µm2 of tissue sampled from the vBNST, 222 single or dual labeled axonal profiles were counted. Of these, 71% were labeled exclusively for BDA, 27% for μOR, and 1% were dually labeled (Table 3B). In 10,837 µm2 of tissue sampled from the dBNST, 191 labeled presynaptic structures were counted, and approximately 71% were exclusively labeled for BDA, 25% for μOR, and 4% were labeled for BDA and μOR. A total of 11 synapses were made by BDA labeled axon terminals in the ventral and dorsal BNST, all of which were symmetric inhibitory-type specializations.
Despite the low levels of NR1 or μOR in axon terminals of CeA-BNST projection neurons, there were numerous instances of neuronal profiles labeled for these proteins in the BNST. These profiles, included somata, as well as proximal and distal dendrites, which were sometimes contacted by anterogradely labeled presynaptic structures (Figs. 8C–D). In all, 311 NR1 labeled profiles were counted in the vBNST, of which 4% were somata, 72% dendritic, and 20% were axons or axon terminals. Approximately 3% of NR1 labeled profiles (n = 8) were contacted by BDA labeled presynaptic structures, the majority (80%) of which were dendrites. In the dBNST, a total of 308 NR1 labeled profiles were counted, of which 8% were somata, 75% dendritic, and 16% axonal. Approximately 3% of NR1 labeled profiles (n = 10) were contacted by BDA labeled axons or axon terminals.
Anterogradely labeled axons also apposed neuronal profiles expressing μOR in the vBNST. A total of 179 μOR labeled neuronal profiles were counted, of which 2% were somata, 62% were dendrites, and 34% were axons or axon terminals. Approximately 4% (n = 7) of μOR labeled profiles, all of which were dendrites, were contacted by BDA labeled axons or axon terminals. A total of 226 μOR labeled neuronal profiles were counted in the dBNST, of which 5% were somata, 70% were dendrites, and 21% were axons or axon terminals. Approximately 4% (n = 9) of μOR labeled profiles, all of which were dendrites, were contacted by BDA labeled axons or axon terminals.
To determine the ultrastructural distribution of NR1 and μOR in the BNST, forebrain tissue sections were processed for dual labeling of these proteins. A mixed population of neuronal profiles was found, including those labeled exclusively for NR1 or μOR, as well as immunoreactive for both antigens (Table 4). In 7225–10,838 µm2 of sampled vBNST tissue processed for immunoperoxidase detection of NR1 and gold–silver detection of μOR, and with markers reversed, 15–18% of all labeled profiles were somata or dendrites co-labeled for NR1 and μOR. In 9176–10,765 µm2 of sampled dBNST tissue processed for immunoperoxidase detection of NR1 and gold–silver detection of μOR, and with markers reversed, 12–16% of all labeled profiles were NR1 and μOR co-labeled cell bodies and dendrites.
Table 4.
Distribution of NR1, μOR, and NR1 + μOR labeled profiles in the ventral and dorsal BNST. Raw values in parentheses.
