Abstract
Purpose
Adjuvant chemotherapy has been associated with mild cognitive decline among a subset of breast cancer survivors. Late cognitive effects after chemotherapy can have a deleterious impact on survivor quality of life and functional health; however, the etiology of chemotherapy-related cognitive dysfunction remains unknown.
Patients and Methods
We present a case of monozygotic twins who are discordant for breast cancer and chemotherapy exposure (ie, one twin contracted breast cancer and underwent chemotherapy, and the other had no breast cancer). As part of a larger study, each was evaluated with standardized, self-report measures of cognitive function, standard neuropsychological tests, and structural and functional magnetic resonance imaging (MRI).
Results
Results indicated small differences in neuropsychological test performance but striking contrasts in self-reported cognitive complaints and structural and functional MRI images. Specifically, the twin who underwent chemotherapy had substantially more subjective cognitive complaints, more white matter hyperintensities on MRI, and an expanded spatial extent of brain activation during working memory processing than her nonaffected twin.
Conclusion
This case illustrates possible physiologic mechanisms that could produce long-term cognitive complaints among chemotherapy recipients and help formulate hypotheses for further empirical study in the area of chemotherapy-associated cognitive dysfunction.
INTRODUCTION
Many cancer survivors report experiencing cognitive changes after chemotherapy; however, the mechanism(s) for chemotherapy-induced cognitive changes are not well understood.1-5 The cognitive changes are often subtle; therefore, despite reports of memory and attention complaints, cancer survivors frequently perform within the normal range on neuropsychological tests. Additionally, although several studies have found evidence for cognitive changes with chemotherapy, two recent studies, including a well-designed longitudinal study with pre-treatment assessments, failed to find differences among breast cancer patients treated with chemotherapy, patients treated with nonchemotherapy-based regimens, and healthy controls.6,7
One potential explanation for this pattern of results is that patients are able to compensate for relatively subtle changes in cognitive ability by recruiting additional brain areas to perform a specific task. A recent positron emission tomography study reported evidence of expanded activation in frontal areas while long-term breast cancer patients treated with chemotherapy performed a memory task.8 These results seem to support the hypothesis of regional brain activation as a possible compensatory process. We had a unique opportunity to evaluate a set of identical twins, one with a history of breast cancer and treatment with chemotherapy and one with no history of cancer. Self-report measures of cognitive function, neuropsychological tests, and structural and functional magnetic resonance imaging (MRI) were used to assess relative differences across these measures of cognitive function.
PATIENTS AND METHODS
Participants were white, 60-year-old, right-handed female twins who were reared together. The participant who had breast cancer (twin A) was enrolled onto a research study examining the efficacy of a brief cognitive-behavioral treatment (CBT) of memory dysfunction after chemotherapy. Twin A identified her sister (no cancer), who was subsequently contacted for participation. Both had no history of head injury, stroke, or other CNS injury or disease. Neither had a smoking or substance abuse history or other systemic illnesses that could affect cognition. Both provided informed written consent for imaging and genetics testing for research approved by Dartmouth Medical School’s Committee for the Protection of Human Subjects. The consent process was in strict accordance with all US Department of Health and Human Services standards.
Twin A completed adjuvant chemotherapy for stage II breast cancer 22 months before enrollment. Treatment consisted of four cycles of doxorubicin 60 mg/m2 (108.6 mg) and cyclophosphamide 600 mg/m2 (1,086 mg), with each cycle administered every 3 weeks. She also received four cycles of docetaxel 100 mg/m2 (178 mg) over a 1-hour infusion administered every 3 weeks. Her post-treatment hormonal therapy consisted of oral tamoxifen at 20 mg/d (she had been taking this medication for 18 months at the time of study).
