Abstract
Object
Deep brain stimulation (DBS) is an established neurosurgical technique used to treat a variety of neurological disorders, including Parkinson disease, essential tremor, dystonia, epilepsy, depression, and obsessive-compulsive disorder. This study reports on the use of intraoperative MR imaging during DBS surgery to evaluate acute hemorrhage, intracranial air, brain shift, and accuracy of lead placement.
Methods
During a 46-month period, 143 patients underwent 152 DBS surgeries including 289 lead placements utilizing intraoperative 1.5-T MR imaging. Imaging was supervised by an MR imaging physicist to maintain the specific absorption rate below the required level of 0.1 W/kg and always included T1 magnetization-prepared rapid gradient echo and T2* gradient echo sequences with selected use of T2 fluid attenuated inversion recovery (FLAIR) and T2 fast spin echo (FSE). Retrospective review of the intraoperative MR imaging examinations was performed to quantify the amount of hemorrhage and the amount of air introduced during the DBS surgery.
Results
Intraoperative MR imaging revealed 5 subdural hematomas, 3 subarachnoid hemorrhages, and 1 intra-parenchymal hemorrhage in 9 of the 143 patients. Only 1 patient experiencing a subarachnoid hemorrhage developed clinically apparent symptoms, which included transient severe headache and mild confusion. Brain shift due to intracranial air was identified in 144 separate instances.
Conclusions
Intraoperative MR imaging can be safely performed and may assist in demonstrating acute changes involving intracranial hemorrhage and air during DBS surgery. These findings are rarely clinically significant and typically resolve prior to follow-up imaging. Selective use of T2 FLAIR and T2 FSE imaging can confirm the presence of hemorrhage or air and preclude the need for CT examinations.
Keywords: deep brain stimulation, intraoperative MR imaging, Parkinson disease, intracranial hemorrhage, functional neurosurgery
Deep brain stimulation (DBS) is an established neurosurgical technique used to treat many neurological disorders such as Parkinson disease,1, 6 tremor, 2, 21 dystonia,10, 26 and chronic pain,13, 20 and is an emerging technique for the treatment of psychiatric disorders such as obsessive-compulsive disorder,17 depression,18 and epilepsy.8 The use of preoperative MR imaging for accurate target planning is well accepted. Intraoperative MR imaging during DBS surgery is a more recent development and offers immediate confirmation of precise lead placement and screening for complications. There are well-known safety concerns regarding complications during MR imaging following DBS lead placement,5, 15, 25, 26 but with strict attention to the imaging protocol, MR imaging can be safely performed. Of particular concern is the choice of field strength, radiofrequency coil, and MR imaging pulse sequence parameters. With proper choice we can limit radiofrequency power deposition, as measured by specific absorption rates (SARs), to within limits set by the DBS unit manufacturer.19 We present the imaging findings obtained using 1.5-T MR imaging in 143 patients who underwent intraoperative MR imaging during DBS surgery.
Methods
The study was approved by our institutional review board for human studies and is in compliance with the Health Insurance Portability and Accountability Act. This retrospective investigation included those patients who underwent DBS surgery during the 46-month period between August 2006 and May 2010 and consented to participation in clinical research; in fact, this group included all 143 patients who underwent DBS surgery during this period. The 143 patients (93 male and 50 female) underwent 152 DBS surgeries, including 289 lead placements, in which intraoperative 1.5-T MR imaging was used. The imaging protocol consisted of a preoperative examination for targeting and a second intraoperative examination following lead placement but before battery implantation. The patients’ mean age was 59 ± 14.9 years (range 3–84 years). The indications for DBS were Parkinson disease (in 76 cases), essential tremor (in 47), dystonia (in 10), Tourette syndrome (in 2), rubral tremor (in 2), chorea (in 1), tremor due to multiple sclerosis (in 1), cluster headache (in 1), phantom limb pain (in 1), post traumatic tremor (in 1), and epilepsy (in 1). The DBS procedure included electrode placement within the subthalamic nucleus (bilateral in 67 procedures, unilateral in 3), ventral intermediate nucleus (VIM) (bilateral in 46, unilateral in 14), globus pallidus interna (bilateral, 11), and other (11).