| vBNST | dBNST | |||
|---|---|---|---|---|
| NR1 (gold) | NR1 (ABC) | NR1 (gold) | NR1 (ABC) | |
| μOR (ABC) | μOR (gold) | μOR (ABC) | μOR(gold) | |
| NR-1 somata | 3% (16) | 2% (7) | 1% (6) | <1% (4) |
| NR1 dendrites | 34% (168) | 34% (100) | 36% (158) | 48% (225) |
| NR1 axons | 7% (38) | 7% (22) | 3% (13) | 9% (43) |
| NR1 glia | <1% (3) | <1% (2) | 0 | 2% (13) |
| μOR somata | 1% (7) | 2% (5) | 1% (6) | 2% (11) |
| μOR dendrites | 27% (134) | 27% (79) | 40% (177) | 15% (71) |
| μOR axons | 7% (35) | 7% (22) | 5% (23) | 6% (28) |
| μOR glia | 3% (15) | 2% (7) | 1% (7) | 0 |
| NR1 + μOR somata | 10% (47) | 15% (43) | 4% (16) | 3% (14) |
| NR1 + μOR dendrites | 5% (26) | 3% (9) | 8% (36) | 13% (61) |
| NR1 + μOR axons | <1% (4) | 0 | 0 | <2% (1) |
| Total | 493 | 296 | 442 | 471 |
Deleting the NR1 gene in CeA neurons is associated with a decrease in morphine-induced neural activation in the BNST
Mice unilaterally microinjected with rAAV-GFP-Cre or rAAV-GFP were acutely administered saline or morphine, and after 2 h they were sacrificed and transcardially perfused in preparation for immunohistochemistry. As a marker of neural activation, Fos expression was measured in several forebrain sites known to play a role in opioid addiction and directly or indirectly connected with the CeA. These areas included the vBNST, dBNST, nucleus accumbens core and shell (Acbc, Acbs), basolateral amygdala (BLA), central amygdala, and the paraventricular nucleus of the hypothalamus (PVN).
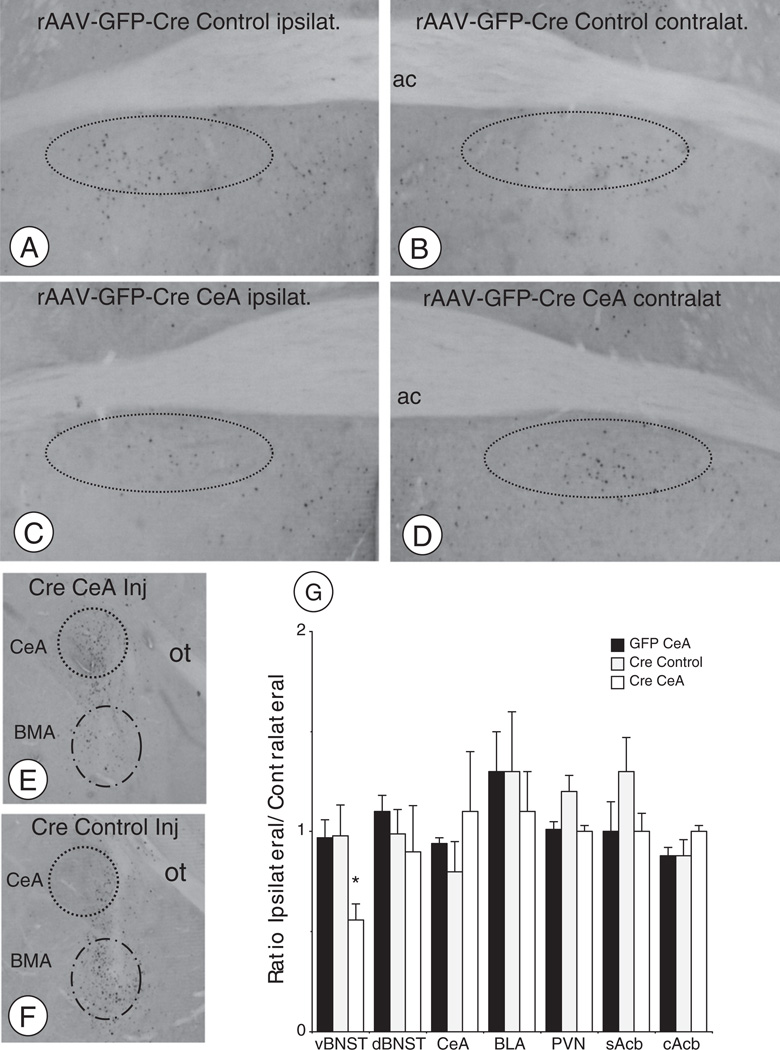
In the vBNST, collapsed across all treatment groups, there was a significant difference in the number of Fos labeled cells between saline and morphine treated groups (9.4 ± 1.2 versus 30.1 ± 4.6, respectively; F(1,38) = 12.2, p < .001). In response to an injection of morphine, mice receiving unilateral rAAV-GFP-Cre in the CeA showed a significant reduction in vBNST activation, calculated as the ratio of ipsilateral/contralateral Fos expression [F(2,9) = 6.2, p < 0.05], when compared to mice receiving rAAV-GFP-Cre injections in a control area near the basomedial amygdala nucleus, or injected with rAAV-GFP in the CeA (Figs. 9A–D). Unilateral microinjection of rAAV-GFP-Cre in the CeA resulted in a local expression of GFP (Figs. 9E–F), and a decrease in the number of cells expressing NR1 mRNA. Expressed as the percent ratio of NR1 mRNA expressing cells in the injected and non-injected hemispheres, there was a greater than 50% reduction in NR1 gene expression in the animals injected with rAAV-GFP-Cre in the CeA versus a ventral control site or receiving rAAV-GFP in the CeA [57 ± 5% versus 99 ± 3% and 104 ± 2%; F(2,17) = 58.1, p < .0001].