Monozygotic status was determined with molecular diagnostic assessment using PowerPlex 16 (Promega, Madison, WI) short tandem repeat analysis (probability of dizygosity, P = .0000305), and genetic testing conducted as part of a larger study revealed that the twins were carriers of the ε-4 allele of apolipoprotein E (APOE). APOE status has been associated with cognitive impairment in other populations and breast cancer patients treated with adjuvant chemotherapy.9-11 A brief battery of standardized neuropsychological tests (domains of verbal memory and processing speed) was administered to assess memory and attention.12-15 Self-reported cognitive function was assessed using the Multiple Ability Self-Report Questionnaire (MASQ), a 48-item self-report measure of cognitive function,16 and anxiety and depressive symptoms were assessed using the Spielberger State-Trait Anxiety Inventory17 and the Center for Epidemiologic Studies Depression Scale.18
Finally, structural and functional MRI scans were acquired during the same session on a GE Horizon 1.5T LX scanner (GE, Waukesha, WI). A gradient echo, echo-planar sequence was used to provide whole-brain coverage for functional MRI (repetition time [TE], 2,500 msec; echo time [TE], 40 msec; field of view [FOV], 24 cm; number of excitations [NEX], 1.29; 5-mm-thick sagittal slices with no skip, yielding a 64 × 64 matrix with 3.75 mm2 in-plane resolution). Initial volumes before spin saturation were discarded. An aurally presented verbal “3-back” task was used to probe working memory. During scanning, participants were asked to listen to a string of consonant letters (except L, W, and Y) presented at a rate of one every 3 seconds. This experiment was presented in a four-condition, blocked design. Conditions were 0, 1, 2, and 3 back. For each consonant heard, participants used a button press device (Photon Control, Burnaby, British Columbia, Canada) to signify whether the current letter was a match (ie, was the same as the designated target or the letter presented 1, 2, or 3 back in the sequence, depending on the condition instructions) or a nonmatch. The number of correct and incorrect responses was recorded, along with reaction times. Each n-back condition was presented in 12 27-second epochs preceded by 3 seconds of instruction (eg, “the match is D” or “the match is one back”). The four experimental conditions were each presented three times in pseudorandom order. During each epoch, there was a possibility of two or three matches, and the number of matches was counterbalanced within and across conditions. In addition, non-target recurrences were presented as foils (eg, a 2-back match during the 3-back condition). Participants rehearsed practice versions of the tasks outside the scanner to ensure comprehension of task demands. We have used this paradigm in several neurologic and neuropsychiatric populations to demonstrate alterations in working memory circuitry.19-22
RESULTS
Results indicated that twin A had substantially more cognitive complaints (MASQ score = 134) than her unaffected twin (MASQ score = 63; higher MASQ scores denote more cognitive complaints; Table 1). The normal control value for the MASQ total score in our research is a mean of 81 (standard deviation = 18). Therefore, twin A scored nearly 3 standard deviations higher on the MASQ total score than healthy control participants. Conversely, there were minimal differences between the twins on standardized neuropsychological test performance. Twin A scored higher on some measures (such as short-and long-delay recall), whereas twin B scored higher on others (such as overall verbal memory as assessed by the California Verbal Learning Test–Total Score). Overall, the clinical significance of these differences was marginal. Both twins had normal anxiety and depression scores, suggesting that these factors had negligible impact on cognitive complaints or neuropsychological test performance, although twin B (the unaffected twin) reported slightly higher state anxiety. Furthermore, both twin A and her sister scored within the normal ranges on all neuropsychological tests (Table 1).
Table 1.
Self-Reported Cognitive Symptoms and Neuropsychological Testing
| Test | Score
|
|
|---|---|---|
| Twin A (chemotherapy) | Twin B (no chemotherapy) | |
| Raw scores | ||
| MASQ total score* | 134 | 63 |
| Language | 20 | 8 |
| Visual-perceptual ability | 13 | 9 |
| Visual memory | 22 | 12 |
| Spatial memory | 27 | 8 |
| Attention-concentration | 20 | 9 |
| Depression and anxiety self-report | ||
| CES-D; < 16 is normal range | 8 | 3 |
| State anxiety, T-score | 43 | 57 |
| Trait anxiety, T-score | 49 | 52 |
| Neuropsychological measures | ||
| CVLT-II trials 1-5 total, T-score | 46 | 56 |
| Short-delay free recall | 11 | 8 |
| Long-delay free recall | 12 | 6 |
| Recognition correct | 12 | 12 |
| Recognize false positive | 0 | 0 |
| Craft stories immediate recall | 46.5 | 50 |
| Craft stories delayed recall | 45 | 41 |
| Age scaled score | ||
| WAIS-III digit symbol coding | 14 | 13 |
| D-KEFS Color-Word Interference Test | ||
| Trial 1 | 14 | 14 |
| Trial 2 | 13 | 13 |
| Trial 3 | 15 | 15 |
| Trial 4 | 13 | 15 |
| D-KEFS Trail Making Test | ||
| Trial 1 | 15 | 13 |
| Trial 2 | 15 | 14 |
| Trial 3 | 14 | 13 |
| Trial 4 | 12 | 12 |
| Trial 5 | 13 | 14 |
Abbreviations: MASQ, Multiple Abilities Self-Report Questionnaire; CES-D, Center for Epidemiologic Studies Depression Scale; CVLT-II, California Verbal Learning Test-II; WAIS-III, Wechsler Adult Intelligence Scale-III; D-KEFS, Delis-Kaplan Executive Function System.
Self-report of cognitive complaints; higher score denotes more complaints.
