Abstract
This study was carried out to determine if, in fasting, an adaptation to utilization of ketones could prevent cerebral dysfunction during periods of acute, insulin-induced glucopenia.
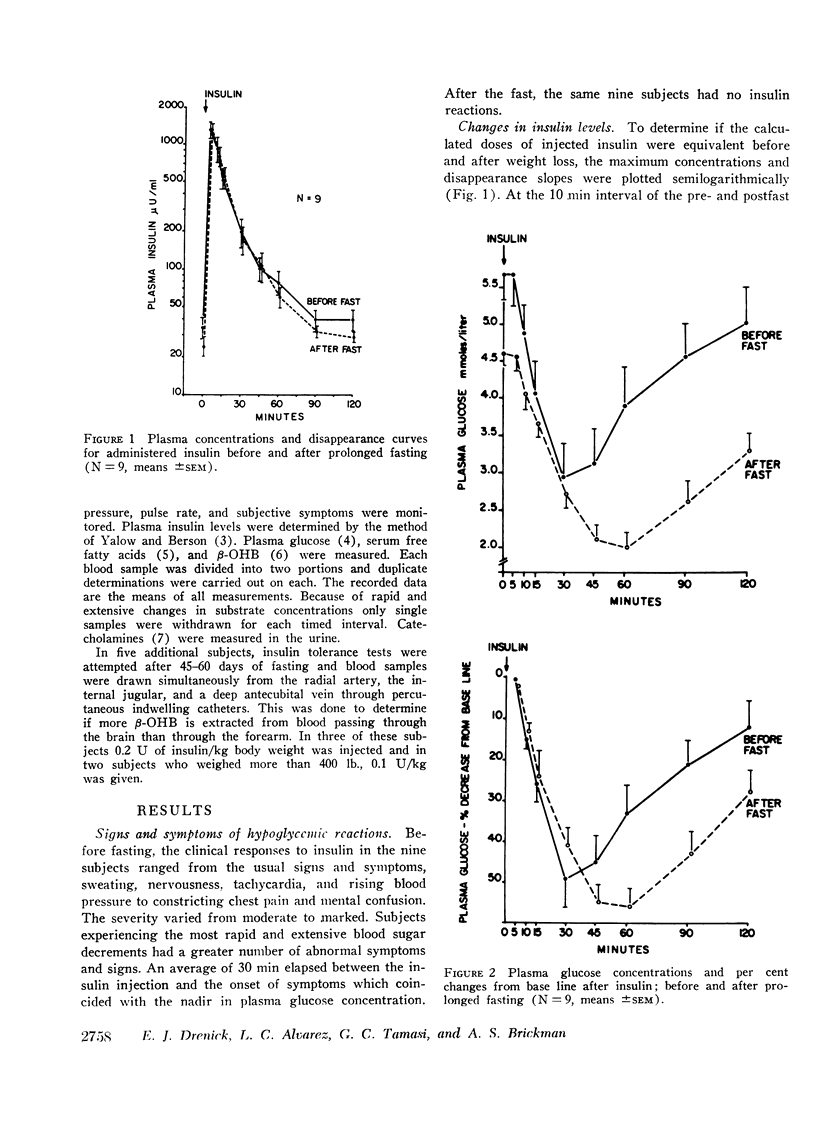
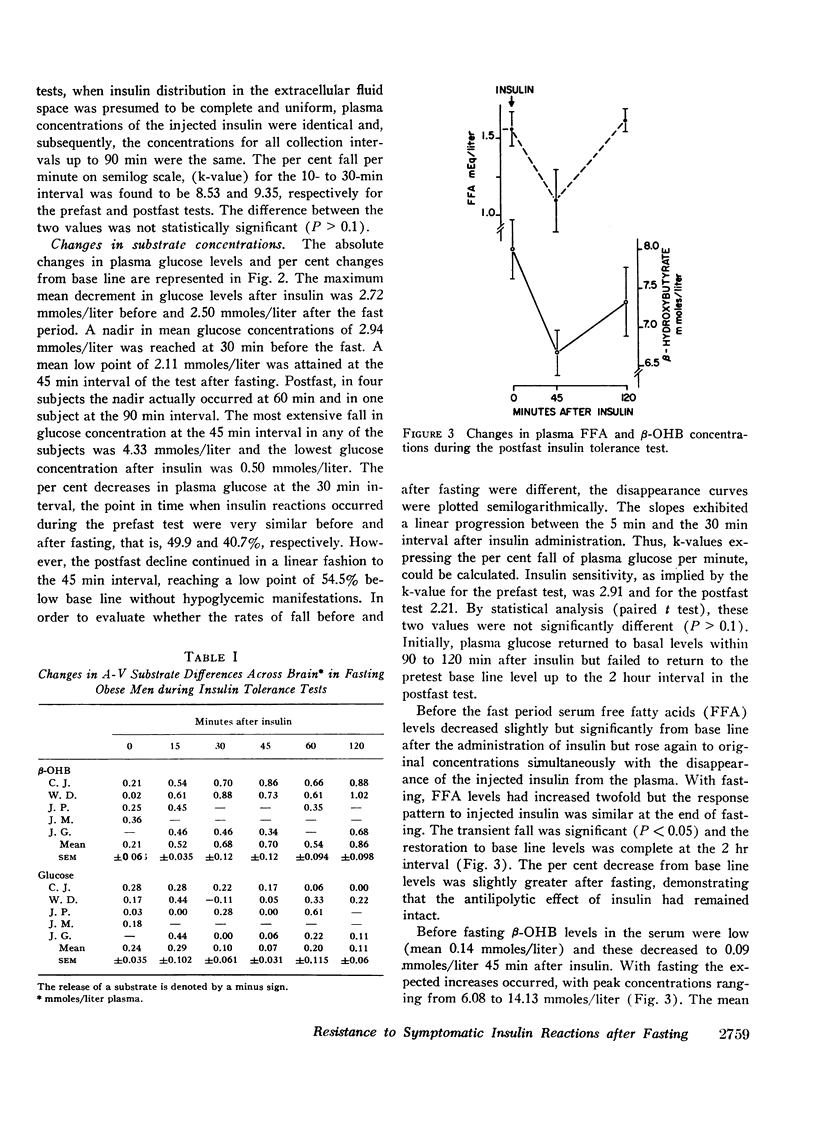
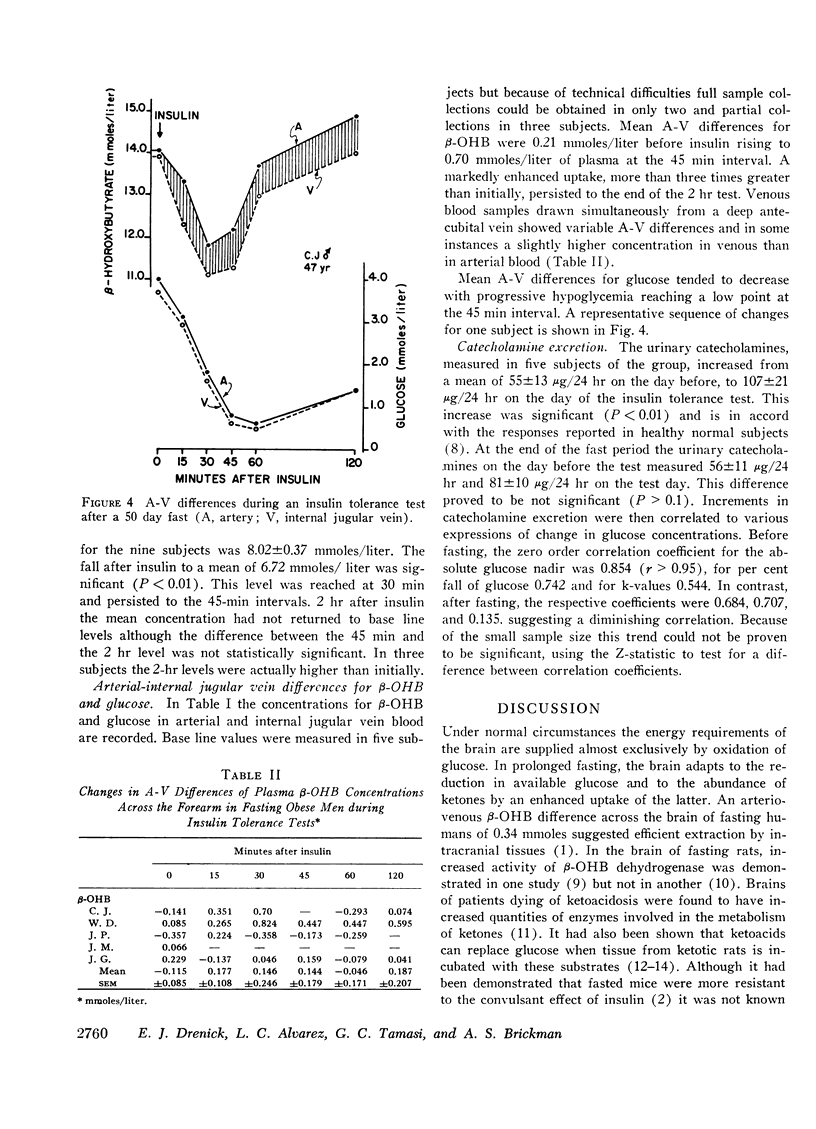
In the course of standard insulin tolerance tests (0.1-0.2 U/kg), nine obese subjects manifested frank hypoglycemic reactions resulting in an increase in urinary catecholamine excretion from 61 to 113 μg/24 hr (P < 0.01). After fasting 2 months, administration of weight-adjusted doses of insulin produced identical maximum insulin concentrations and disappearance curves. However, no insulin reactions nor significant rises in catecholamine excretion occurred despite equal extent and rate of glucose fall. Glucose concentrations as low as 0.5 mmoles/liter (9 mg/100 ml) failed to precipitate hypoglycemic reactions. During the postfast insulin tolerance tests, mean plasma 2-hydroxybutyrate (β-OHB) decreased from 8.02 to 6.69 mmoles/liter (P < 0.01). In another five fasting subjects tested, the A-V difference for β-OHB across brain increased progressively from 0.21 to 0.70 mmoles/liter whereas across the forearm no consistent uptake could be demonstrated. Simultaneously, the A-V difference across the brain for glucose decreased from 0.24 to 0.07 mmoles/liter of plasma.
