Abstract
The mechanism(s) for chemotherapy-induced cognitive changes are largely unknown; however, several candidate mechanisms have been identified. We suggest that shared genetic risk factors for the development of cancer and cognitive problems, including low-efficiency efflux pumps, deficits in DNA-repair mechanisms and/or a deregulated immune response, coupled with the effect of chemotherapy on these systems, might contribute to cognitive decline in patients after chemotherapy. Furthermore, the genetically modulated reduction of capacity for neural repair and neurotransmitter activity, as well as reduced antioxidant capacity associated with treatment-induced reduction in oestrogen and testosterone levels, might interact with these mechanisms and/or have independent effects on cognitive function.
Studies associating cognitive changes with cancer chemotherapy have been reported since the mid 1970s1; however, systematic research on the cognitive side effects of chemotherapy did not appear until the 1990s. Over the past 10 years, neuropsychological studies of cancer survivors2-8 and emerging data from longitudinal studies that include pretreatment neuropsychological assessments9,10 have found evidence supporting the influence of chemotherapy on cognitive functioning, although negative studies have also been reported11-12. The cognitive changes associated with chemotherapy are typically subtle (functioning is reduced but often remains in the normal range), and occur across various domains of cognition, including working memory, executive function and processing speed13-16, but not the retrieval of remote memories (see BOX 1 for a description of the cognitive functions that can be affected by chemotherapy). Furthermore, although acute cognitive changes during chemotherapy are common14,15, long-term post-treatment cognitive changes seem to persist in only a subgroup (17–34%) of cancer survivors. Historically, cognitive changes in cancer patients were assumed to be related to psychological factors such as depression or anxiety, or other side effects of cancer treatments like fatigue. However, most of the studies cited above have found evidence for persistent post-chemotherapy cognitive changes after statistically controlling for psychological factors or fatigue, or by studying patients without significant psychological distress or fatigue.
Box 1.
Cognitive functions and brain regions relevant to chemotherapy-induced cognitive changes
Memory
Working memory
The ability to temporarily store and manipulate information (bilateral prefrontal and parietal regions).
Episodic memory
The ability to learn and retain new context-dependent information (medial temporal lobes and prefrontal cortex).
Remote memory
The ability to retrieve memories from the past (frontal and temporal lobes).
Modality-specific memory
Verbal memory
Memory for words and narrative material presented verbally or in writing (left hemisphere).
Visual memory
Memory for objects, faces, figures or locations presented visually (right hemisphere).
Executive function
The control system that manages other cognitive processes, including planning, rule acquisition, initiating appropriate action, inhibiting competing responses and selecting relevant information (bilateral dorsal lateral prefrontal cortex).
Processing speed
The speed and efficiency with which information is used in completing a task (distributed frontal subcortical network).
Visual, spatial and constructional ability
The ability to visualize and manipulate two and three-dimensional objects (right parietal and bilateral frontal regions).
Attention and concentration
Attention is the ability to focus on certain information or stimuli at the same time as ignoring other information or stimuli. Concentration refers to the ability to maintain attention without being distracted by competing stimuli (distributed frontal subcortical network).
Reaction time
Simple reaction time is the time it takes for a person to react to a stimulus (for example, pressing a button when a light goes on), whereas complex or choice reaction time is the period of latency before a decision is made regarding a stimulus (for example, deciding if a sequence of letters represents a word) (distributed frontal subcortical network).
Motor speed and dexterity
The speed and accuracy with which a person can perform simple motor tasks and manipulate objects (for example, placing pegs in holes on a board) (bilateral frontal and pyramidal and extrapyramidal motor systems).
Studies that have used imaging techniques (FIG. 1) have reported structural and functional changes in the brain associated with chemotherapy17,18. A reduction in the volume of brain structures important for cognitive functioning (such as the frontal cortex) and changes in the integrity of white-matter tracks that connect brain structures have been associated with changes in cognitive functioning, and have been seen using structural magnetic resonance imaging (MRI) on patients after chemotherapy. Recent data from a longitudinal study of breast cancer patients, who were evaluated with structural and functional MRI before treatment and 1 and 12 months after treatment, have suggested a pattern of reduced activation in frontal areas during a working memory task19. Decreased activation in frontal areas is indicative of dysfunction in brain areas crucial for normal working memory functioning. Finally, studies in patients with breast cancer treated with chemotherapy have examined the P-300 event-related brain potential of the electroencephalogram (EEG), which is important for the attention-dependent processing of information. These studies found that decreases in amplitude (intensity of neural activation) and latency (timing and duration of activation) of P-300 were associated with chemotherapy, which is consistent with changes in information-processing capacity20,21.
Figure 1. Neuroimaging methods relevant to the assessment of cognitive changes.
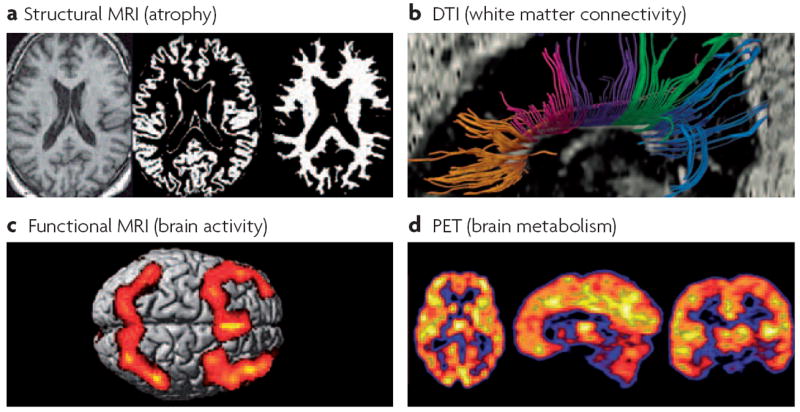
a ∣ Structural magnetic resonance imaging (MRI) provides a high resolution picture of normal neuroanatomic details and atrophy (T1-weighted scans), and visible pathology such as microvascular and inflammatory lesions (T2-weighted scans; fluid-attenuated inversion recovery (FLAIR) scans). Semi-automated methods can be used to segment or classify the structural images into the main tissue compartments, including grey and white matter, cerebrospinal fluid and hyperintense lesions, which reflect microvascular changes or areas of demyelination. Software is available to quantify the volume and other characteristics of each tissue type. b ∣ Diffusion tensor imaging (DTI) is a recently developed technique that can be used to assess pathological changes in grey matter (increased mean diffusivity) and the loss of integrity of white matter fibre bundles (decreased fractional anisotropy). Tractography software enables the identification of directional fibre bundles such as subregions of the corpus callosum (shown). c ∣ Functional MRI (fMRI) uses blood-oxygen-level dependent (BOLD) contrast or perfusion measurements to assess the functional activation of cortical and subcortical regions during the performance of cognitive or sensorimotor tasks in the scanner. Bilateral frontal and parietal activation can be seen, representing the mean activation observed in a group of healthy individuals performing a working memory task. d ∣ Positron emission tomography (PET) using the fluorodeoxyglucose (FDG) radiotracer provides a measure of neuronal metabolism. Other applications of molecular imaging methods such as PET provide data on cerebral blood flow or specific neurotransmitter–receptor systems. Most of these methods have not yet been examined in systematic, prospective studies of chemotherapy-induced or cancer-associated cognitive changes, but these approaches hold promise for identifying the neural bases of such changes.
