Abstract
The concept of the excitatory action of GABA during early development is based on data obtained mainly in brain slice recordings. However, in vivo measurements as well as observations made in intact hippocampal preparations indicate that GABA is in fact inhibitory in rodents at early neonatal stages. The apparent excitatory action of GABA seems to stem from cellular injury due to the slicing procedure, which leads to accumulation of intracellular Cl− in injured neurons. This procedural artifact was shown to be attenuated through various manipulations such as addition of energy substrates more relevant to the in vivo situation. These observations question the very concept of excitatory GABA in immature neuronal networks.
Keywords: GABA, brain slices, in vivo versus in vitro, giant depolarizing potentials, energy substrates
Introduction
Brain slices are widely used to investigate basic processes of brain function. Although being a reduced preparation (i.e., there is no blood flow, oxygen levels are non-physiological, most in vivo metabolites are not present in the artificial cerebrospinal fluid), brain slices provide easier access to cellular phenomena than in vivo models. Many results obtained in vitro (and reproduced by different laboratories) have been verified in vivo, giving ground to the general thought that in vitro results can be generalized to the intact organism. However, although adequate in many cases, this approach may lead to misinterpretation in many others. The concept of the excitatory action of GABA at early postnatal stages of development provides a particular example of correct observations performed in vitro which may not apply to the in vivo situation.
The Concept of Excitatory GABA in the Immature Brain
GABA, the main inhibitory neurotransmitter in vertebrates, activates GABAA receptors (GABAAR) resulting in opening of anion-selective channels and transmembrane fluxes of chloride (Cl) and bicarbonate. Normally, the direction of Cl current determines the hyperpolarizing or depolarizing effect of GABAAR activation on the membrane. If the reversal potential for Cl (ECl) is above (below) the resting membrane potential, Cl leaves (enters) the cell. An outward (inward) flux of negative charges depolarizes (hyperpolarizes) the membrane.
It is important to clarify here the difference between depolarizing and excitatory actions of GABA since there is a widespread misunderstanding of these notions. The concentration of intracellular Cl− measured in different cell types varies from 3 to 60 mM and in mammalian neurons in vitro it is generally low (<10 mM, see Khirug et al., 2008; Bregestovski et al., 2009). As a result, the reversal potential of GABAergic currents, EGABA, is close to the resting membrane potential and activation of GABAAR causes hyperpolarization or weak depolarization. Meanwhile, GABAAR channel opening decreases the input membrane resistance inducing “shunting inhibition” (see Andersen et al., 1980; Staley and Mody, 1992; Tang et al., 2011; Wright et al., 2011) that lowers the neuron’s firing probability. Therefore, a weakly depolarizing GABA may exert an inhibitory effect. In contrast, the “excitatory” GABA action means that GABAAR activation induces a depolarization large enough to generate action potentials.
The inhibitory/hyperpolarizing effects of GABA have been extensively verified in juvenile and adult animals in vivo. At earlier stages of development, the picture appears to be different. In vitro experiments have shown an excitatory action of GABA at early stages of development in kittens (Schwartzkroin and Altschuler, 1977), rabbits (Mueller et al., 1983), and rats (Dunwiddie, 1981; Harris and Teyler, 1983; Mueller et al., 1984; Ben-Ari et al., 1989) in a large number of subsequent studies (for review, Ben-Ari et al., 2007). Experiments performed in rodent brain slices indicated that the switch from the excitatory to inhibitory action of GABA takes place during the second postnatal week (P12–P13; Ben-Ari et al., 2007). The mechanism of this switch was explained as the increased age-dependent expression of KCC2 chloride exporter which takes over the leading role in Cl homeostasis from NKCC1 chloride importer (Blaesse et al., 2009). A hypothesis on the leading role of excitatory GABA in development was proposed by Ben-Ari and co-authors who claimed it as a universal rule: “In all developing animal species and brain structures investigated, neurons have a higher intracellular chloride concentration at an early stage leading to an efflux of chloride and excitatory actions of GABA in immature neurons” (Ben-Ari et al., 2007). These in vitro findings obtained in brain slices or cell cultures were frequently taken for granted. However, several lines of evidence challenge the extrapolation of these conclusions to the intact brain.
GABA is Not Excitatory in the Intact Brain
First, the early study performed in vivo, using intracellular recordings of hippocampal neurons in young kittens, suggested that inhibition is a predominant form of synaptic activity at early postnatal ages (Purpura et al., 1968). However, a high concentration of KCl was used in the pipette solution, which can alter ionic homeostasis.
Second, in vivo recordings using GABAAR antagonists contradict the in vitro observations. A study based on the analysis of more than 200 rat pups at the age of P3–P5 demonstrated that the injection of bicuculline triggered seizures in these pups (Baram and Snead, 1990). Another in vivo study reported that cerebellar Purkinje cells inhibit each other as early as at P5 and that bicuculline abolishes their interaction and increases their spontaneous firing activity (Bernard and Axelrad, 1993). Also, several more recent in vivo studies using specific agonists or antagonists of GABAARs clearly demonstrated the inhibitory action of GABA during the first postnatal week (Minlebaev et al., 2006, 2011; Isaev et al., 2007). For instance, Minlebaev et al. (2006) wrote that in P3–P5 rats: “Blockade of GABAA receptors by gabazine significantly increased spontaneous cortical activity by almost doubling the occurrence of spontaneous spindle-bursts…” However, these results were not mentioned in the subsequent review by the same main authors (Ben-Ari et al., 2007), who instead claimed that GABA “…excites immature neurons and generates primitive oscillations.” It is difficult to state that GABA exerts an excitatory action when GABAAR blockade leads to an increased activity in vivo.
