Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS). Experimental autoimmune encephalomyelitis (EAE) is widely used to dissect molecular mechanisms of MS and to develop new therapeutic strategies. The T helper 17 subset of CD4 T cells play a crucial role in the development of EAE. IL-17, a cytokine produced by Th17 cells, participates in EAE pathogenesis through induction inflammatory gene expression in target cells. Recent work has shown that Act1, a U-box E3 ubiquitin ligase, is recruited to IL-17 receptor (IL-17R) upon IL-17 stimulation and is required for IL-17-mediated signaling. Here, we review the molecular and cellular mechanisms by which IL-17 and Act1-mediated signaling contribute to EAE.
Pathogenic Th17 cells and Autoimmune Diseases
Both environmental and genetic elements are known to trigger the onset of autoimmunity, resulting in the escape of autoreactive lymphocytes from normal selection and the consequent disruption of immune tolerance1-8. Autoreactive lymphocytes are activated and expanded when they encounter their cognate “self” antigens, and infiltrate tissues leading to chronic inflammatory responses and tissue destruction. This eventually leads to organ-specific (such as multiple sclerosis and type 1 diabetes mellitus) or systemic (such as systemic lupus erythematosus and primary sjogren syndrome) autoimmune diseases.
CD4 T helper (Th) lymphocytes play essential regulatory roles in immune responses and autoimmune and inflammatory disease. Upon activation by professional antigen-presenting cells (APCs), naïve CD4 Th cells can differentiate into several subsets including: Th1 cells, characterized by production of IFNγ, which mediate cellular immunity; Th2 cells, which synthesize IL-4, IL-5 and IL-13 and function in humoral immunity and allergic responses. A third lineage of CD4 Th cells, T-helper-17 (Th17) cells, has attracted attention in immunology due to its potent pathogenic role in autoimmune and inflammatory diseases 9-21. The pathogenecity of Th17 cells is conferred by the cytokines that they produce, including IL-17A (also referred as IL-17), IL-I7F, IL-21 and IL-22. While IL-17 is the signature cytokine of Th17, recent studies have clearly shown that other cytokines (IL-21 and IL-22) produced by Th17 cells are important in the pathogenesis of inflammatory responses 22-25. In addition to CD4+ Th17 cells, IL-17 is also produced by other cell types, including CD8+ T cells, δγT and NK cells 26-28. Regardless of the source, IL-17 is a proinflammatory cytokine that up-regulates the expression of inflammatory genes in fibroblasts, endothelial cells, macrophages, epithelial cells and astrocytes. More importantly, IL-17 levels are elevated in various autoimmune diseases such as multiple sclerosis, asthma, inflammatory bowel disease, psoriasis, and rheumatoid arthritis 29,30IL-17 and IL-17R deficiency results in reduced allergen-specific immune responses and autoimmune inflammatory responses, as well as impaired host defense against bacteria and fungus infection 15,31-36. In this review, we examine IL-17 signaling pathways, including recent work that identifies the E3 ubiquitin ligase, Act1, as important for mediating events downstream of the IL-17R. We will focus on the molecular and cellular mechanisms through which IL-17-producing cells contribute to autoiummune demyelinating disease, insights into which have been gained from use of Act1-deficent mice.