Deep Brain Stimulation Surgery
Using local anesthesia a stereotactic head frame (Leksell Model F head frame, Elekta Instruments AB) was placed prior to the stereotactic MR imaging. Imaging identified the anterior commissure, posterior commissure, midcommissural point, and target coordinates. Planning was performed to determine entry points for a safe electrode trajectory that avoided the blood vessels and ventricles. Once imaging was completed the patient was returned to the operating room where under sterile conditions and local anesthesia, bur holes were placed in the skull at the predetermined entry points. After microelectrode recording and placement of DBS 3387 electrodes (Medtronic, Inc.), test stimulation was conducted using a temporary external stimulator. The patient remained awake so that verbal feedback could be obtained to ensure that unwanted adverse effects did not occur. Confirmation of accurate electrode placement was performed first with intraoperative fluoroscopy and subsequently with MR imaging. Once the electrode placement was confirmed and trial stimulation was deemed successful, a Soletra, Kinetra or Activa pulse generator (Medtronic, Inc.) was placed in the chest area under the clavicle.
Magnetic Resonance Imaging
The MR imaging examinations were performed on a 1.5-T intraoperative scanner (GE Healthcare) running version 12.x, 14.x, or 15.x software in a 2-room configuration such that the adjacent MR imaging unit and operating room are separated by a sliding door shielded for radiofrequency penetration. Under the direct supervision of an MR imaging physicist, MR imaging examinations were performed in a transmit-receive head coil and modified such that none of the sequences exceeded 0.1 W/kg average head SAR. The MR imaging parameters for T1-weighted, 3D magnetization-prepared rapid gradient echo (MP-RAGE) included: preparation time 1000 msec; echo time, full minimum; flip angle 8°; total acquisition time 12:49 minutes; bandwidth ± 15.63 kHz; matrix size 256 × 256; slice thickness 1.2 mm with 128 axial slices (nearly equivalently, inversion recovery–fast spoiled gradient echo) can be used, provided the TI is set to 600 msec). A single dose of Gd-based contrast agent (either gadodiamide or gadobenate dimeglumine) was administered for the preoperative examination to facilitate the visualization of vessels. The MR imaging parameters for T2* gradient echo (GRE) imaging included: repetition time 650 msec; echo time 15 msec; flip angle 20°; total acquisition time 1:59 minutes; bandwidth ± 15.63 kHz; matrix size 256 × 192; slice thickness 5 mm/skip 2.5 mm, with a total of 20 axial slices obtained in 2 or 3 packs.
The MR imaging parameters for the optional 2D T2-weighted fluid attenuated inversion recovery (FLAIR) imaging included: repetition time 11,000 msec; echo time 147 msec; inversion time 2250 msec; total acquisition time 3:51 minutes; matrix size 256 × 256; and slice thickness 4 mm. Due to the high SAR associated with FLAIR, only 2 slices were typically obtained during each acquisition. The MR imaging parameters for the optional 2D T2-weighted fast spin echo (FSE) imaging included: repetition time 4350 msec; echo time 101.8 msec; total acquisition time 2:39 minutes; matrix size 256 × 256; and slice thickness 4 mm.
Results
Intraoperative MR imaging demonstrated appropriate placement of the electrodes in all 152 surgeries. Hemorrhages associated with surgery occurred in 9 patients (Table 1). The hemorrhages associated with surgery included 5 subdural hematomas (Fig. 1), 3 subarachnoid hemorrhages (Fig. 2), and 1 intraparenchymal hemorrhage (Fig. 3). In 1 patient the bur hole had to be widened to adequately coagulate a bleeding vessel caused by insertion of the microelectrode at the cortical surface. Otherwise, no other modification of surgery was necessary. Furthermore, no patient required evacuation of any of the intracranial blood. All patients with hemorrhage were admitted to the ICU for close observation. These patients had a follow-up CT scan 24 hours following their surgery. None of the patients had enlargement of their hemorrhage. One of the patients who experienced a subarachnoid hemorrhage developed a severe headache and mild confusion following the surgery, but these symptoms resolved prior to discharge on postoperative Day 3. None of the other hemorrhages resulted in clinical symptoms. Subarachnoid air was identified in 4 patients (Fig. 4) and brain settling or shift in 135 patients. Within these 135 there were a total of 143 instances of brain settling or shift including 109 bilateral, 16 right, and 18 left. Asymmetrical brain settling resulted in midline shift of 4 mm in 1 patient. None of the subarachnoid air or brain settling was clinically apparent.
