Abstract
During development, competition among undifferentiated neurites results in the growth of one neurite at the expense of the rest. This neurite becomes the axon. A similar competitive mechanism later governs the differential outgrowth of the axon and its branches. A long-range signal between different parts of the neuron is required to mediate this competition, but the nature of this signal has been unknown. Recent work has shown that local cAMP signaling promotes neurite growth, but leads to decreased cAMP concentrations in distant neurites, thereby inhibiting their growth. The precise mechanism mediating long-distance cyclic nucleotide signaling is unclear, but may involve localized calcium transients and autocrine signaling. Mututally inhibitory, competitive interactions during outgrowth of neural processes can emerge from these signaling events, both during axon specification and later during axon branching.
Information is transmitted between different regions of the mammalian central nervous system primarily by excitatory projection neurons. These neurons extend single, long axons from cell bodies in one region to dendrites of target neurons in distant regions. During the development of these connections, post-migratory neurons initially extend several short, undifferentiated neurites. One of these neurites grows extensively, and becomes the axon, whereas the other neurites stall [1] (Fig. 1A-C). Because one neurite seems to grow at the expense of the rest, the differential outgrowth of neurites during axon specification is frequently interpreted as a competitive process [2]. Outgrowth is typically considered competitive if inhibiting growth in one neurite promotes the growth of another [3]. This also means that selectively promoting the growth of one neurite will inhibit growth of the others during competition, so either of these patterns of growth/inhibition can be taken as evidence of competition. Supporting the competitive outgrowth interpretation, if the nascent axon is severed, another neurite extends and becomes the axon [4]. Thus, competition among neurites is believed to ensure the specification of one axon among several neurites during the growth of projection neurons.
Fig. 1.
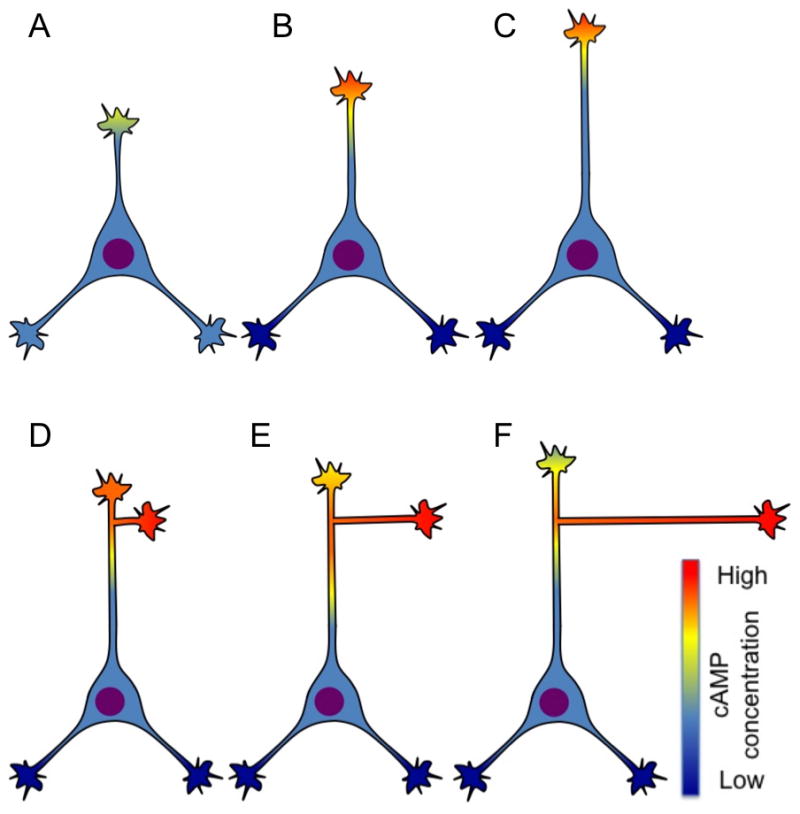
cAMP signals regulate competitive neurite outgrowth. (A-C) Competitive outgrowth of neurites during axon specification. (A) A postmigratory neuron with several undifferentiated neurites. One of these stochastically exhibits higher cAMP concentrations (top). (B) The neurite with high cAMP concentrations reinforces its own cAMP signaling through positive feedback and reduces cAMP concentrations in the other neurites through long-range signaling. (C) Higher cAMP concentrations stimulate growth of the top neurite (which becomes the axon) while suppressing cAMP, and therefore the growth, of the others. (D-F) Proposed model for the competitive outgrowth of primary axons vs. branches regulated by cAMP signaling. (D) An axon branch stochastically exhibits higher cAMP concentrations than the primary axon. (E, F) As cAMP concentrations rise in the branch through positive feedback, cAMP concentrations in the primary axon are reduced by long-range signaling. This causes the branch to extend rapidly while reducing the growth of the primary axon.