Fig. 9.
Deletion of NR1 in CeA neurons results in a decrease in acute morphine-induced Fos expression in the vBNST(A–D). Light micrographs illustrating examples of Fos labeling in the vBNST in morphine administered mice. Area bound by dashed ovals indicates Fos labeling in the vBNST ipsilateral (A) and contralateral (B) to the rAAV-GFP-Cre injection in an area ventral to the CeA (i.e. basomedial amygdala control injection). Expression of Fos is shown in the vBNST ipsilateral (C) and contralateral (D) to the rAAV-GFP-Cre injection in the CeA. (E–F). Expression of GFP in the CeA (E) and BMA (F) after rAAV-GFP-Cre injections. (G). There was a significant reduction in the ratio of ipsilateral/contralateral Fos expression in the vBNST of morphine injected animals receiving rAAV-GFP-Cre in the CeA (Cre-CeA) compared to animals receiving this vector in an area ventral to the CeA (Cre Control), or receiving the rAAV-GFP (GFP CeA) virus in the CeA. Differences were not seen in other brain areas. *p < .05.
Collapsed across all treatments, there were significant differences in the number of Fos labeled cells between saline and morphine treated animals in the dBNST (7.8 ± 1.1 versus 21.9 ± 4.9; F(1,38) = 5.2, p < .05), cAcb (14.6 ± 3.8 versus 30.8 ± 3.3; F(1,38) = 9.9, p < .005), sAcb (16.4 ± 3.8 versus 66.9 ± 7.4; F(1,38) = 27.3, p < .0001), CeA (5 ± 1.9 versus 41.3±6.2; F(1,38) = 23.6, p < .0001), BLA (13.6 ± 4.8 versus 43.9 ± 7.2; F(1,36) = 10.5, p < .005), and the PVN (22.4 ± 7.1 versus 63.3 ± 7.7; F(1,38) = 13.6, p < .001). However, there were no significant reductions in the ratio of ipsilateral/contralateral Fos labeling in the dBNST [F(2,9) = .5, p > .6], cAcb [F(2,9) = .4, p > .6], sAcb [F(2,9) = .1.9, p > .2], CeA [F(2,9) = .3, p > .7], BLA [F(2,9) = .2, p > .8], or PVN [F(2,9) = .2, p > .1] in response to an acute injection of morphine relative to mice receiving rAAV-GFP-Cre in the CeA or control site, or given rAAV-GFP in the CeA (Fig. 9G).
In similarly microinjected mice treated with saline, there were no significant differences in the ratio of ipsilateral/contralateral Fos activation in the vBNST [F(2,9) = 4, p > .05], dBNST [F(2,9) = .5, p > .6], cAcb [F(2,9) = .2, p > .8], sAcb [F(2,9) = .5, p > .6], CeA [F(2,9) = .2, p > .8], BLA [F(2,9) = .01, p > .9], or PVN [F(2,9) = .2, p > .8].