Third, observations on the “intact hippocampus” preparation (in toto) where cellular integrity and connectivity are maintained, also suggest the inhibitory action of GABA. Using recordings from the CA1 area in isolated hippocampus, Wong et al. (2005) showed that synaptically released GABA causes inhibition. Moreover, in contrast to observations made in brain slices (Figure 1A; Ben-Ari et al., 1989), application of bicuculline resulted in epileptiform discharges (Figure 1C; Wong et al., 2005). Interestingly, similar effects were observed by Ben-Ari’s group in the very first study on the intact immature hippocampus (Figure 1B; Khalilov et al., 1997), but they were not discussed in their later publications. Recent experiments using the same preparation from P5–P7 mice confirmed these observations (Dzhala et al., 2010, 2012). Isoguvacine, a selective agonist of GABAARs, transiently reduced spontaneous neuronal activity. Thus, the net effect of GABAAR activation in the intact hippocampal network is inhibitory.
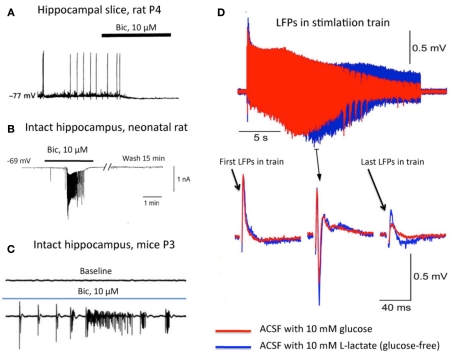
Figure 1.
(A–C) GABA is depolarizing in the slice preparation and hyperpolarizing in the intact hippocampus. (A) Microelectrode recording from hippocampal neuron in a brain slice from a 4-day-old rat (KCl-containing electrode). Note that bicuculline, a GABAA receptor antagonist, caused membrane hyperpolarization and inhibition of spontaneous synaptic activity (from Ben-Ari et al., 1989). (B) Whole-cell voltage-clamp recording with a pipette containing a K-gluconate based solution [(Cl) in the pipette was 4.2 mM] from a neuron in the intact rat hippocampus. Note that bicuculline evokes epileptiform discharges (from Khalilov et al., 1997). (C) GABAergic activities observed from isolated intact neonatal (P3) mouse hippocampus as seen by extracellular recordings from the CA3 area. Top: baseline field potentials. Note the absence of electrical activity. Bottom: note the presence of spontaneous activity and epileptiform discharges in the presence of bicuculline (blue line). To achieve better oxygenation of the preparation, a dual-side perfusion chamber and a fluid rate of 15 ml/min were used (from Wong et al., 2005). (D) Lactate without glucose maintains and even augments synaptic function. Top: local field potentials (LFPs) in response to stimulation trains when ACSF contains 10 mM glucose (red) or 10 mM lactate (blue). Bottom: examples of single LFPs at expanded time scale. Note that in the presence of lactate as the sole energy substrate, LFPs are even better maintained than under glucose-only conditions (from Ivanov et al., 2011).
Together, these results strongly suggest that GABA is inhibitory in the immature intact brain. On the other hand, the excitatory action of GABA has been observed in a number of studies on brain slices (Ben-Ari et al., 2007). What mechanisms may underlie this apparent discrepancy?
Brain Slices are Severely Damaged Brain Tissue
Using brain slices implies that brain tissue will be cut, i.e., that cell processes (dendrites, axons etc.) will be severed, generating a model of traumatic brain injury. According to early histological observation in slices, there is a 50- to 100-μm deep zone of severely disrupted tissue (Garthewaite et al., 1979; Bak et al., 1980; Frotscher et al., 1981). As a consequence of mechanical injury, microglial cells in slices are rapidly activated and become highly mobile (Petersen and Dailey, 2004). This may trigger a cascade of detrimental processes due to the release of a number of neurotoxic substances including cytokines, chemokines, nitric oxide, and superoxide free radicals that generate reactive oxygen species and reactive nitrogen species (Loan and Byrnes, 2010).
While in more recent studies microtomes/vibratomes are used for slices preparation, still the regions close to the surface (30–80 μm deep) contain a large amount of damaged cells (Dzhala et al., 2012). Since most electrophysiological and imaging studies of cell body layers (like hippocampal pyramidal cells) are performed in this region, the results may be biased by the inclusion of these injured cells, thus reflecting pathological rather than physiological processes. Indeed, slicing through brain tissue invariably leads to pathological reorganizations (Hoffman et al., 1994; McKinney et al., 1997).
Damaged Neurons Accumulate Cl
As mentioned above, the net action of GABAAR activation depends upon ECl. Hence, the depolarizing action of GABA in slices may result from intracellular Cl accumulation in traumatized neurons located close to the surface. Indeed, after neuronal trauma, GABA, both synaptically released and exogenously applied, induced depolarization of neurons, and increased intracellular Ca2+ (van den Pol et al., 1996). Using gramicidin perforated-patch recordings, Nabekura et al. (2002), demonstrated that EGABA was more depolarized in axotomized than in intact neurons of the vagus dorsal motor nucleus. The authors concluded that: “axotomy led to …elevation of intracellular Cl, and an excitatory response to GABA. A switch of GABA action from inhibitory to excitatory might be a mechanism contributing to excitotoxicity in injured neurons” (Nabekura et al., 2002). Direct non-invasive measurements of intracellular Cl concentration in Clomeleon-expressing mice (Dzhala et al., 2010, 2012) clearly demonstrated that axotomized and dendrotomized cells proximal to the slice surface have a much higher intracellular Cl concentration than in deeper situated and less injured cells (Figure 2A). In contrast, Cl levels were much lower in the intact hippocampus preparation (Figure 2A), in which a direct activation of GABAAR decreased neuronal firing – an observation consistent with an inhibitory/shunting action of GABA (Dzhala et al., 2012).
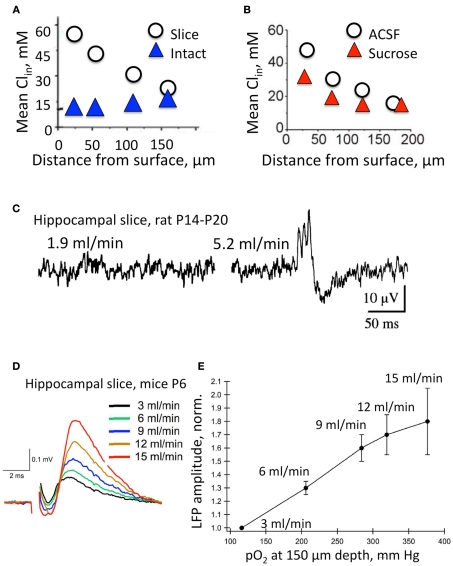
Figure 2.