Interestingly, several studies have found a higher than expected incidence of cognitive problems in cancer patients before the initiation of chemotherapy22-25, and the diagnosis of cancer has been proposed as a potential risk factor for cognitive impairment and Alzheimers disease in the elderly26. These data suggest that cognitive changes associated with chemotherapy need to be examined within the broader context of risk factors and biological processes associated with the development of cancer.
The research so far suggests that performance changes in cognitive functioning can be seen in a subgroup of patients after chemotherapy, and that these changes might be associated with changes in brain structure and function. However, studies that examine the mechanism(s) by which chemotherapy can cause long-term cognitive changes are either in process or are yet to be conducted. When considering mechanisms of chemotherapy-induced cognitive changes, it is important to consider biological pathways that could lead to relatively subtle alterations in cognitive functioning in only a subgroup of people exposed to chemotherapy. The purpose of this Review is to describe candidate mechanisms for chemotherapy-induced cognitive changes (FIG. 2) and potential common risk factors for the development of cancer and cognitive problems. For each of the candidate mechanisms, genetic polymorphisms will be described that might account, in part, for variation in the cognitive effects of chemotherapy (TABLE 1). Unfortunately, data directly supporting the proposed mechanisms are limited; therefore, the goal of this Review is to present mechanisms that lead to logical hypotheses that can be tested empirically. Furthermore, although the focus of the Review is on chemotherapy-associated changes in cognitive function, much of the discussion might be relevant to other treatment modalities (such as radiation therapy, particularly in combination with chemotherapy) and other treatment-related toxicities (for example, peripheral neuropathy).
Figure 2. Candidate mechanisms.
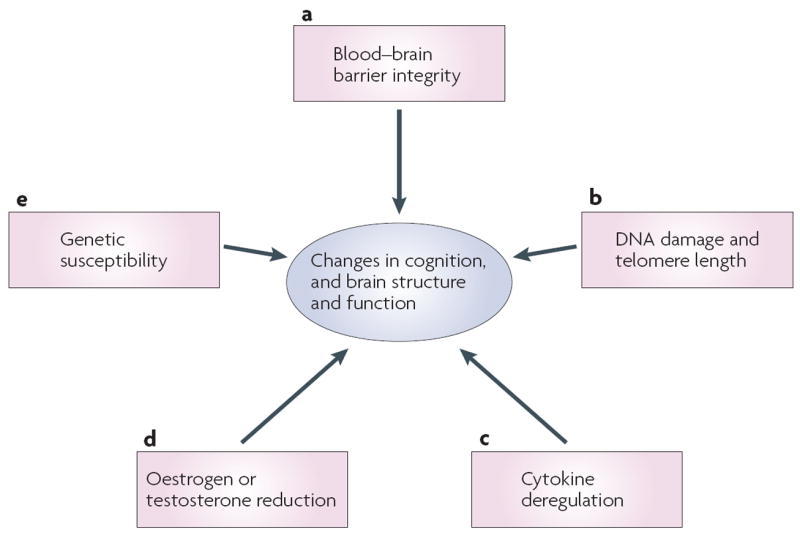
Several candidate mechanisms for chemotherapy-associated changes in cognitive function and brain structure and function are proposed. a ∣ Although common chemotherapeutic agents do not readily cross the blood–brain barrier, recent data from animal studies suggest that even small doses of chemotherapy can cause cell death and reduce cell division in structures relevant for cognition. b ∣ Chemotherapy has been associated with DNA damage and telomere shortening, both of which have been implicated in neural degeneration and the development of neurodegenerative disorders with cognitive components (for example, Alzheimers disease and mild cognitive impairment). c ∣ Chemotherapy-induced cognitive changes might be associated with neurotoxic effects of inflammation and cytokine deregulation. d ∣ Oestrogen and testosterone levels can be reduced secondary to chemotherapy and as a result of hormonal treatments for cancer. Reduction in hormonal levels has been associated with cognitive decline. e ∣ Genetic variability in blood–brain barrier transporters, DNA-repair mechanisms, rate of telomere shortening, cytokine regulation, neuronal repair and plasticity, and neurotransmission could all increase vulnerability to cognitive changes associated with chemotherapy.
Table 1.
Candidate mechanisms for chemotherapy-induced cognitive changes
| Mechanism | Prediction | Findings relevant to cognition in cancer | Candidate genes* | Refs |
|---|---|---|---|---|
| Blood–brain barrier |
|
|
32-34 | |
| DNA damage |
|
|
38,55 | |
| Telomere length |
|
|
57 | |
| Cytokine regulation |
|
|
|
23,66,70,71,79 |
| Neural repair |
|
|
|
80,86,89 |
| Neurotransmitters |
|
|
|
89,93,96 |
Examples of candidate genes are provided. See the references for a more extensive list.
Chemotherapy and the blood–brain barrier
It has generally been assumed that most chemotherapeutic agents do not cross the blood–brain barrier (exceptions include methotrexate and 5-fluorouracil). However, recent reviews have suggested that nearly all frequently used chemotherapeutic agents can cause disorders of the central nervous system (CNS), including encephalopathy, leukoencephalopathy, ototoxicity and cerebellar symptoms, although these are fairly uncommon27,28. In addition, studies using positron emission tomography (PET) have shown that detectable levels of radiolabelled cisplatin29, BCNU (1,3-bis (2-chloroethyl)-1-nitrosourea, also known as carmustine)30 and paclitaxel31 were found in the brain after intravenous administration. The levels of these agents in the brain are low, which might explain why the intravenous administration of chemotherapeutic agents is not usually highly effective in the treatment of brain tumours. However, an unanswered question is whether the level of drug in the brain that could influence cognitive function might be lower than the dose needed to be efficacious for the treatment of tumours.
Related to the last question, Dietrich and colleagues32 administered the commonly used chemotherapy agents BCNU, cisplatin and cytosine arabinoside (cytarabine) systemically to mice. These chemotherapy agents were associated with increased cell death and decreased cell division in the subventricular zone, the dentate gyrus of the hippocampus and the corpus callosum (areas important for normal cognitive functioning) in animals that received treatment; in some cases this pattern persisted for weeks after the discontinuation of the agents. Neural progenitor cells and oligodendrocytes were particularly vulnerable. Importantly, these results were seen at lower doses than were required to cause tumour cell death.