TABLE 1.
Imaging findings during intraoperative MR imaging*
| Finding | No. of Pts (%) | Mean | Range |
|---|---|---|---|
| SDH | 5 (3) | 5.2 mm | 4–8 mm |
| SAH | 3 (2) | 50.5 cm3 | 11.9–116.6 cm3 |
| IPH | 1 (0.6) | 0.25 cm2 | — |
| subarachnoid air | 4 (3) | 19.16 cm3 | 0.74–63.84 cm3 |
| brain shift | |||
| right | 126 (83) | 0.54 cm | 0.1–1.2 cm |
| left | 128 (84) | 0.59 cm | 0.1–1.3 cm |
SAH = subarachnoid hemorrhage; SDH = subdural hematoma; IPH = intraparenchymal hemorrhage.
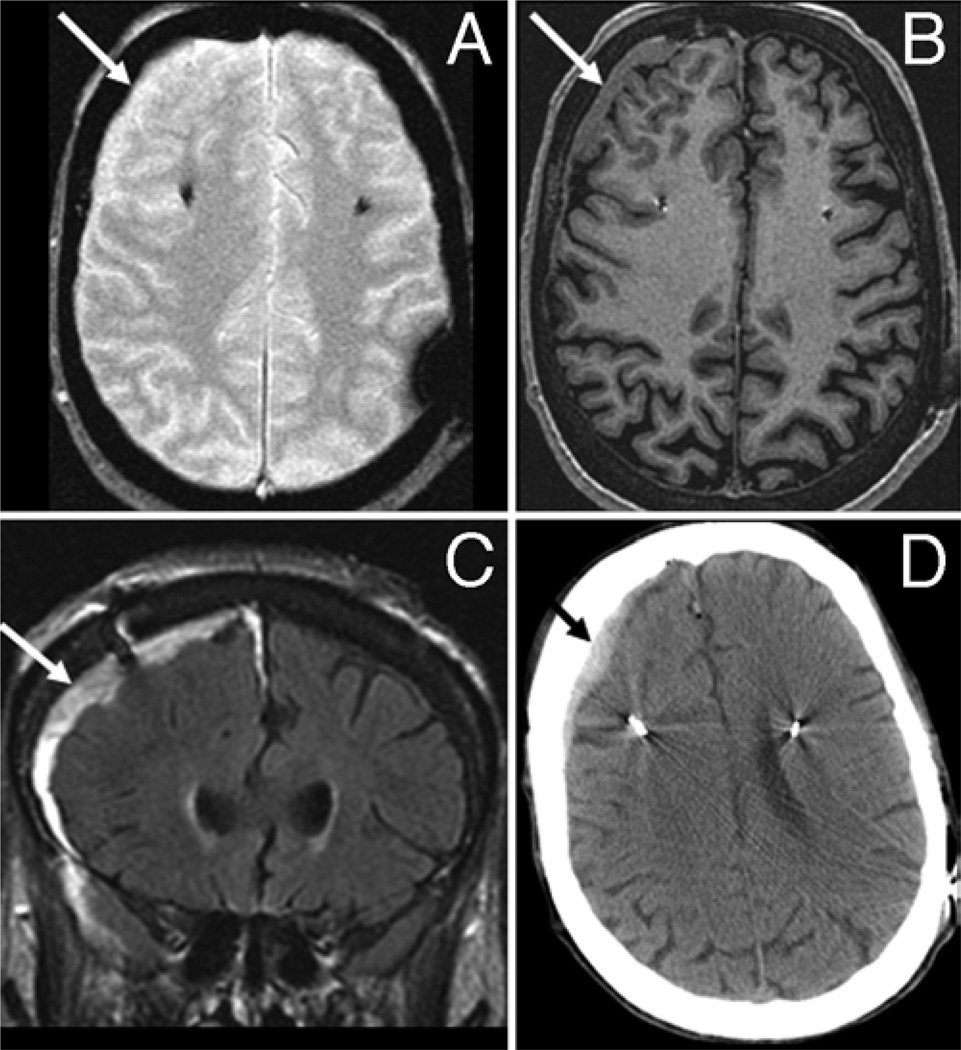
Fig. 1.