Discussion
A combination of immunocytochemical EM, tract tracing, and spatial–temporal gene deletion technology was used to clarify the synaptic organization of the NMDA receptor and the mu-opioid receptor in the pathway between the CeA and the BNST. It was shown that CeA-BNST projection neurons expressed NR1 in their somata and dendritic processes but had low levels in their axonal terminations in the BNST. A similar pattern was also observed for μOR. Using a triple labeling approach, it was found that a population of FG labeled neurons contained both NR1 and μOR. Moreover, expression of the NR1 subunit gene in CeA neurons was required for the morphine-induced production of Fos protein in the BNST. These results indicate that the NMDA receptor and μOR are positioned for the postsynaptic (i.e. somatodendritic) modulation of the CeA-BNST pathway.
Methodological considerations
The tracer FG was used to identify somata and dendritic processes of CeA neurons that project to the BNST. It has been reported that FG displays lower levels of transport to distal dendritic compartments, resulting in lower labeling in smaller processes. However, this potential drawback may be mainly restricted to conditions involving visualization of the tracer through the use of fluorescence microscopy without the use of antibodies. It is less of a concern when FG is labeled with antisera and examined by EM as previously reported (Naumann et al., 2000; Van Bockstaele et al., 1994), and as demonstrated in the present study where FG labeling was readily observed in large and small caliber dendritic profiles.
The triple labeling study was based on an approach that utilized differing silver-enhancement times in tissue labeled with two distinct gold-conjugated secondary antisera (Paspalas and Goldman-Rakic, 2004). Using this strategy, it was possible to obtain two distinct distributions of particle sizes for each silver enhancement time. However, these distributions did overlap at the upper and lower 10% of each respective particle size range. Therefore, particles whose sizes fell within the upper and lower bounds of the short and long enhancement times, respectively, were excluded in order to minimize the number of false positives from the analysis. By eliminating particles from this zone of uncertainty, it was also likely that some valid labeling was excluded (i.e. false negatives). However, since this criterion was applied equally to both antigens and incubation times, a relative, semi-quantitative approximation of the degree of triple labeling was obtained, even if ascertaining absolute values was precluded.
The essential NR1 gene was unilaterally deleted in CeA neurons by local microinjection of a neurotropic rAAV expressing a fusion protein of Cre and a GFP reporter (Glass et al., 2008; South et al., 2003). This approach has been shown to selectively inhibit NMDA receptor-mediated currents (South et al., 2003) as well as pain (South et al., 2003) and addiction related behaviors (Glass et al., 2008), with limited pathogenic/immune reactions (Kaspar et al., 2002) or neuronal loss (Glass et al., 2008; South et al., 2003). By specifically deleting the essential NR1 subunit, this approach provides the ability to selectively target postsynaptic NMDA receptors without the potential for neurobehavioral complications associated with NMDA receptor antagonists (Javitt, 2007), particularly when administered into the CeA (Andrzejewski et al., 2004).
Somata and dendrites of CeA-BNST projection neurons express NR1 and μOR
Injection of FG in the mouse BNST resulted in retrograde labeling in the CeA, a finding in agreement with prior light microscopic studies in rat conducted with several different tracers (Cassell et al., 1986; Roder and Ciriello, 1993; Weller and Smith, 1982). By immunocytochemical EM, it was found that CeA neurons labeled with FG and NR1 were small to medium in size, expressed spherical, as well as, invaginated nuclei and also expressed numerous endomembranous organelles. These morphological and cytological features have been previously described for CeA projection neurons to the brain stem (Glass et al., 2002).