Another factor that might influence the dose of chemotherapy that reaches the CNS is genetic variability in drug transporters at the blood–brain barrier. The gene multidrug resistance 1 (MDR1) encodes the protein P-glycoprotein (P-gp), the role of which is to protect cells from toxic substances by transporting them out of the cell. P-gp is expressed in the capillary endothelial cells in the brain. At the blood–brain barrier, P-gp influences the amount of drug uptake into the brain. Most commonly used chemotherapeutic agents are substrates of P-gp; therefore, the levels and functionality of P-gp at the blood–brain barrier can influence the level of a chemotherapeutic agent in the brain. For the most part, P-gp serves to protect the brain by transporting chemotherapy agents out of the brain. Several polymorphisms of the MDR1 gene have been identified33 that might influence P-gp function. One of the most studied polymorphisms is C3435T in exon 26. People with the T allele have lower levels of P-gp (less efficient efflux); therefore, a higher concentration of a given drug that enters the cell or organ remains because less is pumped out. Studies have shown higher plasma concentrations of several drugs in people homozygous for the T allele compared with people homozygous for the C allele34,35, and animal studies have shown that mice deficient in P-gp have higher concentrations of the chemotherapy agent vincristine36 and steroid hormones in the brain after peripheral administration37. Although studies have not examined the direct influence of MDR1 polymorphisms on concentrations of cytotoxic agents given intravenously to patients, it is possible that such genetic variability might influence the dose of drug delivered to the brain. In our own research, we are examining whether patients treated with chemotherapy who have polymorphisms associated with lower expression or functionality of P-gp are more vulnerable to long-term cognitive side effects, presumably because they are exposed to higher doses of the chemotherapeutic agents. In addition, PET studies using radiolabelled chemotherapeutic agents, such as those described above, could compare concentrations of these agents in the brain across patients with different polymorphisms of MDR1 or other transporter genes (for example, those that encode organic anion transport proteins)35.
Many questions remain about the direct effect of chemotherapy on the brain, and the relationship between the doses of drug delivered to the brain and cognitive functioning. Given the current state of knowledge, it seems unlikely that a direct mechanism for chemotherapy-induced cognitive decline is the only or even the primary mechanism. Therefore, it is important to look for other potential mechanisms for these changes.
DNA damage, oxidative stress and cognition
The integrity of the human genome is crucial for the normal functioning of all biological systems, including the brain38. Mistakes during DNA replication and damage to DNA are common, and elaborate mechanisms exist for base-excision, nucleotide-excision, mismatch and double-strand-break repair39. Oxidative stress is a common source of DNA damage that causes single- or double-strand breaks40,41, and is probably the most frequent cause of DNA damage in neuronal cells42. Oxidative stress occurs through exposure to exogenous toxins, as well as through endogenous mechanisms secondary to normal cellular metabolism41,42. Many chemotherapy agents (for example, alkylating agents) commonly used for the treatment of cancer achieve their therapeutic efficacy, in part, through DNA damage27,43 leading to tumour cell apoptosis. Although the desired goal is to eliminate tumour cells, DNA in normal cells is also affected, resulting in many of the side effects of chemotherapy. Studies have found evidence for oxidative DNA damage in peripheral blood lymphocytes after chemotherapy for breast cancer44,45, and an increased number of point mutations in mitochondrial DNA in patients with various cancer diagnoses treated with chemotherapy with or without radiation therapy46. Furthermore, chemotherapy has been associated with increased levels of non-protein-bound iron47 and free radicals48, and reduced antioxidant capacity48-50, all of which can increase oxidative stress and DNA damage. Therefore, a logical candidate mechanism for chemotherapy-induced cognitive changes is DNA damage affecting the CNS42.
Several lines of evidence have linked DNA damage and repair systems to neurodegeneration. Clinical syndromes such as ataxia telangiectasia and xeroderma pigmentosum are caused by genetic defects in DNA-damage response systems, and are characterized by significant neurological deficits, including cognitive deficits35. Furthermore, oxidative DNA damage has been associated with various neurodegenerative disorders, including Alzheimers disease and Parkinsons disease, that are characterized by significant cognitive symptoms51. Perhaps most relevant for this discussion, elderly patients diagnosed with mild cognitive impairment (a condition characterized by isolated memory deficits without a general loss of function and associated with an increased risk of Alzheimers disease) have been shown to have higher levels of oxidative DNA damage in peripheral leukocytes52 and in the brain on autopsy53. These data suggest that links exist between DNA damage and cognitive problems; therefore, one obvious question is whether cancer patients who show cognitive problems also have higher baseline levels of DNA damage42. So far, no studies have related cognitive functioning to the level of DNA damage after treatment with chemotherapy.
The mechanism(s) by which DNA damage leads to damage in the nervous system is not well understood. The CNS might be vulnerable to increases in oxidative stress because of high endogenous metabolic rates and the production of reactive oxygen species (ROS). One proposed mechanism is the production of defective proteins that eventually lead to neuronal apoptosis. Harrison and colleagues54 showed that a decreased capacity to repair mitochondrial DNA damage was associated with increased apoptosis in neuronal cell cultures. Alternatively, DNA damage might block transcription and cause the loss of essential gene products40. One issue in much of the research that associates DNA damage with neurodegeneration or neurocognitive problems in humans is that DNA damage is assessed in peripheral leukocytes with the assumption that these measurements reflect systemic levels of DNA damage, including in the CNS. In support of this assumption, post-mortem studies have also identified higher levels of DNA damage in the brains of patients with mild cognitive impairment and Alzheimers disease53; however, this issue requires further investigation.
Interestingly, studies have found higher than expected rates of cognitive impairment in cancer patients before the initiation of chemotherapy22-25. In addition, higher levels of oxidative DNA damage have been found in women with breast cancer44,45, and higher levels of oxidative stress were found in dogs with lymphoma55 before the beginning of treatment. These data are consistent with research linking certain DNA-repair polymorphisms associated with a decreased capacity to repair damaged DNA to an increased risk of cancer (for reviews see REFS 39,56). Finally, patients with ataxia telangiectasia and xeroderma pigmentosum are also at a significantly increased risk of developing cancer35. These data raise the possibility that deficits in DNA-repair mechanisms might be a common risk factor for both cancer and neurodegenerative disorders that include cognitive symptoms. Consequently, changes in cognitive function following chemotherapy need to be evaluated within the context of genetic factors that increase the risk of cancer but might also increase the risk of cognitive dysfunction before treatment.
Chemotherapy might also increase cognitive problems through its affect on telomere length. During the DNA replication process, telomeres shorten by 20–200 base pairs per replication. The shortening of telomeres to a crucial point leads to cell senescence and apoptosis, and has been associated with ageing, Alzheimers disease severity, cancer predisposition and mortality of people over 60 years of age57. Several factors influence the rate of telomere shortening, including genetic variation58, oxidative stress59 and chemotherapy59-61. Schroder and colleagues59 reported that standard-dose and high-dose chemotherapy in patients with breast cancer resulted in telomere shortening in leukocytes for most patients, although a minority of patients showed telomere lengthening after treatment. Similarly, high-dose chemotherapy regimens given to patients with haematological malignancies who are undergoing allogeneic stem-cell transplantation have been associated with telomere shortening in haematopoietic stem cells60. Most neuronal cells are post-mitotic; however, certain cells, such as glia in the CNS, remain mitotic and are susceptible to telomere shortening62. Consequently, chemotherapy might have a long-term effect on cognition by accelerating the ageing process, either through the general effect of telomere shortening on biological systems and genomic stability or through the direct effect on telomere length in mitotic cells in the CNS61.