IL-17-induced Act1-mediated signaling
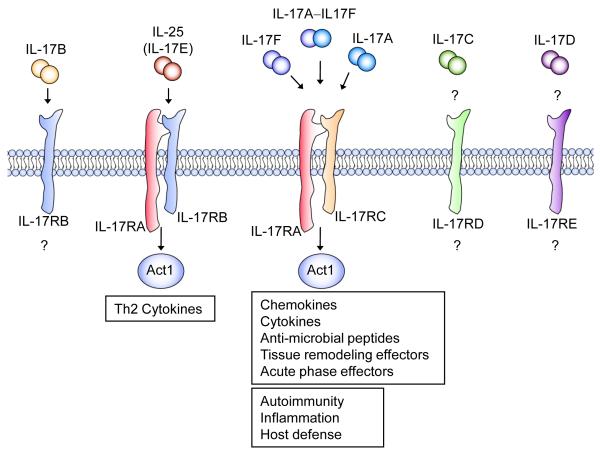
Homology-based cloning has revealed six IL-17 family members, termed IL-17A to IL17F. IL-17 (IL-17A), produced by Th17 cells, is the prototypic IL-17 family member, exerting its actions either as a homodimer or as a heterodimer with IL-17F 37,38. IL-17 coordinates local tissue inflammation through the upregulation of proinflammatory cytokines and chemokines such as IL-6, G-CSF, TNFα, IL-1, CXCL1(KC), CCL2(MCP-1), CXCL2(MIP-2), CCL7(MCP-3), and CCL20(MIP-3A)39. In addition, it induces matrix metalloproteases (MMPs) that allow activated T cells to penetrate the extracellular matrix. Recent studies have shown that IL-17 signals through a heteromeric receptor complex formed by IL-17R (IL-17RA) and IL-17RC, which are single-pass transmembrane proteins expressed by a variety of cells including astrocytes and microglia. IL-17RA can be bound by both IL-17A and IL-17F, but with 10-fold more affinity for IL-17A than for IL-17F. IL-17R (IL-17RA) and IL-17RC belong to the IL-17 receptor family, including three additional members IL-17RB, IL-17RD and IL-17RE. While both IL-17E and IL-17B bind IL-17RB, to induce Th2 cytokines, the ligand for IL-17RD is still unknown. IL-17C may be the ligand for IL-17RE, but the functional link needs to be identified. It is important to note that recent studies have shown that binding of the first IL-17 receptor subunit to the ligand modulates the affinity and specificity of the second-binding event, thereby promoting heterodimeric versus homodimeric complex formation 40.
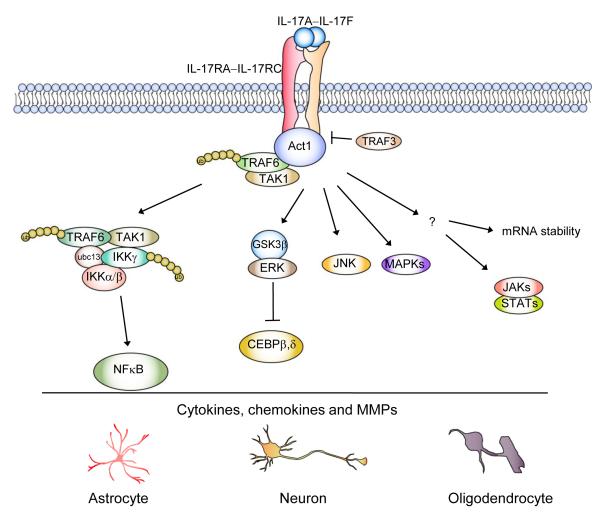
In 2003, a protein was identified in zebrafish that was termed similar expression of fibroblast-growth-factor genes, or SEF, that acts an inhibitor of FGF signaling 41. Interestingly, in mammals, the closest non-orthologous homologue of SEF is found in the IL-17 family of receptors. The sequence of homology in the cytoplasmic region of IL-17 family of receptors is referred to as the SEFIR (SEFs and IL-17Rs) domain. The signaling molecule Act1 also contains a SEFIR domain at the C-terminus, and therefore Act1 is a member of the SEFIR protein family 42-45. It was recently found that Act1 is a key component in IL-17 signaling 46,47. Following IL-17 stimulation, Act1 engages the IL-17R through SEFIR-SEFIR domain interactions. This is followed by recruitment of TRAF6 and TAK1 kinase, which mediate the downstream activation of NF-κB. In addition, Act1 contains a helix-loop-helix domain at the N-terminus, two TRAF binding sites, and a U-box-like region upstream of the SEFIR domain. Importantly, a recent study indicated that Act1 is a bona fide E3 ubiquitin ligase through its U-box-like region, activity of which is essential for IL-17-mediated signalling pathways and inflammatory gene expression 48. By utilizing the Ubc13–Uev1A E2 complex, Act1 mediates Lys 63-linked ubiquitination of TRAF6, which is crucial for the ability of TRAF6 to mediate IL-17-induced NFkB activation through the activation of kinase TAK1 and IkB kinase (IKK). On the other hand, it is intriguing that the IL-17R SEFIR domain alone is not sufficient to reconstitute IL-17-dependent signaling 33. An additional sequence downstream of the SEFIR is also necessary. Further the extended SEFIR region in the IL-17RC is required for the interaction with a phosphorylated isoform of Act1, suggesting importance for the extended SEFIR region in Act1 modification. Future studies are required to identify the kinase that is required for Act1 phosphorylation and the relationship with the extended SEFIR region in the IL-17R.