Dendritic profiles also showed labeling for FG and NR1. In these profiles, NR1 was affiliated with tubulovesicular organelles, in addition to presumably functional sites on the plasmalemma. These profiles varied in size, from large proximal processes to small distal structures with or without spines. They were contacted by axon terminals making non-synaptic appositions or synaptic specializations, which tended to be asymmetric excitatory-type junctions. These properties have also been reported for glutamate (Beckerman and Glass, 2011; Glass et al., 2009), catecholamine (Glass et al., 2002), and peptide receptors (Glass et al., 2009; Jaferi and Pickel, 2009) in the CeA.
Neurons labeled after FG administration in the BNST were typically present along the edge of the medial subregion and throughout the lateral aspects of the CeA, an area previously shown to contain a high density of peptide neuromodulators (Cassell and Gray, 1989), including the endogenous opioid peptide enkephalin (Cassell et al., 1986; Poulin et al., 2006). In the present study it was found that immunoreactivity for μOR was present in CeA-BNST projection neurons in this area. Immunoreactivity for μOR was affiliated with intracellular organelles involved in protein synthesis and transport of somata. It was also found that μOR was present in intracellular and likely functional plasma membrane sites of dendritic profiles.
Prior ultrastructural (Glass et al., 2009) and neurophysiological (Chieng et al., 2006; Zhu and Pan, 2004) reports indicate that NR1 and μOR have a postsynaptic distribution in CeA neurons. However, until now, the phenotypic identity of these neurons has been uncertain. There have been neuropharmacological reports that NMDA receptors are necessary for the learned aversion associated with opioid withdrawal (Glass et al., 2008; Watanabe et al., 2003), behaviors that are relevant to both the CeA and BNST (Delfs et al., 2000). In the present study, an immunocytochemical triple labeling procedure was used to determine if NR1 and μOR were co-expressed in CeA-BNST projection neurons. It was found that individual somata and dendritic profiles expressed labeling for FG, NR1, and μOR. In these retrogradely labeled neurons, NR1 and μOR immunoreactivity were present near plasmalemmal sites in smaller caliber dendritic shafts, including those containing spiny processes receiving asymmetric excitatory-type contacts. In sum, the present results indicate that ligands acting at the NMDA and mu-opioid receptors may co-modulate excitatory signaling involving postsynaptic (i.e. dendritic) compartments of CeA-BNST projection neurons, providing a neuroanatomical substrate for coordinating the experience of stressful events with affective processes, learning, and goal-directed behaviors implicated in opioid addiction.
Axon terminals of CeA-BNST projection neurons are largely devoid of NR1 and μOR labeling
Visualized by light microscopy, BDA administered into the CeA resulted in dense plexuses of labeled fibers and punctate bouton-like structures in posterior ventral and dorsal BNST regions (Gastard et al., 2002). By electron microscopy, the axon terminals of CeA-BNST projection neurons were found to be small to medium in size and expressed aggregates of small synaptic vesicles, and frequently one or more large dense core vesicles, which are known to store and release peptides. Axon terminals labeled for BDA contacted the soma region of BNST neurons, but more frequently made non-synaptic appositions or symmetric inhibitory-type synapses with small and intermediate size dendritic profiles.
Consistent with a prior report (Gracy and Pickel, 1995), we found that NR1 labeling was found in small unmyelinated axons and axon terminals in the BNST. However, in the present study, NR1 was found only in low levels in BDA labeled axon terminals in the BNST, which is also consistent with our finding that FG and NR1 rarely co-labeled endomembrane-like structures in the cell body that likely represent sites of retrograde tracer transport (Wessendorf, 1991). The present finding that axon terminals of CeA-BNST projection neurons form predominantly symmetric inhibitory-type synapses that lack NR1 expression is consistent with suggestions based on neurophysiological evidence that CeA inputs to the BNST have glutamate-independent inhibitory actions in the latter brain region (Egli and Winder, 2003).