Once an axon has been specified, interstitial branches form along the axon shaft. In the mammalian nervous system, it is these branches and not the primary axon that innervate target neurons [5, 6]. Live in vivo and in vitro imaging experiments have revealed that the growth of axonal processes (primary axons and their branches) is unequal. For example, in the developing corpus callosum, interstitial branches grow into the neocortex to form lasting connections [7], whereas the primary axon retracts. In dissociated cultures, growth of axonal processes occurs in a staggered fashion. A study of dissociated hippocampal neurons with an axon and a single branch showed that one process extends while the other stalls [2] (Fig. 1D-F). Which process is favored for growth can change dynamically. When this occurs the stalling process re-extends while the formerly extending process stalls. This suggests that competitive outgrowth emerges from a reciprocal inhibition of growth between primary axons and their branches. Together, these results indicate that competition plays an important role in shaping developing axons, from specification to branching and arborization.
Surprisingly little, however, is known about the intracellular signaling pathways mediating such competitive interactions. An in vitro study of sympathetic neurons showed that local depolarization of axons growing into one compartment of a tissue culture chamber inhibits the growth of axons in a neighboring chamber [8]. Because some of the axonal processes in the unstimulated chamber were branches of axons in the stimulated chamber, this study raised the possibility that activity-dependent mechanisms could regulate competitive axon outgrowth. A later study showed that neurons exhibit activity-dependent, spontaneous calcium transients that are localized to individual axon branches [9]. Axonal processes with higher frequencies of calcium transients rapidly grew at the expense of processes with lower frequencies of calcium transients. Furthermore, experimental stimulation of calcium transients in one axonal process caused stalling or retraction of distant, unstimulated axonal processes. Similarly, during axon specification, stimulation of cyclic adenosine monophosphate (cAMP) signaling in one neurite inhibits growth of distant neurites [10]. Thus, during both axon specification and branching, localized intracellular signals can bias neurite growth in favor of one process. For growth in distant processes to be inhibited, however, a long-range signal must travel between these different neurites and axonal processes. A recent study by Shelly et al. [11] shed some light on these events by investigating the long-distance effects on intracellular signaling during axon specification.
Shelly et al. [11] focused on the role of cyclic nucleotide signaling during specification of an axon from several undifferentiated neurites in dissociated cultures of hippocampal neurons. They found that neurites growing on stripes of F-cAMP, a cell-permeant cAMP analogue, became axons more frequently than did neurites growing off of these stripes, suggesting that cAMP promotes axon formation. Cyclic guanosine monophosphate (cGMP) can antagonize cAMP signaling, and indeed neurites growing on stripes of F-cGMP preferentially became dendrites. Only neurons with the cell body resting on the stripe border were examined, so Shelly et al. [11] were able to distinguish these effects on axon specification from axonal pathfinding. Furthermore, when cAMP and cGMP signals were visualized directly with fluorescence resonance energy transfer (FRET)-based reporters, experimental manipulations that increased cAMP concentration caused a reduction in cGMP concentration in the same neurite and vice versa. This reciprocal inhibition occurred because cAMP and cGMP, acting through protein kinase A and protein kinase G respectively, activated specific phosphodiesterases that hydrolyzed the opposing nucleotide.
If cAMP promotes axon specification, an increase in cAMP concentration restricted to only a single neurite could lead to the emergence of a single axon. To investigate this possibility, Shelly et al. [11] added glass beads coated in forskolin, which increases cAMP production by activating adenylate cyclase, to neuronal cultures. As predicted, cAMP concentrations were increased locally in neurites contacting these beads and tapered off with distance from the site of contact, whereas cGMP concentrations were reduced. Unexpectedly, however, cAMP concentrations in all other neurites were reduced, whereas cGMP concentrations were increased. In contrast, when cGMP concentrations were locally increased in a neurite contacting a glass bead coated with a form of cell-permeant cGMP, no long-distance effects on concentrations of either cAMP or cGMP were apparent in distant neurites. Long-range increases in cAMP concentration in other neurites after local cGMP signaling could promote formation of multiple axons. Thus, lack of a long-range effect of cGMP signaling could explain why many neurites become dendrites whereas only one becomes the axon.