Subdural hematoma. This 69-year-old woman with Parkinson disease underwent bilateral subthalamic nucleus DBS placement. Intraoperative MR imaging revealed a right 8-mm subdural hematoma characterized as isointense (arrows) on both T2* GRE (A) and T1 MPRAGE (B) sequences. The lesion displayed high signal intensity (arrow) on FLAIR imaging (C). Computed tomography confirmed the presence of a subdural hematoma (arrow, D).
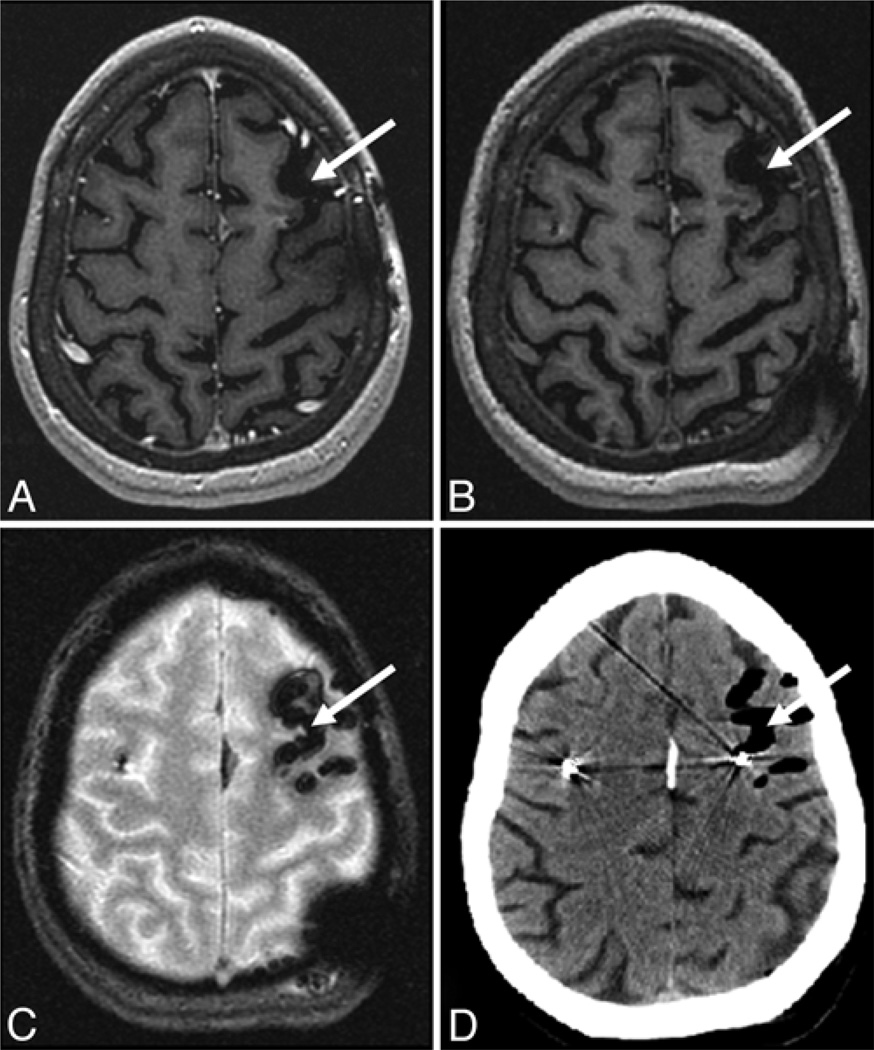
Fig. 2.
Subarachnoid hemorrhage. This 62-year-old woman with Parkinson disease who underwent bilateral subthalamic nucleus DBS electrode placement was found to have a small focus of subarachnoid hemorrhage. The preoperative MR images demonstrated low signal intensity CSF within the left frontal sulcus (arrow, A). The hemorrhage was mildly hyperintense relative to CSF with T1 MP-RAGE (arrow, B), and isointense to CSF on T2* GRE (arrow, C); it demonstrated pronounced FLAIR hyper-intensity (arrow, D). The presence of the subarachnoid hemorrhage was confirmed with CT (arrow, E).