This body of research suggests that both chemotherapy and factors that increase cancer risk might be important in understanding why a subgroup of patients experience long-term chemotherapy-induced cognitive problems. Specifically, people with deficiencies in DNA-repair mechanisms will probably have more DNA damage and shorter telomeres at the time of cancer diagnosis, related to their lifetime accumulation of endogenous and exogenous toxic exposures leading to DNA damage. Chemotherapy is a major cause of systemic DNA damage from which patients with inefficient repair mechanisms will have a more difficult time recovering. Consequently, one could predict that poorer cognitive functioning would be seen in patients with the most DNA damage before and after chemotherapy. As a first step in evaluating this hypothesis, our group is examining the relationships between DNA-repair polymorphisms and cognitive functioning within the context of two longitudinal studies of the effect of chemotherapy on cognitive function in patients with breast cancer and lymphoma. We are particularly focusing on polymorphisms in the base-excision repair pathway because of their importance in modulating oxidative stress42 and the risk of cancer56.
Cytokines and cognition
In addition to their role in the regulation of inflammation, cytokines have important roles in normal CNS function, including the modulation of neuronal and glial cell functioning, neural repair and the metabolism of dopamine and serotonin, both of which are neurotransmitters important for normal cognitive function63. However, the deregulation of cytokine activity has been associated with neurotoxicity and various neurodegenerative disorders, including Alzheimers disease, multiple sclerosis and Parkinsons disease64. In addition, cytokine-induced sickness behaviour is associated with cognitive disturbance, fatigue and depression65,66. Animal and human studies that have used various methods for stimulating a cytokine response (for example, Escherichia coli endotoxin exposure) have shown an association between the deregulation of cytokines and deficits in cognitive performance67-69. For example, Krabbe and colleagues69 reported an inverse association between levels of circulating interleukin 6 (IL6) and memory functioning following the administration of low-dose E. coli endotoxin in healthy volunteers. Other cytokines that have been studied in relation to cognitive function include IL1, IL2, IL10 and tumour necrosis factor-α (TNFα).
For many years, researchers had assumed that the brain was minimally influenced by changes in the peripheral immune system. However, recent research has shown that there is significant communication between cytokines outside the CNS and cytokines in the brain and spinal fluid through various mechanisms, including transport into the brain across the blood–brain barrier or by peripheral cytokines stimulating the release of central cytokines by communication through the vagus nerve 63, 66.
In cancer patients, the relationship between cytokines and cognitive function is most clearly seen in the neuropsychological changes seen in patients who have received immunotherapies such as IL2 or interferon-α. Symptoms associated with immunotherapy include depression, weakness, fatigue and cognitive disruption70. Longitudinal studies of interferon-α and IL2 treatments in cancer populations have shown decrements in cognitive performance, particularly in the domains of information processing speed, executive function, spatial ability and reaction time71,72 (BOX 1). These cognitive changes were independent of symptoms of depression that were also associated with the treatments.
Although not extensively studied, there is evidence that standard-dose chemotherapy is associated with increases in cytokine levels. Specifically, paclitaxel and docetaxel have been associated with increased levels of IL6, IL8 and IL10 (REFS 73-75). The studies cited above examined the acute effects of chemotherapy on cytokine levels; however, there are no direct data examining the associations between chemotherapy-induced cognitive changes and cytokine deregulation in long-term cancer survivors. However, in a related area, breast cancer survivors who experience persistent fatigue two years or more after chemotherapy have been shown to have elevations in several of the same cytokines described above76,77.
Studies have supported the association between inflammation and an increased risk of developing several types of cancer, including bladder, cervical and ovarian78,79. In addition, cytokines have been shown to be increased in cancer patients before treatment. In patients with advanced breast cancer, IL6 and TNFα were increased compared with healthy controls73. Meyers et al.23 studied patients with acute myeloid leukaemia or myelodysplastic syndrome, and found increased levels of IL1, IL1 receptor antagonist (IL1RA), IL6, IL8 and TNFα compared with normal values before treatment. Furthermore, higher levels of IL6 were associated with poorer performance on measures of executive function before treatment. Unfortunately, the number of patients available for post-treatment neuropsychological evaluation was too small to examine the relationships among chemotherapy, cytokine levels and cognitive performance. These data suggest that cytokine deregulation might be related both to the development of cancer and cognitive problems before treatment.
Genetic polymorphisms have been identified that influence cytokine activity and have been associated with disorders such as Alzheimers disease and depression64,80. However, so far no studies have examined the relationships between these polymorphisms and chemotherapy-induced cognitive changes. Within the context of the genetic component of the longitudinal study of the cognitive side effects of chemotherapy described above, we are also examining various polymorphisms related to cytokine activity.
Cognitive changes associated with cytokine deregulation are probably associated with both direct and indirect mechanisms. Neuronal damage secondary to cytokine exposure can be caused by various mechanisms including excitotoxic glutamate receptor-mediated damage and oxidative stress63. Indirect mechanisms include cytokine-induced appetite reduction leading to micronutrient deficiency, and impaired sleep regulation63.
These data raise the possibility that the neurotoxic effects of cytokines triggered during the development of cancer and/or in association with chemotherapy might contribute to the development of post-treatment cognitive problems. Furthermore, cytokines might be triggered in response to DNA damage, which could set up a cycle of increasing DNA damage and cytokine activity38, and chronic inflammation can increase oxidative stress63, which might also contribute to this cycle. Therefore, understanding the independent and interactive effects of DNA damage, inflammation and cytokine deregulation might be important for a complete understanding of chemotherapy-induced cognitive changes.
Genetics of neural repair
Apolipoprotein E (APOE) is a complex glycolipo-protein that facilitates the uptake, transport and distribution of lipids. It seems to have an important role in neuronal repair and plasticity after injury81. A four-exon gene encodes APOE on chromosome 19 in humans. There are three main alleles: E2, E3 and E4. These alleles differ in amino acids at positions 112 and 158: E2 (cysteine–cysteine), E3 (cysteine–arginine) and E4 (arginine–arginine). Animal models suggest a link between the E4 allele and increased mortality, extent of damage and poor repair following brain trauma82-84. The human E4 allele has been associated with various disorders with prominent cognitive dysfunction, including otherwise normal patients with memory complaints, Alzheimers disease and poor outcomes in stroke and traumatic brain injury85,86. Our group evaluated the relationship of the APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard-dose chemotherapy. The results showed that survivors with at least one E4 allele scored significantly lower in the visual memory and spatial ability domains, with a trend to score lower in executive functioning compared with survivors who did not carry an E4 allele87.