In addition to NF-κB activation, IL-17 can also activate the JAK1, JAK2 and PI3K pathway, which coordinates with NFκB activation to upregulate gene expression, especially genes important for host defense (e.g. human defensin 2 in human airway epithelial cells) 49. Moreover, a recent report showed that STAT3 is critical for IL-17-mediated CCL11 expression in human airway smooth muscle cells50. Furthermore, IL-17 can synergize with TNFα or IL-1 in the induction of inflammatory gene expression where post-transcriptional effects through mRNA stability play a major role. Interestingly, while Act1 is required for IL-17-mediated stability of KC mRNA induced by TNFα 51, TRAF6 is dispensable, implicating other signaling intermediates in mediating IL-17-dependent mRNA stability 52.
One important question is how IL-17 signaling is regulated to adequately control Th17-mediated inflammatory diseases. A recent study showed that TRAF3 is a receptor proximal negative regulator in IL-17 receptor (IL-17R) signaling 53. TRAF3 suppressed IL-17-mediated signaling pathways and subsequent induction of inflammatory cytokines and chemokines. The binding of TRAF3 to IL-17R interfered with the formation of the receptor signaling activation complex IL-17R-Act1-TRAF6, resulting in suppression of downstream signaling. Furthermore, IL-17 has been found to directly activate ERK-GSK phosphorylation cascades to inactivate C/EBP beta, a critical transcription factor for mediating induction of IL-17 responsive genes54. Future studies are required to elucidate how IL-17 signaling might be modulated at different stages of its signaling cascades.
Th17 and IL-17 in EAE, a murine model for the immunopathogenesis of MS
Although Th17 cells and IL-17 signaling have been implicated in many inflammatory and autoimmune diseases, experimental autoimmune encephalomyelitis (EAE) is the best characterized animal disease model for the effector function of Th17 cells. EAE is an animal model commonly used to study multiple sclerosis 55 and can reproduce many of the clinical and neuropathological aspects of the disease. Multiple sclerosis (MS) is an autoimmune disease in which self-reactive T-cells specific for myelin antigens elicit an inflammatory response in the central nervous system (CNS) causing damage through demyelination and subsequent axonal injury 56-58. EAE can be induced by active immunization of animals with myelin antigens or by the adoptive transfer of myelin antigen specific T cells. EAE includes an initiation stage, which involves activation and expansion of myelin specific T cells in the periphery, an effector stage in which myelin specific T cells in the CNS are reactivated resulting in CNS inflammatory response and a recovery stage in which the immune response is down regulated 59,60.
During the initiation stage, protein antigens (myelin components) and peptides are presented by antigen-presenting cells (APCs) within secondary lymphoid organs to the neuroantigen-reactive T cells leading to Th1 and Th17 cell activation and expansion9,61-65. Importantly, recent data demonstrate that both Th1 and Th17 cells can independently induce EAE possibly through different mechanisms. Several studies have suggested that Th1 and Th17 cells might induce distinct types of EAE based on histology and CNS chemokine profile66-69. Whereas Th2 cells are viewed as counter-inflammatory70, they have been shown to lead to a mild atypical form of EAE 71-73. Nevertheless, Th17 cells are now recognized at least as one of the major mediators in EAE induction and pathogenesis. The disease-relevance of Th17-mediated EAE was underscored by recent data which established a similar relationship between efficacy of IFN-β immunotherapy and Th17 cells in both EAE and MS. Interferon-β (IFN-β ), the most commonly-used treatment for MS, is ineffective in a substantial subset of patients for unknown reasons 74. In EAE, IFN-β reduced EAE signs caused by Th1 cells but exacerbated disease induced by Th17 cells. Strikingly, serum from patients who had responded poorly to IFN-β treatment showed high levels of IL-17, while those for whom IFN-β was beneficial demonstrated low serum IL-17 levels74. These results suggested that Th17-mediated pathogenesis might define a discrete subset of MS patients for whom IFN-β does not provide effective disease control.