Intracellular Cl concentration and electrical activity strongly depend on the experimental model and conditions. (A) The mean intracellular Cl concentration in neurons at different depth from the surface in the intact hippocampi ( ) and acute hippocampal slice preparations (◯) at P5–P7. Note the highly elevated Cl concentrations in neurons from the surface layers in the slice preparation (Modified from Dzhala et al., 2012). (B) The effects of slicing conditions on intracellular Cl concentration. Mean Cli as a function of depth in the hippocampal slices prepared from P5–P7 mice in control ACSF and in a high sucrose solution (Modified from Dzhala et al., 2012). (C–E) Genesis of network events and amplitude of local field potentials strongly depend upon the flow rate of ACSF. (C) Spontaneous network activity recorded at a low flow rate of 1.9 ml/min (left), and a high flow rate of 5.2 ml/min (right). Note sharp wave–ripple activity only at a high flow rate. Juvenile (P14–P20) transverse hippocampal 400–450 μm thick slices from Wistar rats were used here (from Hájos et al., 2009). (D) Examples of local field potentials measured in the same slice and electrode positions at different flow rates. Note the remarkable increase in amplitude when the flow rate is increased. (E) Summary of the dependence of local field potential (LFP) amplitudes on the oxygen levels and perfusion rates. Slices 400 μm thick from P4–P7 Swiss mice (from Ivanov et al., 2011).
) and acute hippocampal slice preparations (◯) at P5–P7. Note the highly elevated Cl concentrations in neurons from the surface layers in the slice preparation (Modified from Dzhala et al., 2012). (B) The effects of slicing conditions on intracellular Cl concentration. Mean Cli as a function of depth in the hippocampal slices prepared from P5–P7 mice in control ACSF and in a high sucrose solution (Modified from Dzhala et al., 2012). (C–E) Genesis of network events and amplitude of local field potentials strongly depend upon the flow rate of ACSF. (C) Spontaneous network activity recorded at a low flow rate of 1.9 ml/min (left), and a high flow rate of 5.2 ml/min (right). Note sharp wave–ripple activity only at a high flow rate. Juvenile (P14–P20) transverse hippocampal 400–450 μm thick slices from Wistar rats were used here (from Hájos et al., 2009). (D) Examples of local field potentials measured in the same slice and electrode positions at different flow rates. Note the remarkable increase in amplitude when the flow rate is increased. (E) Summary of the dependence of local field potential (LFP) amplitudes on the oxygen levels and perfusion rates. Slices 400 μm thick from P4–P7 Swiss mice (from Ivanov et al., 2011).
Finally, it is important to note that the intracellular Cl concentration may be cell type-dependent (Rohrbough and Spitzer, 1996; Sauer et al., 2012) and location-dependent in a given cell (Duebel et al., 2006). An uneven distribution of Cl ions has been described in hippocampal neurons using electrophysiological recordings (Szabadics et al., 2006; Khirug et al., 2008) and non-invasive monitoring of intracellular Cl (Waseem et al., 2010). Future studies on GABA action in the immature brain should take these factors into account.
Thus, the slicing procedure is clearly associated with damaged cells, which accumulate chloride. Slice quality critically depends upon the slicing procedure and equipment. Recent studies described conditions for better preparation (with microtomes/vibratomes) and preservation of acute slice preparations (Schurr et al., 1989; Hájos and Mody, 2009; Hájos et al., 2009; Maier et al., 2009; Ivanov and Zilberter, 2011). Still, even state-of-the-art procedures do not prevent damage inherent to slicing. For instance, using a vibratome, Taylor et al. (1999) wrote: “Light microscopy of slices fixed immediately after Vibroslice preparation indicated significant swelling of pyramidal neurons, i.e., cell bodies, mitochondria, dendrites, and nuclei were enlarged and hydropic.” While experimentators try to achieve recovery as much as possible after slicing (Taylor et al., 1999; Bischofberger et al., 2006), even after 1.5 h incubation in artificial cerebrospinal fluid (ACSF; typical experimental procedure for recovery of slice integrity) neurons and glial cells are still functionally and energetically defective. This point is supported by the observations of Dzhala et al. (2012) who demonstrated Cl accumulation in slice surface-proximal neurons (Figures 2A,B).
Traumatic Tissue Needs More Energy
Abnormalities induced by tissue trauma in brain slices are exacerbated by several additional factors. The lack of blood flow in slices dramatically changes the way energy substrates and oxygen are delivered to cells. Energy substrates and O2 are instead supplied exogenously by artificial extracellular solution (ACSF), which must diffuse passively from the surface. In the intact brain, blood vessels, astrocytes, and neurons form a complex system supporting and adjusting brain metabolism (Pellerin, 2010; Turner and Adamson, 2011; Zilberter and Bregestovski, 2012) while in brain slices metabolism depends entirely on the experimental conditions. Although experimentalists are trying to create conditions maximally close to the in vivo environment, they are obviously far from ideal. Support normally provided by blood is not entirely compensated by perfusion of ACSF. Glucose-based composition of ACSF was empirically adjusted more than 60 years ago for relatively long-lasting preservation of neuronal function in brain slices and is, obviously, not physiological (Hájos and Mody, 2009; Zilberter et al., 2010). Slices exposed to ACSF exhibit severe abnormalities in energy metabolism. For instance, the rate of glycolysis is reduced by more than 50% in brain slices (Rolleston and Newsholme, 1967; Benjamin and Verjee, 1980) as compared to the in vivo estimates (Ghajar et al., 1982). In addition, the total adenine nucleotide pool is decreased by 30–50% in slices as compared to that observed in vivo (Whittingham et al., 1984) and this effect become less important with increasing of slice thickness (Zur Nedden et al., 2011). Remarkably, the slicing procedure causes a decrease to about 50% of the total content of ATP, creatine, and adenylate, as well as a strong change in intracellular pH from about 6.6–7.2 (Whittingham et al., 1984). Such a deficit in the cell energy supply may directly affect GABAergic action.