The mechanism by which the E4 allele exerts its negative effect is unclear. Rather than an active detrimental effect, it might be that the E4 allele of APOE is less effective than the E3 allele in promoting neuronal repair and neuritic growth and branching. However, data also suggest that E4 carriers, compared with people with other forms of APOE, might have morphological differences in the brain, specifically, lower hippocampal volume (an indication of a reduced functional capacity of the hippocampus, which is important for memory functioning88).
Brain-derived neurotrophic factor (BDNF) is expressed in the brain, particularly in the prefrontal cortex and hippocampus, and has been associated with neuronal repair and survival, dendritic and axonal growth, and long-term potentiation89,90. A functional polymorphism of BDNF is associated with a valine-to-methionine amino-acid substitution at codon 66. The methionine allele has been associated with poorer performance on measures of memory and executive function and lower hippocampal volume91-93 in non-cancer populations. No chemotherapy studies have examined the relationship of BDNF polymorphisms to chemotherapy-induced cognitive changes; however, this seems to be another important target for investigation90.
Genetics of neurotransmission
Polymorphisms in neurotransmitters that affect their activity have also been linked to cognitive performance81. A polymorphism in catechol-O-methyltransferase (COMT) is one of the most commonly studied polymorphisms, and is so far the only polymorphism to be linked to the cognitive side effects of chemotherapy (see REFS 81,90,94 for potential candidate alleles that affect the activity of other neurotransmitters). COMT catalyses the metabolic breakdown of catecholamines through the methylation of dopamine and noradrenaline. A common functional polymorphism of COMT, characterized by a single nucleotide change from G to A at position 472, changes a valine to a methionine. The effect of this amino-acid substitution is a significant change in the efficiency of COMT enzymatic activity95. COMT with the valine allele is almost four times as active as that with the methionine allele. Therefore, individuals homozygous for the valine allele presumably metabolize dopamine much more rapidly than those with the methionine allele. COMT is an important modulator of the amount of dopamine in the frontal cortex, accounting for ~60% of the metabolic degradation of dopamine96. Dopamine is important for executive and memory functioning mediated by the frontal cortex, and research has shown that decreased dopamine levels associated with the valine allele of COMT are associated with poor performance on various measures of cognitive functioning90 in non-cancer populations. Analyses of pilot data from our long-term survivor cohort showed that breast cancer and lymphoma survivors who had been treated with chemotherapy and carried the COMT valine allele scored significantly lower on measures of verbal memory and spatial ability compared with survivors who were homozygous for the methionine allele (T.A.A. and A.J.S., unpublished data).
Oestrogen and testosterone
The influence of low levels of oestrogen and testosterone on cognitive functioning should be briefly mentioned within the context of chemotherapy-induced cognitive changes because chemotherapy can induce early, abrupt menopause in women. Reduction of oestrogen associated with natural menopause has been associated with changes in cognitive functioning, particularly working memory; therefore, although not well studied, one would assume that chemotherapy-induced menopause would have similar effects97. In addition, certain treatments for cancer that reduce levels of these hormones (tamoxifen and aromatase inhibitors for breast cancer, and androgen ablation in prostate cancer) are often given in combination with chemotherapy or as single agents. Initial evidence suggests that these treatments have a detrimental effect on cognitive functioning even when given without chemotherapy98,99. Research also supports the neuroprotective and antioxidant effects of both oestrogen100 and testosterone101, and the importance of oestrogen for maintaining telomere length102. Therefore, reduced oestrogen or testosterone might have an independent effect on cognitive function or might interact with chemotherapy through a reduction of antioxidant capacity or the ability to maintain telomere length.
Conclusions and future directions
Despite increasing research in this area, the mechanisms by which chemotherapy-induced cognitive changes occur remain largely unknown. However, several potentially important candidate mechanisms have been identified that can be evaluated in future research. We suggest that there might be shared genetic factors for the development of cancer and cognitive problems. These include low-efficiency efflux pumps that lead to increased exposure to toxins in the brain, deficits in DNA-repair mechanisms that lead to greater DNA damage, and deregulation of the immune response. Cancer patients with one or more of these risk factors might show cognitive deficits at diagnosis, and are probably the most vulnerable to cognitive side effects of chemotherapy. Exposure to chemotherapy causes DNA damage and telomere shortening in healthy cells and tumour cells, and triggers an immunological response, both of which can have neurotoxic effects. Furthermore, in certain patients, DNA damage and cytokine release could interact to create a cycle of chronic inflammation and DNA damage that is sustained after chemotherapy is discontinued. Reduced capacity for neural repair (for example through polymorphisms in APOE or BDNF) and neurotransmitter activity (for example by polymorphisms in COMT), as well as reduced antioxidant capacity associated with reduction in oestrogen and testosterone levels, might exacerbate this process and/or have independent effects on cognitive function.
Two additional mechanisms deserve to be mentioned, even though they have not been directly studied in relation to chemotherapy-induced cognitive changes. First, many chemotherapy agents are cardiotoxic; therefore, cognitive changes could be secondary to cardiovascular changes that could influence cerebrovascular function103. Second, alterations in the neuroendocrine system have been associated with cognitive deficits104. Corticosteroids, which are known to alter neuroendocrine functioning and cognition, are commonly used as part of chemotherapy regimens or to manage side effects such as nausea105.
The tools for evaluating the candidate mechanisms proposed are, for the most part, currently available. As outlined at the 2003 Banff consensus conference on cognitive side effects of chemotherapy (BOX 2), large scale13, longitudinal studies of various patient groups who are evaluated before receiving chemotherapy and followed after chemotherapy with measures of neuropsychological functioning, brain structure and function (MRI and PET), genetic profiles and biomarkers relevant for candidate mechanisms (for example, DNA damage and immune response) are necessary to identify a large enough subgroup of patients who experience persistent cognitive problems secondary to chemotherapy. Ideally, comparison groups of patients with the same diagnoses who are treated with non-chemotherapy-based regimens and/or healthy controls will be evaluated with the same measures at analogous time periods. However, because investigators have proposed that chemotherapy might accelerate the ageing process61, cross-sectional studies of long-term survivors (for example, five or more years after treatment) who have been treated with chemotherapy compared with well-matched groups of patients not treated with chemotherapy and healthy controls might be necessary to examine the relationships among variables such as cognitive performance, DNA damage and/or telomere shortening, genetic profile and imaging-based measures of brain function. Finally, animal models of chemotherapy-induced cognitive changes, which are currently under development, will improve the ability of researchers to examine mechanistic questions106,107.
Box 2.
Recommendations from the Banff conference13
Conduct longitudinal studies that include pre- and post-treatment neuropsychological assessments that enable the evaluation of acute and longterm cognitive changes, and that include appropriate comparison groups such as cancer patients not treated with chemotherapy and/or matched healthy controls.
Design studies that examine factors that increase the risk of cognitive changes (for example, genetic factors) and define the mechanism(s) that underlies chemotherapy-induced cognitive changes.
Use advanced imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) to define structural and functional changes in the brain associated with chemotherapy.
Develop animal models that will aid in identifying the mechanism(s) of chemotherapy-induced cognitive changes.