Recent studies have shown that EAE is substantially reduced in mice lacking IL-17 or the IL-17 receptor and IL-17-specific inhibition suppresses inflammation13,35,35,36,75. Similar results were observed in mice deficient in Act1, the key signaling molecule in IL-17 signaling. Th17 cells are robustly generated in Act1-deficient mice and normally infiltrate the Act1-deficient CNS but fail to recruit hematogenously derived lymphocytes, neutrophils, and macrophages into the CNS. Taken together, these results indicate that whereas IL-17-mediated signaling is not required for the activation of T cells (the initiation stage of EAE), the IL-17-induced Act1-mediated signaling plays an essential role in the effector stage of EAE. While recent studies indicate Th1 cells can also induce EAE, it is important to point out that, in an adoptive transfer experiment, Act1-deficient recipient mice of Th1 cells exhibited similar onset and severity of EAE as wild-type recipients, indicating that Act1 deficiency has no obvious impact on the effector stage of Th1 cell-mediated EAE.
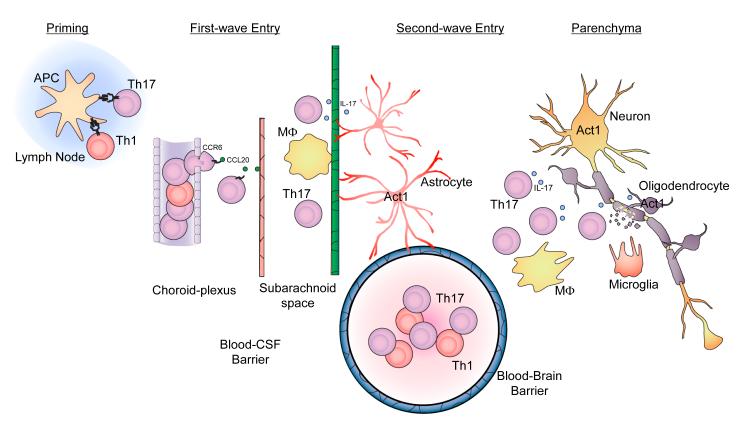
To define the effector stage of EAE, a “Two Waves hypothesis” has been proposed. After priming in peripheral lymph nodes, antigen-specific memory “pioneer” T cells traffic through the choroid plexus into the subarachnoid space (Wave 1), where they encounter antigen, presented by macrophages (meningeal APCs), are restimulated and undergo clonal expansion 76,77. Following expansion, inflammatory cytokines are upregulated and released into the subarachonoid space, acting on adjacent CNS tissue, The ensuing activation of parenchymal vasculature by this cytokine flux allows perivascular leukocyte infiltrates to accumulate (Wave 2) leading to the detrimental inflammatory cascade, a hallmark of the onset of EAE. One recent study demonstrated how Th17 cells traffic through the choroid plexus into the subarachnoid space (Wave 1). After priming in peripheral lymph nodes, CCR6+ Th17 cells escape from choroid-plexus blood vessels, migrate towards choroid-plexus epithelial cells, which express CCL20 (ligand for CCR6). Signaling through CCR6 allows T cells to cross the tight junctions between the choroid-plexus epithelial cells and enter the cerebral ventricles, from which they migrate to the subarachnoid space. In the first wave, Th17 cells cross the blood-CSF barrier 76,77 (Figure 2). Th17 cells and IL-17 signaling are thought to contribute to Wave 2 of the effector stage during EAE. Act1-deficient mice have been utilized as a model to characterize IL-17-dependent events in EAE pathogenesis.
Figure 2. Model of IL-17 signaling in CNS resident cells.
Upon IL-17 stimulation, Act1 is recruited to IL-17R through SEFIR-SEFIR domain interaction. Act1 then exerts its E3 ligase activity to mediate K63-linked ubiquitination of TRAF6, allowing TRAF6 to interact with TAK1 for subsequent NFkB activation. IL-17 activation of the other signaling events (including ERK, JNK and p38 pathways) are also Act1 dependent, however the exact mechanism leading to their activation has yet to be elucidated. During Th17-mediated EAE pathogenesis, these various IL-17-dependent signaling events impinge on the CNS resident cells to promote the production of inflammatory cytokines and chemokines and consequent demyelination and neuron degeneration.