To test this hypothesis, Zilberter and collaborators analyzed whether improving energy supply to neurons with glucose oxidative energy substrates (OES) can modulate the response to GABA. In neocortical and hippocampal slices from neonatal (P3–P8) rats and mice, supplementing ACSF with β-hydroxybutyrate, lactate, or pyruvate significantly hyperpolarized EGABA, switching the GABA action from excitatory to inhibitory (Holmgren et al., 2010). Moreover, OES inhibited giant depolarizing potentials (GDPs; Holmgren et al., 2010; Mukhtarov et al., 2011), a spontaneous network activity pattern characteristic for neonatal hippocampal slices (Ben-Ari et al., 2007). The beneficial effect of OES on energy metabolism status in neurons was confirmed by direct simultaneous measurements of oxygen consumption and NADH fluorescence during neuronal activity (Ivanov and Zilberter, 2011; Ivanov et al., 2011). For instance, in the presence of glucose, lactate was effectively utilized as an energy substrate (Ivanov et al., 2011), causing an augmentation of oxidative metabolism (Figure 1D). Moreover, in the absence of glucose, lactate was fully capable of maintaining synaptic function (Schurr et al., 1988; Ivanov et al., 2011). These observations demonstrate that neuronal function can definitely be improved in both neonatal (Ivanov et al., 2011) and adult (Ivanov and Zilberter, 2011) slices by supplementing glucose with OES. Glucose alone, even at strongly hyperglycemic concentrations as in standard ACSF (10 versus 1–2 mM in the brain extracellular fluid (Abi-Saab et al., 2002; Zilberter et al., 2010) cannot fully cover energy demands during neuronal activation.
These studies have ignited a controversy (Kirmse et al., 2010; Ruusuvuori et al., 2010; Tyzio et al., 2011). However, although Tyzio and co-authors failed to reproduce the effects of b-hydroxybutyrate on EGABA, they did reproduce the EGABA-hyperpolarizing effect of 5 mM pyruvate. Kirmse et al. (2010) did not find any effect of β-hydroxybutyrate or pyruvate on GABA-induced Ca2+ fluorescent transients; but measurements for control and BHB-treated cells were performed on different slices with a slow ACSF perfusion rate leading to improper oxygenation (see Ivanov et al., 2011; Ivanov and Zilberter, 2011). Ruusuvuori et al. observed the inhibitory effect of lactate on GDP generation but suggested that this effect is induced by intracellular acidification. Indeed, OES caused pHi changes of less than −0.05 pH units (Ivanov et al., 2011; Mukhtarov et al., 2011). However, the 0.25–0.35 reduction in pHi obtained by substituting bicarbonate-containing solution with HEPES-based HCO3-free solution did not eliminate GDPs (Mukhtarov et al., 2011). Therefore, a significant contribution of pHi to the effects of OES on GDPs is unlikely (Ivanov et al., 2011; Mukhtarov et al., 2011). Certainly, the controversy needs to be resolved by independent groups. But the results clearly demonstrate that metabolic processes are central to the reorganization of cell function after making brains slices.
Altogether, these observations demonstrate that the slicing procedure injures cells and disrupts brain metabolism, leading to intracellular Cl accumulation in neurons and rendering GABA strongly depolarizing or even excitatory as has been reported during the first postnatal week in rodents.
This, however, does not rule out the possibility that GABA may be depolarizing, in particular at very early stages of development. For example, treatment of mice with bumetanide during the period of embryonic cortical developmental results in disruption of excitatory synapse formation (Wang and Kriegstein, 2011). As bumetanide antagonizes the Na+–K+–2Cl− cotransporter (NKCC1), which accumulates intracellular Cl, these observations suggest that Cl in embryonic neurons is elevated and plays an important signaling role in developmental processes.
GABA and Early Network Activities
Oscillations/correlated neuronal discharges are a hallmark of network activity at any stage of development (Buzsáki, 1986, 2002; Spitzer, 1994; Chrobak and Buzsáki, 1998; Leinekugel et al., 2002; Khazipov et al., 2004; Adelsberger et al., 2005; Sipilä et al., 2006). At early stages of development, this synchronized activity may be important for brain maturation, regulating multiple processes including neuronal migration (Komuro and Rakic, 1998) and directing neuronal differentiation (Gu and Spitzer, 1997; Spitzer et al., 2000), dendritic growth and patterning (Katz and Shatz, 1996; Wong and Ghosh, 2002), activation of transmitter receptors (Liao et al., 2001), and the pattern of specific connections (Penn et al., 1998). The most prominent synchronized activity, early network oscillations (ENOs) associated with changes in neuronal intracellular Ca2+ concentration, were observed in small groups of neurons and in large populations in vitro (Garaschuk et al., 1998, 2000; Corlew et al., 2004) and in vivo (Adelsberger et al., 2005). Spindle-bursts were described in the neonatal rat neocortex in vivo (Khazipov et al., 2004). Thus, waves of spontaneous electrical activity propagating across many regions of the brain are a hallmark of developing networks, and actively contribute to cortical development and plasticity (Katz and Shatz, 1996; Mizuno et al., 2007). Distinct mechanisms underlie generation of synchronized events, including synaptic interaction, gap junction communication, the presence of pacemaker-like neurons as well as activation of metabotropic glutamate and ACh receptors (Kandler and Katz, 1998; Flint et al., 1999; Blankenship and Feller, 2010).
However, the reports that GABA is depolarizing/excitatory in slices from the immature brain led to a very popular theory, which inspired many researches in the neurodevelopment field and provided a conceptual framework to explain early network activities recorded in vivo. Excitatory GABA (i.e., its ability to drive the membrane potential to firing threshold) would be essential for developing networks. In vitro experiments revealed the occurrence of spontaneous network events involving large populations of neurons. This phenomenon was first described by Harris and Teyler (1983) who called it “spontaneous unison firing.” It was also observed by Mueller et al. (1984), who wrote: “Immature neurons often demonstrated spontaneous depolarizations of up to 30 mV amplitude and 30 to 60 sec duration.” Several years later, Ben-Ari et al. (1989) also described this phenomenon in immature brain slices, which they named GDPs. GDPs were infrequent or absent after P12. It was proposed that depolarizing GABA plays a key role in the generation of GDPs and that this spontaneous activity results from the synergistic excitatory activities mediated by GABAA and glutamate N-methyl-d-aspartate (NMDA) receptors (Ben-Ari et al., 1997). Since GDPs were not observed after postnatal days 10–11, at the time close to the “excitation/inhibition switch,” it was postulated that GDPs represent a primitive activity pattern of the developing brain and that it is “largely based on excitatory GABA” (Ben-Ari et al., 2007).