Identify the neuropsychological tests that are the most sensitive to the cognitive side effects of chemotherapy and develop new tests that are more closely related to cognitive functioning in the real world.
Develop and evaluate medication and cognitive rehabilitation interventions.
In summary, several candidate mechanisms for chemotherapy-induced cognitive changes have been proposed, and it is likely that there are several pathways to cognitive decline depending on the treatment regimens and the particular vulnerabilities of the individual. Given that only some individuals seem to experience long-term cognitive changes following chemotherapy, it might be that several interacting mechanisms are necessary to produce changes in biological systems that translate into changes in cognitive ability.
At a glance.
Evidence for chemotherapy-induced cognitive changes comes from studies that have used neuropsychological testing, imaging (structural and functional magnetic resonance imaging (MRI) and positron emission tomography (PET)) and electrophysiological (electroencephalogram) assessments. Emerging data from animal studies also support the effect of chemotherapy on cognitive function.
Most chemotherapy agents administered systemically do not cross the blood–brain barrier in significant doses; however, the amount that enters the brain can be modified by genetic variability in blood–brain barrier transporters. In addition, recent data from animal studies suggest that very small doses of common chemotherapy agents can cause cell death and reduced cell division in brain structures crucial for cognition, even at doses that do not effectively kill tumour cells.
Chemotherapy might cause cognitive changes through DNA damage caused directly by the cytotoxic agents or through increases in oxidative stress. Many chemotherapeutic agents also cause the shortening of telomeres, thereby accelerating cell ageing. Genetic variability in DNA-repair genes might influence the extent of, and recovery from, chemotherapy-associated DNA damage.
Chemotherapy-induced cognitive changes might also be related to the neurotoxic effects of cytokine deregulation. Cytokine deregulation and inflammation can also lead to increased oxidative stress, which could establish a cycle of increased DNA damage that triggers additional cytokine release.
Variability in genes that regulate neural repair and/or plasticity, such as apolipoprotein E (APOE) and brain-derived neurotrophic factor (BDNF), and neurotransmission, such as catechol-O-methyltransferase (COMT), might increase the vulnerability of an individual to chemotherapy-induced cognitive changes.
Changes in levels of oestrogen and testosterone are associated with cognitive decline, and can be influenced by chemotherapy (for example, chemotherapy-induced menopause) or hormonal treatments, such as tamoxifen or aromatase inhibitors for breast cancer or androgen ablation for prostate cancer.
The effects of chemotherapy-associated cardiovascular toxicity and alterations in neuroendocrine function on cognitive function require investigation.
Acknowledgments
The authors are supported by grants from the Office of Cancer Survivorship of the US National Cancer Institute and a National Institutes of Health Roadmap U54 Grant.
Glossary
- Magnetic resonance imaging
A noninvasive technique that produces high-resolution, computerized images of internal body tissues. Structural MRI enables abnormalities of brain structure to be evaluated and volumetric measurements of specific structures to be made. Functional MRI enables activation patterns in various brain areas in response to the performance of cognitive or motor tasks to be examined.
- P-300
The P-300 is a neural-evoked potential component of the EEG. The P-300 is an event-related potential that is triggered approximately 300 milliseconds after the presentation of an unexpected or novel stimulus.
- Encephalopathy
Encephalopathy refers to alterations in brain structure and/or function that can have several causes, including infection, exposure to toxic chemicals (for example, chemotherapy), poor nutrition or lack of oxygen or blood flow to the brain. The primary symptoms of encephalopathy are alterations in mental status.
- Leukoencephalopathy
Alterations of the white matter of the brain owing to infection or exposure to toxic chemicals.
- Ototoxicity
Toxicity associated with organs or nerves involved with hearing or balance.
- Cerebellar symptoms
The cerebellum is an area of the brain that is important for the integration of sensory input and motor output. Disorders of the cerebellum include symptoms associated with equilibrium, posture, motor learning and fine motor control.
- Positron emission tomography
A nuclear medicine imaging technique that produces a three-dimensional image of functional or metabolic processes in the body by scanning for a radioactive isotope (for example, a radiolabelled chemotherapy agent) that has been injected into the bloodstream.
- Ataxia telangiectasia
A disorder caused by a mutation of the ataxia telangiectasia mutated (ATM) gene, which is important for DNA repair. Ataxia telangiectasia causes progressive immunological and neurological problems, including cognitive symptoms, and people with ataxia telangiectasia have a significantly increased risk of cancer.
- Xeroderma pigmentosum
A genetic DNA-repair disorder in which the body is unable to repair DNA damage or mutations caused by ultraviolet light.
- Glia
A group of non-neuronal cells in the brain that provide support and nutrition, form myelin and influence signal transmission in the nervous system.
- Sickness behaviour
Physiological response to infection that includes symptoms such as decreased activity level, fatigue, decreased motivation and cognitive problems.
- Vagus nerve
The vagus nerve is the only cranial nerve that extends from the brainstem to all the organs in the abdomen.
- Excitotoxicity
The process by which neurons are damaged or killed through the over-activation of receptors for the excitatory neurotransmitter glutamate.