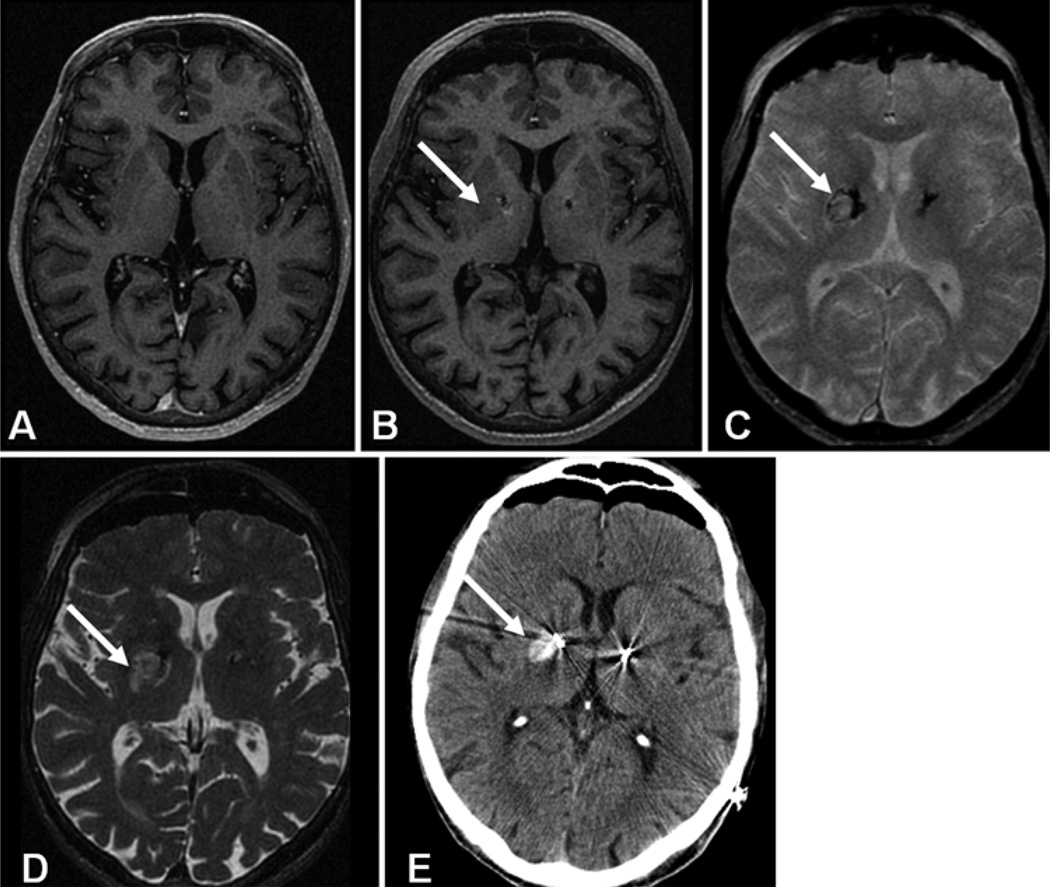
Fig. 3.
Intraparenchymal hemorrhage. This 70-year-old male with Parkinson disease underwent bilateral subthalamic nucleus DBS electrode placement. The intraoperative MR images showed a 5 × 5 mm hematoma within the right basal ganglia. Intraoperative T1 MP-RAGE demonstrated the DBS lead but the adjacent hemorrhage (arrow, B) was isointense and nearly undetectable when compared with the preoperative image (A). On T2* GRE the hematoma was characterized as isointense with a peripheral margin of low signal intensity (arrow, C) and high signal intensity on axial T2 FSE sequences (arrow, D). Computed tomography confirmed the presence of the hematoma (arrow, E). In addition the CT scan shows bilateral brain settling or shift that is also present but not as evident on the intraoperative MR images.