The role of Act1 in Th17-mediated EAE pathogenesis
At the onset of Wave 2, after activation of BBB-type parenchymal and subpial vessels, infiltrating effector Th17 cells transmigrate across the endothelium and endothelial basement membrane. They are then re-activated by cognate antigen presented by local CNS APCs including perivascular or meningeal macrophages and dendritic cells. These re-activated Th17 cells subsequently migrate across the glial limitans basement membrane, deep into the parenchymal CNS white matter, and initiate tissue destruction including demyelination and axonal injury. To define the mechanistic role of IL-17 signaling in this process, one important question is what specific cell types are critical for IL-17-dependent autoimmune inflammation of the CNS.
Human Th17 lymphocytes have been shown to promote BBB disruption78. However, no differences are noted in the distribution of antigen-specific Th17 cells in the perivascular space of wild-type compared to that in Act1-deficient recipient mice 79. It seems that Act1 does not influence the access of myelin-specific T cells through the BBB into the CNS parenchyma. Although previous studies have shown that BBB endothelial cells express IL-17 receptor 78, endothelial-derived Act1 has no impact on Th17-induced BBB disruption and is dispensable for EAE development 79. After the Th17 cells transmigrate across the BBB, they are then re-activated by cognate antigen presented by local CNS APCs, resulting in Th17 cell expansion. Importantly, Act1 deficiency does not affect the proliferation and survival of Th17 cells in the CNS during EAE. The reduced accumulation of Th17 cells in the CNS of Act1-deficient mice at the peak of disease probably reflects impaired chemokine and/or cytokine mediated recruitment of leukocytes. As the major effector cells of CNS damage during EAE, perivascular macrophages and microglia are essential APC residing within the CNS that produce inflammatory cytokines and chemokines when activated 80,81. Interestingly, although IL-17 responsiveness has been detected in microglia and macrophages, myeloid-derived Act1 is also dispensable for EAE development.
Because Act1 signalling in either endothelial cells or myeloid cells is not the major source contributing to Th17-mediated EAE pathogenesis, IL-17-responsiveness of the CNS resident cells and their participation in EAE development and pathogenesis has been examined[ET1]79. Antigen-specific Th17 cells traffic through the choroid plexus into the subarachnoid space where they are reactivated. As a consequence, Th17 signature cytokines, including IL-17, are produced and act on the adjacent CNS tissue. It is possible that astrocytes with processes in the glial limitans, and around cerebral blood vessels are positioned to transduce signals from meningeal Th17 cells to activate the BBB endothelium and to drive perivascular leukocyte infiltration and concomitant inflammatory cascade associated with the onset of EAE79,82. [ET2] The large numbers of activated Th17 cells and other inflammatory cells subsequently migrate deeper into the white matter of the CNS parenchyma, resulting in tissue destruction including demyelination and eventually neurologic deficit. Th17 signature cytokines, including IL-17, may directly and/or indirectly act on oligodendrocytes and neurons, leading to demyelination and neurodegeneration. Indeed, it is found that CNS-restricted (including astrocytes, oligodendrocytes and neurons) Act1 ablation reduces EAE disease severity induced by active immunization or adoptive transfer of myelin-specific Th17 cells[ET3] 79. Consistent with reduced clinical disease, mononuclear cell infiltrates are substantially decreased in the white matter of spinal cords from CNS-restricted Act1-deficient mice relative to control mice. Signature IL-17-responsive inflammatory genes (cytokines, chemokines and matrix metalloproteinases, including CXCL1, CXCL2, CCL20, CXCL-12, GM-CSF, IL-6, MMP3 and MMP9) are greatly reduced in CNS-restricted Act1-deficient mice compared to that in control mice. One important question is which CNS resident cell type(s) is responsive to IL-17 and responsible for IL-17-dependent EAE pathogenesis. Astrocytes are responsive to IL-17 and play a major role in the production of cytokines and chemokines during EAE. IL-17- and IL-17 and TNF-induced inflammatory gene expression is reduced in Act1-deficient astrocytes as compared to that in control mice. Astrocytes have direct contacts with the glial limitans as well as the cerebral vasculature, which enables them to couple inflammatory cytokine expression to the infiltration of the CNS by leukocytes. Thererfore, IL-17-induced Act1-mediated inflammatory gene expression in astrocytes might play a crucial role for the conversion of Wave 1 to Wave 2 during the effector stage of EAE, contributing to Th17-mediated EAE pathogenesis. In addition to astrocytes, IL-17-induced Act1-mediated signaling cascades in other CNS resident cells (including oligodendrocytes and neurons) might also coordinately mediate CNS inflammation, demyelination and neurodegeneration, contributing to Th17-mediated pathogenesis of EAE.