- Long-term potentiation
Long-lasting increase in the functioning of a synapse, which is thought to be related to neural plasticity and the cellular basis for learning and memory.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Silberfarb PM. Chemotherapy and cognitive defects in cancer patients. Annu Rev Med. 1983;34:35–46. doi: 10.1146/annurev.me.34.020183.000343. [DOI] [PubMed] [Google Scholar]
- 2.Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology. 1995;4:61–66. [Google Scholar]
- 3.van Dam FS, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. comment. [DOI] [PubMed] [Google Scholar]
- 4.Schagen SB, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Brezden CB, et al. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, et al. Neuropsychological impact of standard-dose chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 7.Tchen M, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 8.Castellon SA, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 10.Schilling V, et al. The effects of adjuvant chemotherapy on cognition in women with breast cancer — preliminary results of an observational study. The Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins V, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan KA, et al. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early stage breast carcinoma. Cancer. 2005;104:2499–2507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannock IF, Ahles TA, Ganz PA, van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. This paper provides an overview of a consensus conference that included most of the researchers from around the world who were conducting research on chemotherapy-induced cognitive changes. [DOI] [PubMed] [Google Scholar]
- 14.Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3(Suppl):S84–S90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson RJ, Ahles TA. Low neuropsychologic performance among adult cancer survivors treated with chemotherapy. Curr Neurol Neurosci Rep. 2003;3:215–222. doi: 10.1007/s11910-003-0081-2. [DOI] [PubMed] [Google Scholar]
- 16.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VV, Compas BE. Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 17.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological and neuroimaging perspectives. Sem Clin Neuropsych. 2003;8:201–216. [PubMed] [Google Scholar]
- 18.Stemmer S, et al. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. Am J Neuroradiol. 1994;15:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 19.Saykin AJ, et al. Altered brain activation following systemic chemotherapy for breast cancer: interim analysis from a prospective study. J Int Neuropsychol Soc. 2006;12:131. [Google Scholar]
- 20.Kreukels BP, et al. Electrophysiological correlates of information processing in breast-cancer patients treated with chemotherapy. Breast Cancer Res Treat. 2005;94:53–61. doi: 10.1007/s10549-005-7093-3. [DOI] [PubMed] [Google Scholar]
- 21.Kreukels BP, et al. Effects of high-dose and conventional-dose adjuvant chemotherapy on long-term cognitive sequelae in patients with breast cancer: an electrophysiologic study. Clin Breast Cancer. 2006;7:67–78. doi: 10.3816/CBC.2006.n.015. [DOI] [PubMed] [Google Scholar]
- 22.Wefel JS, et al. Chemobrain in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 23.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 24.Ahles TA, et al. Psychological and neuropsychological functioning of patients with limited small-cell lung cancer treated with chemotherapy and radiation therapy with or without warfarin, a study for Cancer and Leukemia Group B. J Clin Oncol. 1998;16:1954–1960. doi: 10.1200/JCO.1998.16.5.1954. [DOI] [PubMed] [Google Scholar]
- 25.Wagner LI, et al. Trajectory of cognitive impairment during breast cancer treatment: a prospective analysis. J Clin Oncol Suppl. 2006;24:8500. [Google Scholar]
- 26.Heflin LH, et al. Cancer as a risk factor for long-term cognitive deficits and dementia. J Natl Cancer Inst. 2005;97:854–856. doi: 10.1093/jnci/dji137. [DOI] [PubMed] [Google Scholar]
- 27.Verstappen CCP, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: Clinical signs and optimal management. Drugs. 2003;63:1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Troy L, et al. Cisplatin-based therapy: A neurological and neuropsychological review. Psychooncology. 2000;9:29–39. doi: 10.1002/(sici)1099-1611(200001/02)9:1<29::aid-pon428>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Ginos JZ, et al. [13N]cisplatin PET to assess pharmacokinetics of intro-arterial versus intravenous chemotherapy for malignant brain tumors. J Nucl Med. 1987;28:1844–1852. [PubMed] [Google Scholar]
- 30.Mitsuki S, et al. Pharmacokinetics of 11C-labelled BCNU and SARCNU in gliomas studied by PET. J Neurooncol. 1991;10:47–55. doi: 10.1007/BF00151246. [DOI] [PubMed] [Google Scholar]
- 31.Gangloff A, et al. Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with 18F-Fluoropaclitaxel. J Nucl Med. 2005;46:1866–1871. [PubMed] [Google Scholar]
- 32.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. Epub ahead of print, This is an excellent paper describing in vitro and in vivo (mice) studies showing that common chemotherapy agents caused increased cell death and decreased cell division in the subventricular zone and in the dentate gyrus of the hippocampus, and in the corpus callosum. These effects were seen with doses that were not effective in causing cell death in tumor cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamroziak K, Robak T. Pharmacogenomics of MDR1/ABCB1 gene: the influence on risk and clinical outcome of haematological malignancies. Hematology. 2004;9:91–105. doi: 10.1080/10245330310001638974. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmeyer S, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006;234:4–33. doi: 10.1016/j.canlet.2005.06.051. This manuscript describes evidence for genetic variability in drug transporters and their influence on drug disposition and clinical response. [DOI] [PubMed] [Google Scholar]
- 36.Muramatsu T, et al. Age-related differences in vincristine toxicity and biodistribution in wild-type and transporter-deficient mice. Oncol Res. 2004;14:331–343. doi: 10.3727/0965040041292387. [DOI] [PubMed] [Google Scholar]
- 37.Uhr M, Holsboer F, Muller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b p-glycoproteins. J Neuroendocrinol. 2002;14:753–759. doi: 10.1046/j.1365-2826.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- 38.Rolig RL, McKinnon PJ. Linking DNA damage and neurodegeneration. Trends Neurosci. 2000;23:417–424. doi: 10.1016/s0166-2236(00)01625-8. This paper provides an overview of the evidence linking DNA damage to neurodegeneration and cognitive function. [DOI] [PubMed] [Google Scholar]
- 39.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 40.Caldecott KW. DNA single-strand breaks and neurodegeneration. DNA Repair. 2004;3:875–882. doi: 10.1016/j.dnarep.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Abner CW, McKinnon PJ. The DNA double-strand break response in the nervous system. DNA Repair. 2004;3:1141–1147. doi: 10.1016/j.dnarep.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutat Res. 2007;614:24–36. doi: 10.1016/j.mrfmmm.2006.06.007. This paper reviews the relevance of DNA-repair pathways to DNA damage in post-mitotic neurons, and the impact of DNA damage on neuronal survival and brain ageing. Additionally, these authors relate DNA repair to neurotoxicity associated with chemotherapy, including cognitive side effects and peripheral neuropathy. [DOI] [PubMed] [Google Scholar]
- 43.Sedletska Y, Giraud-Panis M-J, Malinge J-M. Cisplatin is a DNA-damaging antitumour coumpound triggering multifactorial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr Med Chem Anticancer Agents. 2005;5:251–265. doi: 10.2174/1568011053765967. [DOI] [PubMed] [Google Scholar]
- 44.Blasiak J, et al. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutat Res. 2004;554:139–148. doi: 10.1016/j.mrfmmm.2004.04.001. Using the comet assay, these investigators showed greater DNA damage and lower DNA repair efficacy in patients with breast cancer, both before and after chemotherapy. [DOI] [PubMed] [Google Scholar]
- 45.Nadin SB, Vargas-Roig LM, Drago G, Ibarra J, Ciocca DR. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in response to chemotherapy. Cancer Lett. 2006;239:84–87. doi: 10.1016/j.canlet.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Wardell TM, et al. Changes in human mitochondrial genome after treatment of malignant disease. Mutat Res. 2003;525:19–27. doi: 10.1016/s0027-5107(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 47.Weijl NI, et al. Non-protein bound iron release during chemotherapy in cancer patients. Clin Sci. 2004;106:475–484. doi: 10.1042/CS20030271. [DOI] [PubMed] [Google Scholar]
- 48.Kaya E, et al. Oxidant/antioxidant parameters and their relationship with chemotherapy in Hodgkin’s lymphoma. J Int Med Res. 2005;33:687–692. doi: 10.1177/147323000503300611. [DOI] [PubMed] [Google Scholar]
- 49.Papageorgiou M, et al. Cancer chemotherapy reduces plasma total antioxidant capacity in children with malignancies. Leukemia Res. 2005;29:11–16. doi: 10.1016/j.leukres.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy DD, Ladas EJ, Rheingold SR, Blumberg J, Kelly KM. Antioxidant status decreases in children with acute lymphoblastic leukemia during the first six months of chemotherapy treatment. Ped Blood Cancer. 2005;44:378–385. doi: 10.1002/pbc.20307. [DOI] [PubMed] [Google Scholar]