Long-range modulation of cAMP concentrations can also explain how competitive growth (e.g. the growth of one neurite inhibiting the growth of the others) emerges from local intracellular signaling pathways during axon specification. Before axon specification, different neurites may stochastically bear different amounts of cAMP (Fig. 1A). Because neurites with higher cAMP concentrations suppress cAMP signaling in other neurites, one would be expected to emerge as the "victor" of the ensuing long-range battle of reciprocal cAMP inhibition (Fig. 1B, C). Increases in cGMP concentration in the losing neurites would further decrease their cAMP concentrations, thereby inhibitingtheir growth relative to the newly specified axon [10].
Although Shelly et al. did not investigate the initiation of cAMP signaling in this system, one mechanism is suggested by a study by Davare et al. [12], showing that calcium influx through a transient receptor potential (TRP) channel initiates axon specification in hippocampal neurons by signaling through calcium-calmodulin-dependent protein kinase I (CaMKI). It is unclear whether a mechanism of long-distance cAMP suppression similar to those described by Shelly et al. [11] regulates competition among different axonal processes (Fig. 1 D-F), but there is evidence to support this possibility. Activation of TRP channels can stimulate the growth of axons and existing branches in dissociated cortical neurons [13]. In Xenopus spinal axons, repetitive bursts of calcium transients occurring spontaneously or evoked by stimulation with KCl increase cAMP concentrations [14]. This bursting pattern of calcium activity, when present in some axonal processes but not others from the same axon, also induces competitive outgrowth among these processes in cultures of mammalian cortical neurons [9]. Together, these results suggest that calcium signaling is one way of activating a local increase and (ultimately) a long-range decrease in cAMP concentrations resulting in competitive outgrowth among neurites during axon specification. This mechanism may also mediate calcium-dependent competition among axonal processes during axon branching and arborization.
One remaining question concerns how cAMP concentrations are reduced in distant neurites during long-distance signaling. One possibility is that an autocrine signaling mechanism [8, 15] globally activates cGMP signaling to reduce cAMP concentrations. This mechanism would not be expected to reduce cAMP concentration in the neurite initiating the signal because the local increase in cAMP concentration would decrease cGMP concentration. This hypothesis also explains why local cAMP concentration tapers off along the neurite rather than initiating a positive feedback loop that propagates high cAMP concentration throughout the neuron. (Positive feedback might be expected because cAMP inhibits its own hydrolysis by reducing cGMP signaling to cAMP-specific phosphodiesterases [11].) A global activation of cGMP by autocrine mechanisms would preclude this possibility. Although the mechanisms underlying competitive axon outgrowth remain incompletely understood, the study by Shelly et al. [11] offers insight into some of the long distance signals underlying the mutually inhibitory signaling events leading to the emergence of competitive behaviors during the growth of neural processes.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. I thank Drs. Susan Wray and Filippo Casoni for helpful comments on the manuscript.
References
- 1.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8(4):1454–68. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruthel G, Hollenbeck PJ. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J Neurosci. 2000;20(6):2266–74. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sengpiel F, Kind PC. The role of activity in development of the visual system. Curr Biol. 2002;12(23):R818–26. doi: 10.1016/s0960-9822(02)01318-0. [DOI] [PubMed] [Google Scholar]
- 4.Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330(6145):254–6. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–55. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- 6.Hutchins BI, Li L. EphrinA and TrkB interact to promote axon branching. J Neurosci. 2009;29(14):4329–31. doi: 10.1523/JNEUROSCI.0238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14(4):2161–77. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh KK, Miller FD. Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron. 2005;45(6):837–45. doi: 10.1016/j.neuron.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J Neurosci. 2008;28(1):143–53. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng JQ, Zheng Z, Poo M. Long-range signaling in growing neurons after local elevation of cyclic AMP-dependent activity. J Cell Biol. 1994;127(6 Pt 1):1693–701. doi: 10.1083/jcb.127.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327(5965):547–52. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 12.Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, Soderling TR, Wayman GA. Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci. 2009;29(31):9794–808. doi: 10.1523/JNEUROSCI.1544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29(18):5873–83. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca(2+) spikes. Nature. 2002;418(6893):93–6. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- 15.Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11(6):649–58. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]