These observations led to the broadly accepted idea that the excitatory action of GABA underlies neuronal maturation of immature neuronal networks. According to this concept, the elevated Cl concentration and, consequently, the excitatory action of GABA, represent necessary steps in the development of the nervous system. This viewpoint is epitomized in the recent review of van Welie et al. (2011), who wrote: “Depolarizing GABA is required for normal brain development, as it contributes to the morphological maturation of neurons (Cancedda et al., 2007) and neuronal circuits (Ben-Ari, 2001; Akerman and Cline, 2006). Depolarizing GABA can drive juvenile neurons to fire action potentials (Ben-Ari, 2002) and conversely, neuronal activity can regulate EGABA, by either specific patterns of synaptic activation (Woodin et al., 2003; Balena and Woodin, 2008), or alterations in postsynaptic activity levels via changes in intracellular Ca2+ (Fiumelli et al., 2005).”
This statement relies on the axiom that the nature of GDPs observed in brain slices correlates with network activities recorded in vivo in developing networks. While the general patterns of this activity may be similar in vitro and in vivo, the underlying mechanisms may be different. The presence and character of oscillatory activity in brain slices highly depend upon energy support, oxygenation, and perfusion rate (Hájos and Mody, 2009; Hájos et al., 2009; Holmgren et al., 2010; Mukhtarov et al., 2011). For instance, sharp wave (SPW) oscillations are a hallmark of hippocampal activity in developing and adult hippocampus in vivo (Leinekugel et al., 2002). SPWs are usually not observed or very infrequent in slices when using slow perfusion rates of ACSF (1.6–2.4 ml/min; Hájos et al., 2009; Maier et al., 2009). However, SPWs appear (or become more frequent) at high speed of perfusion (Figure 2C), suggesting that a proper delivery of oxygen to the whole slice is critical for the genesis of SPWs in vitro (Hájos et al., 2009). The importance of oxygen delivery at elevated flow rates was further demonstrated by Ivanov et al. (2011). A decrease from 15 to 3.25 ml/min in the perfusion rate resulted in strong decrease of oxygen and a two-fold reduction of the local field potential amplitude in brain slices from P6 mice (Figures 2D,E).
Particularly convincing arguments were obtained in a recent study demonstrating that while GDPs can be recorded both in slices and the intact hippocampus during the first postnatal week, the mechanism of their genesis is different (Dzhala et al., 2012). Isoguvacine application dramatically increased GDP frequency in brain slices (in keeping with the excitatory action of GABA); whilst in the intact hippocampus isoguvacine completely abolished GDPs (in keeping with the inhibitory action of GABA).
Since the slicing procedure also lesions superficial neurons that leads to Cl accumulation in mature networks (Dzhala et al., 2012), one would expect GDPs to occur in adult slices. However, the study by Dzhala et al. (2012) shows that, whilst superficial neurons remain connected to the network in immature slices, they are functionally disconnected in mature slices. Hence, superficial cells with high internal Cl do not contribute much to network activity in mature slice.
Together, these observations strongly suggest that ENOs do not rely upon excitatory GABA. Hence, the mechanistic insights regarding GDP genesis/propagation/function gained from slice studies should be re-evaluated. As underlined in the recent review: “Usage of brain slice preparations has significantly contributed to a deeper understanding of neuronal functions at the cellular and network level in the recent decades. However, given factors such as absence of blood circulation, longer diffusion distances, steep interstitial pO2 gradients, and composition of the recording solution have to be kept in mind when interpreting data from slice preparations” (Kann, 2011).
Resume
Remaining uncertainties notwithstanding, studies utilizing the intact hippocampus preparation with more functional neurons, glial cells, and network activity, as well as the few available in vivo studies, suggest that GABA plays an inhibitory role in the immature brain (at least during the first postnatal week in rodents). Perhaps, the most important take-home message is that our understanding of brain function is based on experimental methods and measurements that inevitably distort/perturb the system. The observations are correct, but their interpretation may not be. The concept of excitatory GABA and its alleged role for neuronal network maturation provides a perfect example of how cautious we should be when interpreting experimental results.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Kevin Staley for critical reading of the manuscript and valuable suggestions. This study was supported by the grant from the European Union Seventh Framework: NEUROCYPRES, HEALTH-F2-2008-202088 (to Piotr Bregestovski).