- 51.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 52.Migliore L, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26:567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. The results of this study showed that patients with mild cognitive impairment, a condition characterized by a relatively isolated impairment in memory in the context of normal functioning in other areas, had higher levels of DNA damage compared with older adults without mild cognitive impairment. [DOI] [PubMed] [Google Scholar]
- 53.Keller JN, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. This study showed that patients who had been diagnosed with mild cognitive impairment had elevated levels of DNA damage in the brain at autopsy, suggesting a relationship between DNA damage seen peripherally in this population and DNA damage in the CNS. [DOI] [PubMed] [Google Scholar]
- 54.Harrison JF, et al. Oxidative stress-induced apoptosis in neurons correlates with mitcochondrial DNA base exicision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vajdovich P, et al. Redox status in dogs with non-hodgkin lymphomas. An ESR study. Cancer Lett. 2005;224:339–346. doi: 10.1016/j.canlet.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 56.Hung R, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk: A HuGE review. Am J Epidemiol. 2005;162:925–942. doi: 10.1093/aje/kwi318. [DOI] [PubMed] [Google Scholar]
- 57.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 58.Vasa-Nicotera M, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroder CP, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplation. Br J Cancer. 2001;84:1348–1353. doi: 10.1054/bjoc.2001.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lahav M, et al. Nonmyeloablative conditioning does not prevent telomere shortening after allogeneic stem cell transplantation. Transplantation. 2005;80:969–976. doi: 10.1097/01.tp.0000173649.99261.df. [DOI] [PubMed] [Google Scholar]
- 61.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67:212–215. doi: 10.1016/j.mehy.2006.01.045. This manuscript reviews the evidence that morbidity associated with chemotherapy might be related to acceleration of the ageing process. [DOI] [PubMed] [Google Scholar]
- 62.Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- 63.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition — the case for head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 64.Tonelli LH, Postolache TT, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Front Biosci. 2005;10:675–680. doi: 10.2741/1562. [DOI] [PubMed] [Google Scholar]
- 65.Cleeland CS, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 66.Kelley KW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 67.Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatments. Brain Behav Immun. 2003;17:S125–S131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- 68.Reichenberg A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psych. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 69.Krabbe KS, et al. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316–2326. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 71.Scheibel RS, Valentine AD, O’Brien S, Meyers CA. Cognitive dysfunction and depression during treatment with interferon-α and chemotherapy. J Neuropsych Clin Neurosci. 2004;16:185–191. doi: 10.1176/jnp.16.2.185. [DOI] [PubMed] [Google Scholar]
- 72.Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-α treatments in cancer patients. Psychosom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pusztai L, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Penson RT, et al. Chtokines IL-1β, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFα in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 76.Callado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 77.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 78.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 79.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. This paper presents evidence in support of a role for chronic inflammation in enhancing the predisposition to develop cancer. Furthermore, the authors present evidence that genetic polymorphisms that regulate immune function can affect cancer risk. [DOI] [PubMed] [Google Scholar]
- 80.McGeer PL, McGeer EG. Polymorphisms in inflammatory genes and risk of Alzheimer Disease. Arch Neurol. 2001;58:1790–1792. doi: 10.1001/archneur.58.11.1790. [DOI] [PubMed] [Google Scholar]
- 81.Morley KI, Montgomery GW. The genetics of cognitive processes: candidate genes in humans and animals. Behav Genet. 2001;31:511–531. doi: 10.1023/a:1013337209957. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, et al. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997;80:1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 83.Hartman RE, et al. Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer’s disease. J Neurosci. 2002;22:10083–10087. doi: 10.1523/JNEUROSCI.22-23-10083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sabo T, et al. Susceptibility of transgenic mice expressing human apolipoprotein E to closed head injury: the allele E3 is neuroprotective whereas E4 increases fatalities. Neuroscience. 2000;101:879–884. doi: 10.1016/s0306-4522(00)00438-3. [DOI] [PubMed] [Google Scholar]
- 85.Laws SM, et al. APOE-epsilon4 and APOE-491A polymorphisms in individuals with subjective memory loss. Mol Psych. 2002;7:768–775. doi: 10.1038/sj.mp.4001083. [DOI] [PubMed] [Google Scholar]
- 86.Nathoo N, et al. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol. 2003;56:132–136. doi: 10.1136/mp.56.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. This is the first study to show an association between APOE genotype and cognitive functioning in long-term cancer survivors. [DOI] [PubMed] [Google Scholar]
- 88.Lind J, et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E ε4: relation to chronological age and recognition memory. Neurosci Lett. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 89.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and ageing hippocampus: role of secreted proteins tPA and BDNF. Age Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT, and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. This paper provides an excellent review of the research examining the relationship between genetic variability and cognitive functioning with an emphasis on dopamine receptor genes, catechol-O-methyltransferase (COMT) and brain-derived neurotrophic factor (BDNF) [DOI] [PubMed] [Google Scholar]
- 91.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 92.Hariri AR, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pezawas L, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McAllister TW, et al. Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psych Rep. 2004;6:364–371. doi: 10.1007/s11920-004-0023-y. [DOI] [PubMed] [Google Scholar]
- 95.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 96.Malhotra AK, et al. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psych. 2001;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 97.Zec RF, Trivedi MA. The effects of estrogen replacement therapy on neuropsychological functioning in postmenopausal women with and without dementia: a critical and theoretical review. Neuropsychol Rev. 2002;12:65–109. doi: 10.1023/a:1016880127635. [DOI] [PubMed] [Google Scholar]
- 98.Bender DM, Paraska KK, Sereika SM, Ryan CM, Berga SL. Cognitive function and reproductive hormones in adjuvant therapy for breast cancer: a critical review. J Pain Sympt Manage. 2001;21:407–424. doi: 10.1016/s0885-3924(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 99.Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. Br J Urol. 2005;96:48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- 100.Unfer TC, et al. Influence of hormone replacement therapy on blood antioxidant enzymes in menopausal women. Clin Chim Acta. 2006;369:73–77. doi: 10.1016/j.cca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Chisu V, Lepore MG, Zedda M, Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-Nitro-L-styrosine incorporation into α-tubulin in a mouse neuroblastoma cell line. Arch Ital Biol. 2006;144:63–73. [PubMed] [Google Scholar]
- 102.Lee D-C, Im J-A, Kim J-H, Lee H-R, Shim J-Y. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–479. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Theodoulou M, Seidman AD. Cardiac effects of adjuvant therapy for early breast cancer. Semin Oncol. 2003;30:730–739. doi: 10.1053/j.seminoncol.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 104.Miller DB, O’ Callaghan JP. Aging, stress and the hippocampus. Age Res Rev. 2005;4:123–140. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Hukovic N, Brown ES. Effects of prescription corticosteroids on mood and cognition. Adv Psychosom Med. 2003;24:161–167. doi: 10.1159/000073786. [DOI] [PubMed] [Google Scholar]
- 106.Lee GD, et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- 107.Winocur G, Vardy J, Bims MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. These investigators developed an animal model of chemotherapy-induced cognitive deficits and showed deficits in memory and learning tasks in mice treated with chemotherapy that were similar to deficits seen in breast cancer survivors. [DOI] [PubMed] [Google Scholar]