With respect to imaging results, both structural and functional differences between the twins were noted. Structural imaging results revealed white matter hyperintensities in both twins, which were read as a nonspecific finding of uncertain clinical significance by a neuroradiologist blinded to participant identity. Of note, white matter hyperintensities are common among APOE ε-4 carriers.10 Interestingly, white matter lesion volumes were greater for twin A than twin B in both the right (3,725.68 mm3 v 2,897.75 mm3, respectively) and left (6,075.00 mm3 v 3,343.36 mm3, respectively) cerebral hemispheres (Fig 1). Volumetric analyses of other brain regions of interest (ie, hippocampus, amygdala, entorhinal cortex, and corpus callosum) were also conducted, with findings demonstrating no consistent pattern of difference between participants.
Fig 1.
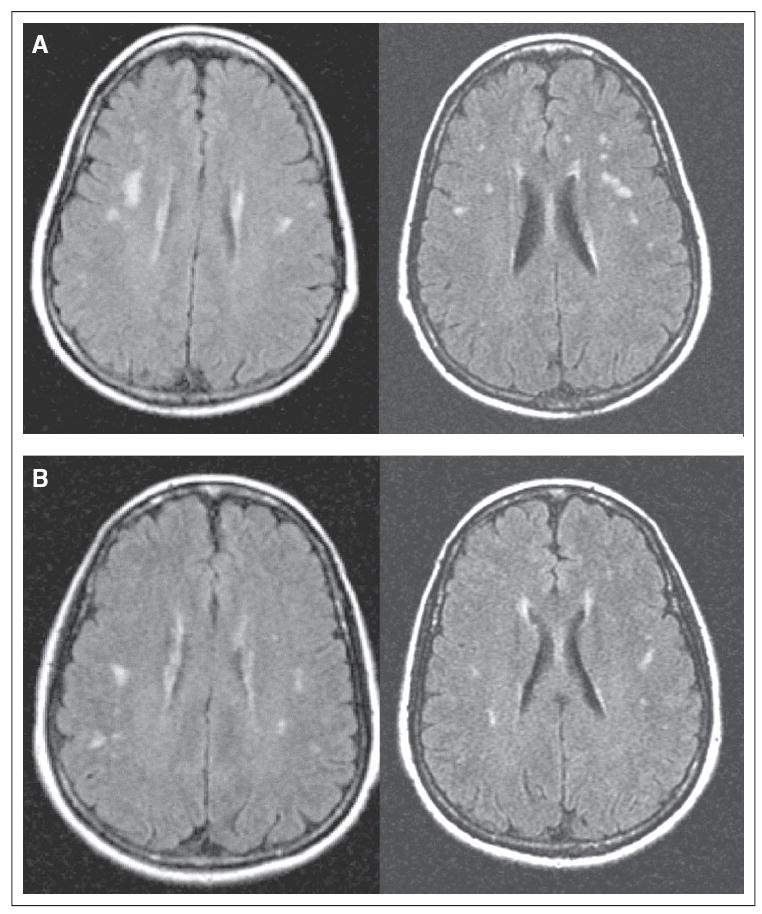
Fluid attenuated inversion recovery magnetic resonance images of white matter hyperintensities. The hyperintensities were read by the study neuroradiologist as of uncertain clinical significance, but they appeared somewhat more prominent throughout white matter in (A) the chemotherapy-treated twin than in (B) the twin who did not receive chemotherapy as confirmed by volumetric analysis.
Figure 2 shows functional MRI images of both twins engaged in the n-back working memory task. Across task conditions with increasing working memory load, twin A demonstrated much broader spatial extent of activation in typical working memory circuitry (bifrontal and biparietal regions) than twin B. However, twins A and B did not differ in task performance accuracy. Thus, although task performances were the same, more brain activation was noted in the twin who underwent chemotherapy (Fig 2).
Fig 2.

Functional magnetic resonance images of 60-year-old identical twins during a working memory task with incrementally increasing levels of difficulty (left to right). Colored regions denote increased brain activation during working memory relative to a simple vigilance task. (A) Twin treated with chemotherapy; (B) twin who did not receive chemotherapy. Note the expanded spatial extent of cortical activation in the chemotherapy-treated twin.
DISCUSSION
Cancer survivors treated with chemotherapy frequently self-report higher levels of cognitive problems but perform within normal limits on neuropsychological tests, as was seen in this report. The imaging data provide a potential explanation. Twin A’s pattern of increased cortical activity may represent recruitment of a broader neural network needed to accomplish performance comparable to the unaffected twin. More cortical activation may indicate a compensation for dysfunction in neural circuitry affected by chemotherapy. We have observed an expanded spatial extent of activation in functional MRI studies in other disorders (eg, multiple sclerosis, traumatic brain injury, mild cognitive impairment), which also seems to reflect a compensatory process in individuals with known brain dysfunction.19-23 It is unclear to what degree the observed white matter structural changes may be reflective of normal aging versus APOE status or neural pathology (eg, chemotherapy exposure).