References
- Abi-Saab W. M., Maggs D. G., Jones T., Jacob R., Srihari V., Thompson J., Kerr D., Leone P., Krystal J. H., Spencer D. D., During M. J., Sherwin R. S. (2002). Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J. Cereb. Blood Flow. Metab. 22, 271–279 [DOI] [PubMed] [Google Scholar]
- Adelsberger H., Garaschuk O., Konnerth A. (2005). Cortical calcium waves in resting newborn mice. Nat. Neurosci. 8, 988–990 10.1038/nn1502 [DOI] [PubMed] [Google Scholar]
- Akerman C. J., Cline H. T. (2006). Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 26, 5117–5130 10.1523/JNEUROSCI.0319-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. (1980). Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J. Physiol. (Lond.) 305, 279–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak I. J., Misgeld U., Weiler M., Morgan E. (1980). The preservation of nerve cells in rat neostriatal slices maintained in vitro: a morphological study. Brain Res. 197, 341–353 10.1016/0006-8993(80)91120-8 [DOI] [PubMed] [Google Scholar]
- Balena T., Woodin M. A. (2008). Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize E(Cl) during development. Eur. J. Neurosci. 27, 2402–2412 10.1111/j.1460-9568.2008.06194.x [DOI] [PubMed] [Google Scholar]
- Baram T. Z., Snead O. C. (1990). Bicuculline induced seizures in infant rats: ontogeny of behavioral and electrocortical phenomena. Brain Res. Dev. Brain Res. 57, 291–295 10.1016/0165-3806(90)90055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. (2001). Developing networks play a similar melody. Trends Neurosci. 24, 353–360 10.1016/S0166-2236(00)01743-4 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. (2002). Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 10.1038/nrm937 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 416, 303–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Gaiarsa J. L., Tyzio R., Khazipov R. (2007). GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284 10.1152/physrev.00017.2006 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Khazipov R., Leinekugel X., Caillard O., Gaiarsa J. L. (1997). GABAA, NMDA and AMPA receptors: a developmentally regulated ‘ménage à trois.’ Trends Neurosci. 20, 523–529 10.1016/S0166-2236(97)01147-8 [DOI] [PubMed] [Google Scholar]
- Benjamin A. M., Verjee Z. H. (1980). Control of aerobic glycolysis in the brain in vitro. Neurochem. Res. 5, 921–934 10.1007/BF00966133 [DOI] [PubMed] [Google Scholar]
- Bernard C., Axelrad H. (1993). Effects of recurrent collateral inhibition on Purkinje cell activity in the immature rat cerebellar cortex – an in vivo electrophysiological study. Brain Res. 626, 234–258 10.1016/0006-8993(93)90584-A [DOI] [PubMed] [Google Scholar]
- Bischofberger J., Engel D., Li L., Geiger J. R., Jonas P. (2006). Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat. Protoc. 1, 2075–2081 10.1038/nprot.2006.312 [DOI] [PubMed] [Google Scholar]
- Blaesse P., Airaksinen M. S., Rivera C., Kaila K. (2009). Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838 10.1016/j.neuron.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Blankenship A. G., Feller M. B. (2010). Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 11, 18–29 10.1038/nrn2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P., Waseem T., Mukhtarov M. (2009). Genetically encoded optical sensors for monitoring of intracellular chloride and chloride-selective channel activity. Front. Mol. Neurosci. 2:15. 10.3389/neuro.02.015.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. (1986). Hippocampal sharp waves: their origin and significance. Brain Res. 398, 242–252 10.1016/0006-8993(86)91483-6 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. (2002). Theta oscillations in the hippocampus. Neuron 33, 325–340 10.1016/S0896-6273(02)00586-X [DOI] [PubMed] [Google Scholar]
- Cancedda L., Fiumelli H., Chen K., Poo M. M. (2007). Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 27, 5224–5235 10.1523/JNEUROSCI.5169-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak J. J., Buzsáki G. (1998). Gamma oscillations in the entorhinal cortex of the freely behaving rat. J. Neurosci. 18, 388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R., Bosma M. M., Moody W. J. (2004). Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J. Physiol. (Lond.) 560, 377–390 10.1113/jphysiol.2004.071621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duebel J., Haverkamp S., Schleich W., Feng G., Augustine G. J., Kuner T., Euler T. (2006). Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron 49, 81–94 10.1016/j.neuron.2005.10.035 [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V. (1981). Age-related differences in the in vitro rat hippocampus: development of inhibition and the effects of hypoxia. Dev. Neurosci. 4, 165–175 10.1159/000112753 [DOI] [PubMed] [Google Scholar]
- Dzhala V., Valeeva G., Glykys J., Khazipov R., Staley K. (2012). Traumatic alterations in GABA signaling disrupt hippocampal network activity in the developing brain. J. Neurosci. 32, 4017–4031 10.1523/JNEUROSCI.5139-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala V. I., Mail M. E., Staley K. J. (2010) “Chloride imbalance in the acute hippocampal slice model of brain trauma,” in Abstract SFN Meeting 255.25/R10, San Diego [Google Scholar]
- Fiumelli H., Cancedda L., Poo M. M. (2005). Modulation of GABAergic transmission by activity via postsynaptic Ca2+ -dependent regulation of KCC2 function. Neuron 48, 773–786 10.1016/j.neuron.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Flint A. C., Dammerman R. S., Kriegstein A. R. (1999). Endogenous activation of metabotropic glutamate receptors in neocortical development causes neuronal calcium oscillations. Proc. Natl. Acad. Sci. U.S.A. 96, 12144–12149 10.1073/pnas.96.21.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M., Misgeld U., Nitsch C. (1981). Ultrastructure, of mossy fiber endings in in vifro hippocampal slices. Exp. Brain Res. 41, 247–255 10.1007/BF00238890 [DOI] [PubMed] [Google Scholar]
- Garaschuk O., Hanse E., Konnerth A. (1998). Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. (Lond.) 507, 219–236 10.1111/j.1469-7793.1998.219bu.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O., Linn J., Eilers J., Konnerth A. (2000). Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 3, 452–459 10.1038/74823 [DOI] [PubMed] [Google Scholar]
- Garthewaite J., Woodhams P. L., Collins M. J., Balazs R. (1979). On the preparation of brain slices: morphology and cyclic nucleotides. Brain Res. 173, 373–377 10.1016/0006-8993(79)90641-3 [DOI] [PubMed] [Google Scholar]