Future Pespective
In EAE, as in MS, the majority of neurological signs reflect spinal cord dysfunction: during the initial stages of EAE this dysfunction is caused by inflammation, whereas axon loss causes impairment during the chronic phase of disease 83. Demyelination underlies potentially reversible neuronal dysfunction early in disease, whereas neurodegeneration results in permanent neurological deficit. It is tempting to propose that IL-17 signaling to specific CNS resident cells via Act1 produces both inflammatory and neurodegenerative consequences during EAE. In support of this hypothesis, it has been reported that IL-17 promotes oxidative stress-mediated oligodendrocyte apoptosis 84 and neuronal injury induced by oxygen-gucose deprivation, a condition manifested during stroke 85. By using two-photon microscopy, direct interaction was detected between MOG-specific Th17 and neuronal cells associated with extensive axonal damage86. Therefore, one important future study will be to generate astrocyte-, oligodendrocyte and neuron-specific Act1-deficient mice to delineate the cell-type specific functions of IL-17 signaling during EAE pathogenesis. On the other hand, it is important to note that Act1 is also required for signaling pathway(s) mediated by other IL-17 family member(s), such as the IL-25 (IL-17E)87,88. It is intriguing that IL-25 inhibits Th17 cell differentiation and function in an EAE model 70. While the role of Act1 in CNS resident cells is probably restricted to its signaling in the IL-17 pathway, future studies are required to carefully compare and contrast the phenotypes of the CNS cell-type specific IL-17RC- and Act1-deficient mice. Such future research will offer the first opportunity to create EAE models both for the inflammatory and neurodegenerative aspects of MS, and to further define how inflammatory cytokine IL-17 is associated with neurodegenerative tissue injury in the CNS.
Epithelial-derived Act1 is required for IL-25-mediated pulmonary inflammation
Allergic asthma is a chronic inflammatory disorder of the lung. The symptoms include bronchial hyperreactivity, infiltration of CD4+ Th2 cells, neutrophils, eosinophils and mast cells, as well as hyperplasia of smooth muscle, often associated with elevated serum IgE concentrations. The sensitization and progression towards allergic asthma are a result of reactivity of the airway epithelial cells and innate immune cells to allergens, and the subsequent induction of adaptive immunity. Recent studies have shown that IL-25 (also known as IL-17E), a member of the structurally related IL-17 family, functions as an important mediator of Th2 responses. IL-25 is produced by airway epithelial cells, T lymphocytes of the CD4+ subset with a Th2 profile, and by innate effector eosinophils and basophils. While recombinant IL-25 can induce Th2 immunity: elevated IL-4, IL-5, IL-13, eosinophilia and IgE, endogenous IL-25 is critical for allergen-induced pulmonary inflammation in a mouse asthma model. These findings clearly demonstrate that IL-25 is an important mediator of the Th2 responses, suggesting that IL-25 lies upstream of the classical Th2 cytokine pathway. We recently reported that Act1 is an essential signaling molecule for IL-25 receptor (IL-25R) signaling87. Mice deficient in Act1 have abolished IL-25-induced expression of Th2 cytokines (IL-4, IL-5, and IL-13) and chemokines (Eotaxin, TARC and RANTES) in the airway and reduced allergen-induced pulmonary eosinophilia. Importantly, Act1 deficiency in epithelial cells resulted in diminished IL-25-induced Th2 responses and lung inflammation87. Therefore, epithelial-derived Act1 is required for IL-25-mediated pulmonary inflammation.