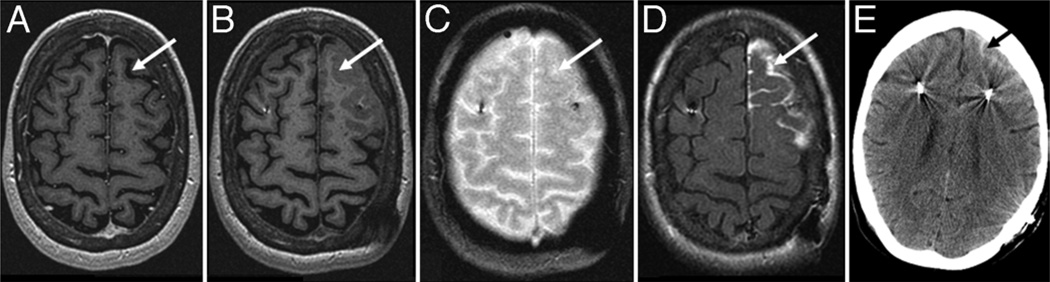
Fig. 4.
Pneumocephalus. This 58-year-old woman underwent bilateral VIM DBS for treatment of an essential tremor. The procedure included replacement of a previously placed left VIM lead. Preoperative MR imaging showed postoperative changes including a prominent left frontal sulcus (arrow, A) with low signal intensity CSF. Intraoperative T1 MP-RAGE showed low signal intensity within this sulcus due to air, similar to the preoperative study (arrow, B). T2* GRE represented the air as an absence of signal with blooming due to susceptibility effects (arrow, C). The CT scan confirmed the presence of subarachnoid air (arrow, D).
Discussion
Intraoperative MR imaging during DBS lead placement can be performed safely to confirm lead placement prior to completing the surgery and to assess for intraoperative complications. In this series, appropriate positioning of the leads was confirmed in all 152 surgeries. The intraoperative imaging allowed immediate evaluation of potential complications that in our series included 9 intracranial hemorrhages, only one producing symptoms, which lasted only 2 days. When patients return for post-surgical programming, knowledge of the lead placements from the intraoperative MR imaging examination allows more rapid and accurate programming.
Magnetic resonance imaging with DBS leads in place requires extreme caution and careful attention to imaging technique.5, 15, 25 Previous reports have documented the risk of MR imaging in these patients due to radiofrequency lesioning resulting from heating of DBS leads.11, 22 The examination must be performed in a transmit/receive head coil because performing it with a body-coil transmit and surface-coil receive mode results in much greater energy deposition. The energy deposited is measured by the SAR and according to the manufacturer’s guidelines is kept below 0.1 W/kg average head SAR. We always require the presence of an MR imaging physicist during the intraoperative MR imaging examination to ensure the sequences and the protocol are tailored for the individual patient.9 We have previously shown that placement of DBS leads does not significantly affect the radiofrequency power calculation, as measured by the change in transmit gain provided by the automatic prescan. This suggests that the leads do not significantly detune the radiofrequency coil or significantly increase reflected power.3
We have developed an imaging strategy to screen for the presence of hemorrhagic complications. The intraoperative imaging includes T1 MP-RAGE and T2* GRE sequences, both of which are associated with relatively low SAR. These sequences allow for evaluation of intraparenchymal hemorrhage with T2* GRE, while the T1 MP-RAGE sequence demonstrates the presence of extra-parenchymal subarachnoid and subdural hemorrhage. Intraparenchymal hemorrhage identified during this study displayed the expected imaging features for hyperacute blood.4, 14, 16 Intraparenchymal hemorrhage may be virtually invisible on T1 MP-RAGE imaging (Fig. 3) but is seen as subtly increased signal with a thin rim of dark signal on T2* GRE.7, 12, 24 When an intraparenchymal hemorrhage is suspected, its presence or absence can be confirmed with T2 FSE imaging where the hyperacute blood is seen as mildly increased T2 signal (Fig. 3). Subarachnoid and subdural extraparenchymal hemorrhage was demonstrated to be isointense or nearly so with both T1 MP-RAGE and T2* GRE sequences. Extraparenchymal hemorrhage can be seen displacing the CSF when subarachnoid in location or as a curvilinear extraaxial collection when subdural in location on the T1 MP-RAGE sequence (Figs. 1 and 2). Whenever there is a concern for subarachnoid or subdural hemorrhage, T2-weighted FLAIR imaging can be used, allowing the extraparenchymal blood to be easily detected due to its high signal intensity (Figs. 1 and 2). It should be noted that that the FLAIR sequence is very SAR intensive, which limits the acquisition to approximately 2 slices to remain within the 0.1 W/kg limit. This strategy of screening for intra- and extraparenchymal hemorrhage with standard T1 MP-RAGE and T2* GRE and then selectively using T2-weighted FSE and FLAIR for confirmation precludes the need for CT imaging.