- Ghajar J. B. G., Plum F., Duffy T. E. (1982). Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J. Neurochem. 38, 397–409 10.1111/j.1471-4159.1982.tb08643.x [DOI] [PubMed] [Google Scholar]
- Gu X., Spitzer N. C. (1997). Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev. Neurosci. 19, 33–41 10.1159/000111214 [DOI] [PubMed] [Google Scholar]
- Hájos N., Ellender T. J., Zemankovics R., Mann E. O., Exley R., Cragg S. J., Freund T. F., Paulsen O. (2009). Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur. J. Neurosci. 29, 319–327 10.1111/j.1460-9568.2008.06577.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N., Mody I. (2009). Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J. Neurosci. Methods 183, 107–113 10.1016/j.jneumeth.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Teyler T. J. (1983). Evidence for late development of inhibition in area CA1 of the rat hippocampus. Brain Res. 268, 339–343 10.1016/0006-8993(83)90500-0 [DOI] [PubMed] [Google Scholar]
- Hoffman S. N., Salin P. A., Prince D. A. (1994). Chronic neocortical epileptogenesis in vitro. J. Neurophysiol. 71, 1762–1773 [DOI] [PubMed] [Google Scholar]
- Holmgren C. D., Mukhtarov M., Malkov A. E., Popova I. Y., Bregestovski P., Zilberter Y. (2010). Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. J. Neurochem. 112, 900–912 10.1111/j.1471-4159.2009.06506.x [DOI] [PubMed] [Google Scholar]
- Isaev D., Isaeva E., Khazipov R., Holmes G. L. (2007). Shunting and hyperpolarizing GABAergic inhibition in the high-potassium model of ictogenesis in the developing rat hippocampus. Hippocampus 17, 210–219 10.1002/hipo.20259 [DOI] [PubMed] [Google Scholar]
- Ivanov A., Mukhtarov M., Bregestovski P., Zilberter Y. (2011). Lactate effectively covers energy demands during neuronal network activity in neonatal hippocampal slices. Front. Neuroenergetics 3:2. 10.3389/fnene.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A., Zilberter Y. (2011). Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity Front. Neuroenergetics 3:9 10.3389/fnene.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K., Katz L. C. (1998). Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 18, 1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O. (2011). The energy demand of fast neuronal network oscillations: insights from brain slice preparations. Front. Pharmacol. 2:90. 10.3389/fphar.2011.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C., Shatz C. J. (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- Khalilov I., Khazipov R., Esclapez M., Ben-Ari Y. (1997). Bicuculline induces ictal seizures in the intact hippocampus recorded in vitro. Eur. J. Pharmacol. 319, R5–R6 10.1016/S0014-2999(96)00964-8 [DOI] [PubMed] [Google Scholar]
- Khazipov R., Sirota A., Leinekugel X., Holmes G. L., Ben-Ari Y., Buzsáki G. (2004). Early motor activity drives spindle bursts] in the developing somatosensory cortex. Nature 432, 758–761 10.1038/nature03132 [DOI] [PubMed] [Google Scholar]
- Khirug S., Yamada J., Afzalov R., Voipio J., Khiroug L., Kaila K. (2008). GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na–K–2Cl cotransporter NKCC1. J. Neurosci. 28, 4635–4639 10.1523/JNEUROSCI.0908-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmse K., Witte O. W., Holthoff K. (2010). GABA depolarizes immature neocortical neurons in the presence of the ketone body ß-hydroxybutyrate. J. Neurosci. 30, 16002–16007 10.1523/JNEUROSCI.2534-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H., Rakic P. (1998). Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J. Neurobiol. 37, 110–130 [DOI] [PubMed] [Google Scholar]
- Leinekugel X., Khazipov R., Cannon R., Hirase H., Ben-Ari Y., Buzsáki G. (2002). Correlated bursts of activity in the neonatal hippocampus in vivo. Science 296, 49–52 10.1126/science.1069837 [DOI] [PubMed] [Google Scholar]
- Liao D., Scannevin R. H., Huganir R. (2001). Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 21, 6008–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loan D. J., Byrnes K. R. (2010). Role of microglia in neurotrauma. Neurotherapeutics 7, 366–377 10.1016/j.nurt.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N., Morris G., Johenning F. W., Schmitz D. (2009). An approach for reliably investigating hippocampal sharp wave-ripples in vitro. PLoS ONE 4, e6925. 10.1371/journal.pone.0006925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney R. A., Debanne D., Gähwiler B. H., Thompson S. M. (1997) Lesion-induced axonal sprouting and hyperexcitability in the hippocampus in vitro: implications for the genesis of posttraumatic epilepsy. Nat. Med. 3, 990–996 10.1038/nm0997-990 [DOI] [PubMed] [Google Scholar]
- Minlebaev M., Ben-Ari Y., Khazipov R. (2006). Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J. Neurophysiol. 97, 692–700 10.1152/jn.00759.2006 [DOI] [PubMed] [Google Scholar]
- Minlebaev M., Colonnese M., Tsintsadze T., Sirota A., Khazipov R. (2011). Early γ oscillations synchronize developing thalamus and cortex. Science. 334, 226–229 10.1126/science.1210574 [DOI] [PubMed] [Google Scholar]
- Mizuno H., Hirano T., Tagawa Y. (2007). Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J. Neurosci. 27, 6760–6770 10.1523/JNEUROSCI.1215-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A. L., Chesnut R. M., Schwartzkroin P. A. (1983). Actions of GABA in developing rabbit hippocampus: an in vitro study. Neurosci. Lett. 39, 193–198 10.1016/0304-3940(83)90076-9 [DOI] [PubMed] [Google Scholar]
- Mueller A. L., Taube J. S., Schwartzkroin P. A. (1984). Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J. Neurosci. 4, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtarov M., Ivanov A., Zilberter Y., Bregestovski P. (2011). Inhibition of spontaneous network activity in neonatal hippocampal slices by energy substrates is not correlated with intracellular acidification. J. Neurochem. 116, 316–321 10.1111/j.1471-4159.2010.07111.x [DOI] [PubMed] [Google Scholar]
- Nabekura J., Ueno T., Okabe A., Furuta A., Iwaki T., Shimizu-Okabe C., Fukuda A., Akaike N. (2002). Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 22, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L. (2010). Food for thought: the importance of glucose and other energy substrates for sustaining brain function under varying levels of activity. Diabetes Metab. 36, S59–S63 10.1016/S1262-3636(10)70233-0 [DOI] [PubMed] [Google Scholar]
- Penn A. A., Riquelme P. A., Feller M. B., Shatz C. J. (1998). Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112 10.1126/science.279.5359.2108 [DOI] [PubMed] [Google Scholar]