Act1, the double-edged sword
While Act1 is necessary for IL-17-mediated inflammatory responses, Act1 deficiency actually causes mice to develop spontaneous autoimmune disease. This intriguing observation adds another layer of complexity to the role of Act1. In fibroblasts, endothelial cells, epithelial cells, astrocytes, and macrophages, Act1 serves as a component of the IL-17 receptor-signaling cascade. However, in B cells, Act1 serves as a negative regulator of CD40-CD40L and BAFF-BAFFR signaling to control B cell maturation and survival, respectively47. The loss of Act1 thus results in increased numbers of B cells, culminating in splenomegaly, lymphadenopathy, hypergammaglobulinemia, and autoantibody production. In fact, BALB/c mice develop Sjogren-like disease as early as 3wks of age, while C57BL/6 mice exhibit autoimmune phenotype by 9mos of age47,89. This observation of autoimmune phenotype has also been seen in mice with a spontaneous mutation in the Act1 gene 90. It is exciting to note that three recent independent cohort studies of psoriasis patients found a genetic mutation in Act1 that predisposes them to develop this autoimmune disease91-93. Psoriasis is a skin disease characterized by epidermal hyper-proliferation and chronic inflammation of the skin. Future studies are required to investigate the molecular mechanisms for the precise role of Act1 in modulating autoimmunity.
Figure 1. IL-17 cytokine and receptor family.
The IL-17 cytokine family includes IL-17 A-F, which are predicted to form homo- and heterodimeric interactions that are necessary for signaling. There are also five known IL-17 receptor subunits. To date, IL-17RA, -RC and –RB are the best characterized. IL-17RA is the common receptor subunit for IL-17A, IL-17F and IL-17E (IL-25) driven gene expression. IL-17A and IL-17F bind the receptor complex IL-17RA-IL-17RC to drive inflammatory gene expression. IL-25 binds to the IL-17RA-IL-17RB complex to mediate its effects on Th2 homeostasis.
Figure 3. Th17 cells in the initiation and effector stages of EAE.
(A) T cell priming and activation. Protein antigens (myelin components) and peptides are presented by Ag-presenting cells (APCs) within secondary lymphoid organs to the neuroantigen-reactive T cells leading to Th1 and Th17 cell activation and expansion. (B) First wave of Th17 cell entry. After priming in peripheral lymph nodes, CCR6+ Th17 cells escape from choroid-plexus blood vessels, migrate towards choroid-plexus epithelial cells, which express CCL20 (ligand for CCR6). Restimulation of T cells by macrophages in the subarachniod space leads to expansion of antigen-specific Th17 cells. In the first wave, Th17 cells cross the blood-CSF barrier. (C) Second wave of Th17 cell entry hypothesized in the proposal. At the onset of Wave 2, after activation of the blood-brain barrier, Th17 cells adhere at the endothelium via upregulated adhesion molecules and begin penetration of the capillary endothelium. Once the activated lymphocytes have extravasated, they are then re-activated by their cognate antigens presented by local APCs in the CNS, leading to the amplification of the inflammatory cascade in the CNS. The large numbers of activated Th17 cells and other inflammatory cells subsequently migrate deeper into the white matter of the CNS parenchyma, resulting in tissue destruction including demyelination and eventually neurologic deficit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Rizzi M, et al. Disruption of immunological tolerance: role of AIRE gene in autoimmunity. Autoimmun. Rev. 2006;5:145–147. doi: 10.1016/j.autrev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Carson MJ, et al. CNS immune privilege: hiding in plain sight. Immunol. Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassan C, Liblau RS. Immune tolerance and control of CNS autoimmunity: from animal models to MS patients. J. Neurochem. 2006 doi: 10.1111/j.1471-4159.2006.04270.x. [DOI] [PubMed] [Google Scholar]
- 4.Pagan AJ, et al. T cell-mediated activation and regulation of anti-chromatin B cells. Autoimmun. Rev. 2006;5:373–376. doi: 10.1016/j.autrev.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Rudensky AY, et al. FOXP3 and NFAT: partners in tolerance. Cell. 2006;126:253–256. doi: 10.1016/j.cell.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Gauld SB, et al. Silencing of autoreactive B cells by anergy: a fresh perspective. Curr. Opin. Immunol. 2006;18:292–297. doi: 10.1016/j.coi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Brink R. Regulation of B cell self-tolerance by BAFF. Semin. Immunol. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Cose S. T-cell migration: a naive paradigm? Immunology. 2007;120:1–7. doi: 10.1111/j.1365-2567.2006.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 10.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 15.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 16.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Weaver CT, et al. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Harrington LE, et al. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 21.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 23.Caruso R, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat. Med. 2009;15:1013–1015. doi: 10.1038/nm.1995. [DOI] [PubMed] [Google Scholar]