Axons of CeA-BNST projection neurons terminated in areas previously shown to exhibit preproenkephalin gene expression (Poulin et al., 2009) and enkephalin containing fibers (Moga et al., 1989). This area has also been shown to contain neurons that express μOR mRNA (Poulin et al., 2009) and respond to opioids (Casada and Dafny, 1993). However, in the present study, only a low incidence of μOR labeling was found in axons and axon terminals of CeA-BNST projection neurons. This finding is consistent with a prior report in the anterolateral BNST that μOR labeling was present in low levels in axon terminals making symmetric synapses (Jaferi and Pickel, 2009), the primary type of synaptic specialization formed by CeA inputs to the BNST.
Although it was infrequently expressed in BDA terminals, NR1 was present in somata and dendrites of BNST neurons, including those contacted by CeA inputs. These results are compatible with neurophysiological reports that NMDA receptors modulate postsynaptic signaling in the BNST (Egli and Winder, 2003). These events may be relevant to μOR, which was found to be co-expressed with NR1 in BNST neurons. The dendritic distribution of μOR and NR1 in postsynaptic compartments of BNST neurons, including those receiving excitatory contacts, provides a subcellular basis for the ability of opioid agonists to modulate excitatory signaling (Dalsass and Siegel, 1990) and influence opioid-dependent plasticity in response to exposure to opioid exposure (Dumont et al., 2008) and drug procurement behavior (Dumont et al., 2005).
Expression of the NR1 gene in CeA neurons is required for morphine-induced immediate early gene expression in the BNST
There was a decrease in morphine-induced Fos protein expression in the BNST of animals with CeA NR1 deletion, which is consistent with a prior lesion study (Nakagawa et al., 2005). The reduced Fos expression found with CeA NR1 deletion was not seen in mice that received rAAV-GFP microinjection in the CeA, or rAAV-GFP-Cre microinjection in the adjacent basomedial amygdala. Among several brain areas examined, reduced morphine-induced Fos expression was found only in the vBNST. This area of the BNST has been reported to contain opioid responsive neurons (Casada and Dafny, 1993) and play a critical role in aversive aspects of opioid dependence (Delfs et al., 2000), in addition to having an important role in neurobehavioral responses to stress (Fendt et al., 2005). These results demonstrate that the morphine-induced activity of the CeA-BNST pathway requires functional NMDA receptors in the CeA, although the possibility that these actions are, to some extent, mediated by an indirect multisynaptic route between the CeA and BNST cannot as yet be ruled out.
Functional considerations
The CeA and BNST are emerging as important components of neural pathways that coordinate the experience of stressful events with memory, learning, and goal-directed behaviors. In particular, the CeA and BNST are implicated in cue-conditioned fear and anxiety (Davis et al., 2010), phenomena that may parallel the aversive conditioned place learning associated with opioid withdrawal. Given the potential significance of withdrawal-associated cues in drug craving and drug seeking behaviors (Koob, 2009), aversive learning may have an important impact on opioid addiction. The critical role that NMDA receptors play in the development (Higgins et al., 1992) and extinction (Myers and Carlezon, 2010) of withdrawal place aversion, coupled with the present ultrastructural findings, indicates that the CeA-BNST pathway may be a critical neural substrate linking NMDA receptors, opioid withdrawal, and affective learning processes involved in opioid addiction.
Footnotes
Supported by: DA-016735, DA-024030 (MJG).