It is important to point out that twin A was enrolled onto a brief CBT program aimed at helping her compensate for daily memory and attention performance problems. However, she only completed two sessions of CBT, which may not have been enough treatment to have produced the expanded spatial extent observed in the functional MRI. Furthermore, twin A’s neuropsychological testing and self-report of cognitive complaints (total MASQ score; Table 1) were obtained before this intervention, so CBT could not have affected neuropsychological test scores or number of daily cognitive complaints reported here. In addition, twin A was also on a standard hormonal treatment after adjuvant chemotherapy (tamoxifen). Therefore, although there have been mixed reports on the relationship between tamoxifen and cognitive functioning, it is possible that tamoxifen contributed to the observed differences in activation pattern.
Although these case results must be interpreted with caution, they suggest intriguing hypotheses that can guide future research examining the relationships among self-report measures of cognitive functioning, performance on neuropsychological testing, and structural and functional changes evaluated with imaging techniques in patients experiencing cognitive difficulties associated with chemotherapy. Future research using functional MRI and the neuropsychological methods outlined here may help clarify neural processes contributing to the problem of late cognitive effects of chemotherapy.
Acknowledgments
We gratefully acknowledge the assistance of Fred Briccetti, MD, of New Hampshire Oncology-Hematology for participant recruitment. We also thank Tara McHugh, MA, John MacDonald, MA, Paul Wang, BA, Alexander Mamourian, MD, Clifford Eskey, MD, PhD, C. Harker Rhodes, MD, PhD, and Gregory Tsongalis, PhD.
Supported by grants from the Office of Cancer Survivorship, National Cancer Institute; Grants No. R01 CA101318, R03 CA90151, and R01 CA87845 from the National Institutes of Health; and the Lance Armstrong Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin, Tim A. Ahles
Financial support: Robert J. Ferguson, Andrew J. Saykin, Tim A. Ahles
Administrative support: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin, Tim A. Ahles
Provision of study materials or patients: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin
Collection and assembly of data: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin
Data analysis and interpretation: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin
Manuscript writing: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin, Tim A. Ahles
Final approval of manuscript: Robert J. Ferguson, Brenna C. McDonald, Andrew J. Saykin, Tim A. Ahles
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
References
- 1.Ferguson RJ, Ahles TA. Low neuropsychologic performance among adult cancer survivors treated with chemotherapy. Curr Neurol Neurosci Rep. 2003;3:215–222. doi: 10.1007/s11910-003-0081-2. [DOI] [PubMed] [Google Scholar]
- 2.Minisini A, Atalay G, Bottomley A, et al. What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol. 2004;5:273–282. doi: 10.1016/S1470-2045(04)01465-2. [DOI] [PubMed] [Google Scholar]
- 3.Phillips KA, Bernhard J. Adjuvant breast cancer treatment and cognitive function: Current knowledge and research directions. J Natl Cancer Inst. 2003;95:190–197. doi: 10.1093/jnci/95.3.190. [DOI] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 5.van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high risk breast cancer: High dose versus standard dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan KA, Small BJ, Andrykowski MA, et al. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104:2499–2507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 9.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Reed T, Miller BL, et al. Impact of apolipoprotein E e-4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999;30:1548–1553. doi: 10.1161/01.str.30.8.1548. [DOI] [PubMed] [Google Scholar]
- 11.Haan MN, Shemanski L, Jagust WJ, et al. The role of APOE e-4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 12.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test-II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 13.Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler D. Wechsler Adult Intelligence Scale–3rd Edition (WAIS-III) San Antonio, TX: Harcourt, Brace & Co; 1997. [Google Scholar]
- 15.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 16.Seidenberg M, Haltiner A, Taylor MA. Development and validation of a multiple ability self-report questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 17.Spielberger CD, Gorsuch RL, Lushene RG. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Press; 1971. [Google Scholar]
- 18.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.McAllister TW, Saykin AJ, Flashman LA, et al. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 20.McAllister TW, Sparling MB, Flashman LA, et al. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- 21.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 22.Wishart HA, Saykin AJ, McDonald BC, et al. Brain activation patterns associated with working memory in relapsing-remitting MS. Neurology. 2004;62:234–238. doi: 10.1212/01.wnl.0000103238.91536.5f. [DOI] [PubMed] [Google Scholar]
- 23.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]