- 24.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 26.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz SM, et al. IL-17A is produced by Th17, gammadelta T cells and other CD4-lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 28.Passos ST, et al. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J. Immunol. 2010;184:1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 30.Hemdan NY, et al. Interleukin-17-producing T helper cells in autoimmunity. Autoimmun. Rev. 2010;9:785–792. doi: 10.1016/j.autrev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin. Immunopathol. 2010;32:33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho AW, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J. Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Garcia I, et al. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J. Immunol. 2009;182:2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y, et al. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2010;184:4307–4316. doi: 10.4049/jimmunol.0903614. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi M, et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J. Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 38.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruddy MJ, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 40.Ely LK, et al. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 2009;10:1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novatchkova M, et al. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 42.Novatchkova M, et al. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 43.Li X, et al. Act1, an NF-kappa B-activating protein. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10489–10493. doi: 10.1073/pnas.160265197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian Y, et al. Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9386–9391. doi: 10.1073/pnas.142294499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leonardi A, et al. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10494–10499. doi: 10.1073/pnas.190245697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang SH, et al. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 47.Qian Y, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 48.Liu C, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang F, et al. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 50.Saleh A, et al. Critical role for STAT3 in IL-17A-mediated CCL11 expression in human airway smooth muscle cells. J. Immunol. 2009;182:3357–3365. doi: 10.4049/jimmunol.0801882. [DOI] [PubMed] [Google Scholar]
- 51.Hartupee J, et al. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 52.Hartupee J, et al. IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. J. Immunol. 2009;182:1660–1666. doi: 10.4049/jimmunol.182.3.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu S, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen F, et al. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci. Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 56.Becher B, et al. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J. Mol. Med. 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 57.Gold R, et al. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 58.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 59.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 60.Steinman L. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 61.Steinman L. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 62.Agrawal A, et al. ERK1-/- mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:5788–5796. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 63.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korn T, et al. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 2009 doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lees JR, et al. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J. Exp. Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segal BM. Th17 cells in autoimmune demyelinating disease. Semin. Immunopathol. 2010;32:71–77. doi: 10.1007/s00281-009-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kroenke MA, et al. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kleinschek MA, et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das MP, et al. Autopathogenic T helper cell type 1 (Th1) and protective Th2 clones differ in their recognition of the autoantigenic peptide of myelin proteolipid protein. J. Exp. Med. 1997;186:867–876. doi: 10.1084/jem.186.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jager A. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinman L. A rush to judgment on Th17. J. Exp. Med. 2008;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 76.Ransohoff RM. Immunology: In the beginning. Nature. 2009;462:41–42. doi: 10.1038/462041a. [DOI] [PubMed] [Google Scholar]
- 77.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 78.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang Z, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 81.Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma X, et al. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J. Immunol. 2010;184:4898–4906. doi: 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wujek JR, et al. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- 84.Paintlia MK, et al. Synergistic Activity of Interleukin-17 and Tumor Necrosis Factor-alpha Enhances Oxidative Stress-Mediated Oligodendrocyte Apoptosis. J. Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.07136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang DD, et al. IL-17 potentiates neuronal injury induced by oxygen-glucose deprivation and affects neuronal IL-17 receptor expression. J. Neuroimmunol. 2009;212:17–25. doi: 10.1016/j.jneuroim.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Siffrin V, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Swaidani S, et al. The Critical Role of Epithelial-Derived Act1 in IL-17- and IL-25-Mediated Pulmonary Inflammation. J. Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Claudio E, et al. The Adaptor Protein CIKS/Act1 Is Essential for IL-25-Mediated Allergic Airway Inflammation. J. Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qian Y, et al. Deficiency of Act1, a critical modulator of B cell function, leads to development of Sjogren’s syndrome. Eur. J. Immunol. 2008;38:2219–2228. doi: 10.1002/eji.200738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsushima Y, et al. An atopic dermatitis-like skin disease with hyper-IgE-emia develops in mice carrying a spontaneous recessive point mutation in the Traf3ip2 (Act1/CIKS) gene. J. Immunol. 2010;185:2340–2349. doi: 10.4049/jimmunol.0900694. [DOI] [PubMed] [Google Scholar]
- 91.Ellinghaus E, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat. Genet. 2010;42:991–995. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huffmeier U, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet. 2010;42:996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strange A, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]