References
- Aicher SA, Goldberg A, Sharma S, Pickel VM. μ-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J. Comp. Neurol. 2000;422:181–190. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Sadeghian K, Kelley AE. Central amygdalar and dorsal striatal NMDA receptor involvement in instrumental learning and spontaneous behavior. Behav. Neurosci. 2004;118:715–729. doi: 10.1037/0735-7044.118.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman MA, Glass MJ. Ultrastructural relationship between the AMPA-GluR2 receptor subunit and the mu-opioid receptor in the mouse central nucleus of the amygdala. Exp. Neurol. 2011;227:149–158. doi: 10.1016/j.expneurol.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot G, Chahl LA. Induction of Fos-like immunoreactivity by opioids in guineapig brain. Brain Res. 1996;731:45–56. doi: 10.1016/0006-8993(96)00457-x. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Responses of neurons in bed nucleus of the stria terminalis to microiontophoretically applied morphine, norepinephrine and acetylcholine. Neuropharmacology. 1993;32:279–284. doi: 10.1016/0028-3908(93)90112-g. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J. Comp. Neurol. 1989;281:320–333. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J. Comp. Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J. Neurosci. Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RKW, Peto CA, Sawchenko PE. Fine structure and plasticity of barosensitive neurons in the nucleus of solitary tract. J. Comp. Neurol. 2000;422:338–351. [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J. Comp. Neurol. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Dalsass M, Siegel A. Opioid peptide regulation of neurons in the bed nucleus of the stria terminalis: a microiontophoretic study. Brain Res. 1990;531:346–349. doi: 10.1016/0006-8993(90)90799-h. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat. Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J. Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J. Comp. Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J. Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray MI, Andres ME, Bustos G, Gysling K. Regulation of endogenous noradrenaline release from the bed nucleus of stria terminalis. Biochem. Pharmacol. 1995;49:687–692. doi: 10.1016/0006-2952(94)00498-b. [DOI] [PubMed] [Google Scholar]
- Gastard M, Jensen SL, Martin JR, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Res. 2002;957:207–222. doi: 10.1016/s0006-8993(02)03513-8. [DOI] [PubMed] [Google Scholar]
- Glass MJ. The role of functional postsynaptic NMDA receptors in the central nucleus of the amygdala in opioid dependence. Vitam. Horm. 2010;82:145–166. doi: 10.1016/S0083-6729(10)82008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Colago EE, Pickel VM. Alpha-2A-adrenergic receptors are present on neurons in the central nucleus of the amygdala that project to the dorsal vagal complex in the rat. Synapse. 2002;46:258–268. doi: 10.1002/syn.10136. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Kruzich PJ, Kreek M, Pickel VM. Decreased plasma membrane targeting of NMDA-NR1 receptor subunit in dendrites of medial nucleus tractus solitarius neurons in rats self-administering morphine. Synapse. 2004;53:191–201. doi: 10.1002/syn.20049. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Hegarty DM, Oselkin M, Quimson L, South SM, Xu Q, Pickel VM, Inturrisi CE. Conditional deletion of theNMDA-NR1 receptor subunit gene in the central nucleus of the amygdala inhibits naloxone-induced conditioned place aversion in morphine dependent mice. Exp. Neurol. 2008;213:57–70. doi: 10.1016/j.expneurol.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Vanyo L, Quimson L, Pickel VM. Ultrastructural relationship between NMDA-NR1 and mu-opioid receptor in the mouse central nucleus of the amygdala. Neuroscience. 2009;163:857–867. doi: 10.1016/j.neuroscience.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. Eur. J. Pharmacol. 2001;422:87–90. doi: 10.1016/s0014-2999(01)01066-4. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Comparative ultrastructural localization of the NMDAR1 glutamate receptor in the rat basolateral amygdala and bed nucleus of the stria terminalis. J. Comp. Neurol. 1995;362:71–85. doi: 10.1002/cne.903620105. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Nguyen P, Sellers EM. The NMDA antagonist dizocilpine (MK801) attenuates motivational as well as somatic aspects of naloxone precipitated opioid withdrawal. Life Sci. 1992;50:PL167–PL172. doi: 10.1016/0024-3205(92)90452-u. [DOI] [PubMed] [Google Scholar]
- Hof PR, Young EG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/SV mouse brains. Elsevier, Elsevier; 2000. [Google Scholar]
- Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience. 2009;159:526–539. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-Methyl-d-aspartate receptors, and dopamine–glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee K-F, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl. Acad. Sci. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl. 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborszky L, editors. Tract Tracing Methods 2, Recent Progress. New York: Plenum; 1989. pp. 129–172. [Google Scholar]
- Ma YY, Chu NN, Guo CY, Han JS, Cui CL. NR2B-containing NMDA receptor is required for morphine-but not stress-induced reinstatement. Exp. Neurol. 2007;203:309–319. doi: 10.1016/j.expneurol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J. Comp. Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribuiton of N-methyl-d-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J. Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA. d-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol. Psychiatry. 2010;67:85–87. doi: 10.1016/j.biopsych.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Naumann T, Hartig W, Frotscher M. Retrograde tracing with Fluoro-Gold: different methods of tracer detection at the ultrastructural level and neurodegenerative changes of back-filled neurons in long-term studies. J. Neurosci. Methods. 2000;103:11–21. doi: 10.1016/s0165-0270(00)00292-2. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J. Neurosci. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System. Oxford University Press, Oxford University Press; 1991. [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J. Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur. J. Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J. Comp. Neurol. 2006;496:859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1356–1365. doi: 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav. Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Golhasani-Keshtan F, Haeri-Rohani A, Zarrindast MR. Morphine-induced place preference: involvement of the central amygdala NMDA receptors. Brain Res. 2007;1133:34–41. doi: 10.1016/j.brainres.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Roder S, Ciriello J. Contribution of bed nucleus of the stria terminalis to the cardiovascular responses elicited by stimulation of the amygdala. J. Auton. Nerv. Syst. 1993;45:61–75. doi: 10.1016/0165-1838(93)90362-x. [DOI] [PubMed] [Google Scholar]
- Samson RD, Paré D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J. Neurosci. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Mick G, Kiyama H, Tohyama M. Expression patterns of a glutamate-binding protein in the rat central nervous system: comparison with N-methyl-d-aspartate receptor subunit 1 in rat. Neuroscience. 1995;64:459–475. doi: 10.1016/0306-4522(94)00335-3. [DOI] [PubMed] [Google Scholar]
- Sawada S, Yamamoto C. Title postsynaptic inhibitory actions of catecholamines and opioid peptides in the bed nucleus of the stria terminalis. Exp. Brain Res. 1981;41:264–270. doi: 10.1007/BF00238883. [DOI] [PubMed] [Google Scholar]
- Semenova S, Danysz W, Bespalov A. Low-affinity NMDA receptor channel blockers inhibit acquisition of intravenous morphine self-administration in naive mice. Eur. J Pharmacol. 1999;378:1–8. doi: 10.1016/s0014-2999(99)00431-8. [DOI] [PubMed] [Google Scholar]
- South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J. Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. N-methyl-d-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neurosci. Lett. 1995;193:37–40. doi: 10.1016/0304-3940(95)11662-g. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Neurosci. Rev. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Wright AM, Cestari DM, Pickel VM. Immunolabeling of retrogradely transported Fluoro-Gold: sensitivity and application to ultrastructural analysis of transmitter-specific mesolimbic circuitry. J. Neurosci. Methods. 1994;55:65–78. doi: 10.1016/0165-0270(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neuroscience. 2003;118:695–708. doi: 10.1016/s0306-4522(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Okuda K, Sakurada C, Ando R, Sakurada T, Sakurada S. Evidence that nitric oxide-glutamate cascade modulates spinal antinociceptive effect of morphine: a behavioural and microdialysis study in rats. Brain Res. 2003;990:77–86. doi: 10.1016/s0006-8993(03)03440-1. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wessendorf MW. Fluoro-Gold: composition and mechanism of uptake. Brain Res. 1991;553:135–148. doi: 10.1016/0006-8993(91)90241-m. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR. Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur. J. Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Zhu W, Pan ZZ. Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience. 2004;127:871–879. doi: 10.1016/j.neuroscience.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Zhu W, Pan ZZ. μ-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience. 2005;133:97–103. doi: 10.1016/j.neuroscience.2005.02.004. [DOI] [PubMed] [Google Scholar]