In addition to insulin-induced suppression of hepatic ketogenesis, the augmented cerebral ketone uptake during insulin hypoglycemia contributes to the net fall in plasma β-OHB. Ketoacids, extracted by the fast-adapted brain, supplant glucose as a metabolic substrate preventing overt hypoglycemic reactions during acute glucopenia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasse E. O., Havel R. J. Evidence for an effect of inulin on the peripheral utilization of ketone bodies in dogs. J Clin Invest. 1971 Apr;50(4):801–813. doi: 10.1172/JCI106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberdorf F. A., Chernick S. S., Scow R. O. Effect of insulin and acute diabetes on plasma FFA and ketone bodies in the fasting rat. J Clin Invest. 1970 Sep;49(9):1685–1693. doi: 10.1172/JCI106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P. C., Albrink M. J. Insulin resistance in hyperglyceridemia. Metabolism. 1965 Oct;14(10):1059–1070. doi: 10.1016/0026-0495(65)90154-x. [DOI] [PubMed] [Google Scholar]
- Foster D. W. Studies in the ketosis of fasting. J Clin Invest. 1967 Aug;46(8):1283–1296. doi: 10.1172/JCI105621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuth S. M. Effects of prolonged fasting on insulin secretion. Diabetes. 1966 Nov;15(11):798–806. doi: 10.2337/diab.15.11.798. [DOI] [PubMed] [Google Scholar]
- Gibbard S., Watkins P. J. A micro-method for the enzymatic determination of D-beta-hydroxybutyrate and acetoacetate. Clin Chim Acta. 1968 Mar;19(3):511–521. doi: 10.1016/0009-8981(68)90280-5. [DOI] [PubMed] [Google Scholar]
- Gottstein U., Müller W., Berghoff W., Gärtner H., Held K. Zur Utilisation von nicht-veresterten Fettsäuren und Ketonkörpern im Gehirn des Menschen. Klin Wochenschr. 1971 Apr 1;49(7):406–411. doi: 10.1007/BF01484996. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Steinke J., Cahill G. F., Jr Metabolic interactions of glucose, lactate, and beta-hydroxybutyrate in rat brain slices. Am J Physiol. 1969 Sep;217(3):784–792. doi: 10.1152/ajplegacy.1969.217.3.784. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Effects of fatty acids, ketone bodies, lactate and pyruvate on glucose utilization by guinea-pig cerebral cortex slices. Biochem J. 1967 Aug;104(2):519–523. doi: 10.1042/bj1040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOTZ M. C., MASSON G. M., PAGE I. H. ACTH in vitro on release of nonesterified fatty acids from adipose tissues of adrenalectomized rats. Proc Soc Exp Biol Med. 1959 May;101(1):159–161. doi: 10.3181/00379727-101-24866. [DOI] [PubMed] [Google Scholar]
- SUNDERMAN F. W. Further modifications in the measurement of blood glucose. Am J Clin Pathol. 1952 Feb;23(2):193–196. doi: 10.1093/ajcp/23.2_ts.193. [DOI] [PubMed] [Google Scholar]
- Tzagournis M., Skillman R. G. Glucose intolerance mechanism after starvation. Metabolism. 1970 Feb;19(2):170–178. [PubMed] [Google Scholar]
- VON EULER U. S., LUFT R. Effect of insulin on urinary excretion of adrenalin and noradrenalin; studies in ten healthy subjects and in six cases of acromegaly. Metabolism. 1952 Nov;1(6):528–532. [PubMed] [Google Scholar]
- Wiener R., Hirsch H. J., Spitzer J. J. Cerebral extraction of ketones and their penetration into CSF in the dog. Am J Physiol. 1971 May;220(5):1542–1546. doi: 10.1152/ajplegacy.1971.220.5.1542. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]