- Petersen M. A., Dailey M. E. (2004). Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia 46, 195–206 10.1002/glia.10362 [DOI] [PubMed] [Google Scholar]
- Purpura D. P., Prelevic S., Santini M. (1968). Postsynaptic potentials and spike variations in the feline hippocampus during postnatal ontogenesis. Exp. Neurol. 22, 408–422 10.1016/0014-4886(68)90006-X [DOI] [PubMed] [Google Scholar]
- Rohrbough J., Spitzer N. C. (1996). Regulation of intracellular Cl- levels by Na(+)-dependent Cl- cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor mediated responses in spinal neurons. J. Neurosci. 16, 82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. (1967). Effects of fatty acids, ketone bodies, lactate and pyruvate on glucose utilization by guinea-pig cerebral cortex slices. Biochem. J. 104, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusuvuori E., Kirilkin I., Pandya N., Kaila K. (2010). Spontaneous network events driven by depolarizing GABA action in neonatal hippocampal slices are not attributable to deficient mitochondrial energy metabolism. J. Neurosci. 30, 15638–15642 10.1523/JNEUROSCI.3355-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J. F., Strüber M., Bartos M. (2012). Interneurons provide circuit-specific depolarization and hyperpolarization. J. Neurosci. 32, 4224–4229 10.1523/JNEUROSCI.5702-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A., West C. A., Rigor B. M. (1988). Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240, 1326–1328 10.1126/science.3375817 [DOI] [PubMed] [Google Scholar]
- Schurr A., West C. A., Rigor B. M. (1989). Electrophysiology of energy metabolism and neuronal function in the hippocampal slice preparation. J. Neurosci. Methods 28, 7–13 10.1016/0165-0270(89)90002-2 [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Altschuler R. J. (1977). Development of kitten hippocampal neurons. Brain Res. 134, 429–444 10.1016/0006-8993(77)90820-4 [DOI] [PubMed] [Google Scholar]
- Sipilä S. T., Schuchmann S., Voipio J., Yamada J., Kaila K. (2006). The cation-chloride cotransporter NKCC1 promotes sharp waves in the neonatal rat hippocampus. J. Physiol. 573, 765–773 10.1113/jphysiol.2006.107086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. (1994). Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 17, 115–118 10.1016/0166-2236(94)90120-1 [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lautermilch N. J., Smith R. D., Gomez T. M. (2000). Coding of neuronal differentiation by calcium transients. Bioessays 22, 811–817 [DOI] [PubMed] [Google Scholar]
- Staley K. J., Mody I. (1992). Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABA(A) receptor-mediated postsynaptic conductance, J. Neurophysiol. 68, 197–212 [DOI] [PubMed] [Google Scholar]
- Szabadics J., Varga C., Molnar G., Olah S., Barzo P., Tamas G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235 10.1126/science.1121325 [DOI] [PubMed] [Google Scholar]
- Tang Z. Q., Dinh E. H., Shi W., Lu Y. (2011). Ambient GABA-activated tonic inhibition sharpens auditory coincidence detection via a depolarizing shunting mechanism. J. Neurosci. 31, 6121–6131 10.1523/JNEUROSCI.4265-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. P., Weber M. L., Gaughan C. L., Lehning E. J., LoPachin R. M. (1999). Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage. J. Neurosci. 19, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. A., Adamson D. C. (2011). Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 70, 167–176 10.1097/NEN.0b013e31820e1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R., Allene C., Nardou R., Picardo M. A., Yamamoto S., Sivakumaran S., Caiati M. D., Rheims S., Minlebaev M., Milh M., Ferré P., Khazipov R., Romette J. L., Lorquin J., Cossart R., Khalilov I., Nehlig A., Cherubini E., Ben-Ari Y. (2011). Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate. J. Neurosci. 31, 34–45 10.1523/JNEUROSCI.3314-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Obrietan K., Chen G. (1996). Excitatory actions of GABA after neuronal trauma. J. Neurosci. 16, 4283–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Welie I., Smith I. T., Watt A. J. (2011). The metamorphosis of the developing cerebellar microcircuit. Curr. Opin. Neurobiol. 21, 245–253 10.1016/j.conb.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. D., Kriegstein A. R. (2011). Blocking early GABA depolarization with bumetanide results in permanent alterations in cortical circuits and sensorimotor gating deficits. Cereb. Cortex 21, 574–587 10.1093/cercor/bhq176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem T., Mukhtarov M., Buldakova S., Medina I., Bregestovski P. (2010). Genetically encoded Cl-Sensor as a tool for monitoring of Cl-dependent processes in small neuronal compartments. J. Neurosci. Methods 193, 14–23 10.1016/j.jneumeth.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Whittingham T. S., Lust W. D., Christakis D. A., Passonneau J. V. (1984). Metabolic stability of hippocampal slice preparations during prolonged incubation. J. Neurochem. 43, 689–696 10.1111/j.1471-4159.1984.tb12788.x [DOI] [PubMed] [Google Scholar]
- Wong R. O., Ghosh A. (2002). Activity-dependent regulation of dendritic growth and patterning. Nat. Rev. Neurosci. 3, 803–812 10.1038/nrn941 [DOI] [PubMed] [Google Scholar]
- Wong T., Zhang X. L., Asl M. N., Wu C. P., Carlen P. L., Zhang L. (2005). Postnatal development of intrinsic GABAergic rhythms in mouse hippocampus. Neuroscience 134, 107–120 10.1016/j.neuroscience.2005.04.019 [DOI] [PubMed] [Google Scholar]
- Woodin M. A., Ganguly K., Poo M. M. (2003). Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in ClS transporter activity. Neuron 39, 807–820 10.1016/S0896-6273(03)00507-5 [DOI] [PubMed] [Google Scholar]
- Wright R., Raimondo J. V., Akerman C. J. (2011). Spatial and temporal dynamics in the ionic driving force for GABA(A) receptors. Neural Plast. 2011, 728395. 10.1155/2011/728395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y., Bregestovski P. (2012). Fueling brain neuronal activity. Biol. Membr. 29, 59–64 [Google Scholar]
- Zilberter Y., Zilberter T., Bregestovski P. (2010). Neuronal activity in vitro and the in vivo reality: the role of energy homeostasis. Trends Pharmacol. Sci. 31, 394–401 10.1016/j.tips.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Zur Nedden S., Hawley S., Pentland N., Hardie D. G., Doney A. S., Frenguelli B. G. (2011). Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors. J. Neurosci. 31, 6221–6234 10.1523/JNEUROSCI.4039-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]