Occasionally, subarachnoid air is present on the intraoperative examination. This is typically difficult to visualize on the T1 MP-RAGE images where low signal intensity CSF is displaced by low signal intensity air (Fig. 4). However, the very low signal intensity with ballooning due to susceptibility on the T2* GRE sequence makes the subarachnoid air easy to detect. One must be careful not to confuse this low signal intensity on T2* GRE images with deoxyhemoglobin seen in acute hemorrhage. If there is still uncertainty, a FLAIR sequence can be used, because subarachnoid pneumocephalus is characterized by low signal intensity in contrast to the marked high signal intensity of subarachnoid hemorrhage on FLAIR images.
Although the acquisition time for the 3D T1-weighted MP-RAGE scan is quite long (nearly 13 minutes), the entire pre- or intraoperative examination is typically completed within 15–20 minutes of scan time. The exclusive use of the transmit/receive head coil precludes use of parallel imaging methods, which does extend the acquisition time. The pre- and intraoperative MR imaging examinations have been well tolerated, with all patients completing both stages. Because the patient is situated in the head frame, image artifacts caused by patient head motion are not a concern, even with the longer scan time, so the higher spatial resolution obtained with the longer scan can be fully realized.
Starr et al.23 have recently reported an innovative DBS procedure that utilizes an intraoperative 1.5-T MR imaging unit and a skull-mounted aiming device for direct electrode placement into the subthalamic nucleus while the patient is within the magnet bore. In 29 patients with Parkinson disease, the authors reported directly targeting the subthalamic nucleus, which is characterized by T2 hypointensity due to its iron content. They reported no hemorrhagic complications and improved tip placement compared with the traditional frame-based approach. Furthermore, this approach does not include microelectrode measurements, which have been thought to increase the risk of brain hemorrhage. Thus, this raises the important possibility of performing DBS lead implantation without microelectrode recording when intraoperative confirmation of lead placement is obtained. Indeed, it is possible that our technique, as reported here, might ultimately allow the elimination of the slight risk of hemorrhage associated with microelectrode recording, which adds to the cost of surgery and is a time-intensive part of the operation, although this requires further investigation.
Conclusions
Intraoperative MR imaging during DBS surgery can be safely performed when strict attention is paid to imaging parameters and when the examination is supervised by an on-site MR imaging physicist. Intraoperative MR imaging offers improved patient care with immediate evaluation for potential complications.
Acknowledgments
This work was supported by the National Institutes of Health (K08 NS 52232 award to K.H.L.) and the Mayo Foundation (2008– 2010 Research Early Career Development Award for Clinician Scientists to K.H.L.).
Abbreviations used in this paper
- DBS
deep brain stimulation
- FLAIR
fluid attenuated inversion recovery
- FSE
fast spin echo
- GRE
gradient echo
- MR-RAGE
magnetization-prepared rapid gradient echo
- SAR
specific absorption rates
- VIM
ventral intermediate nucleus
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Watson, J Huston. Acquisition of data: Watson, J Huston, Gorman, Lee. Analysis and interpretation of data: Watson, J Huston, Bernstein, McGee, Stead, O Huston. Drafting the article: Watson, J Huston, O Huston. Critically revising the article: Watson, J Huston, Bernstein, Stead, Lee, O Huston. Statistical analysis: O Huston. Study supervision: Watson.
References
- 1.Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson’s disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien) 1993;58:39–44. doi: 10.1007/978-3-7091-9297-9_8. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein MA, Ward HA, McGee KP, Felmlee JP, Knudsen VL, Gorny KR, et al. Changes in RF transmit gain before and after DBS lead placement in 36 consecutive patients. Proc Int Soc Mag Reson Med. 2009;17:3105. (Abstract) [Google Scholar]
- 4.Bradley WG., Jr MR appearance of hemorrhage in the brain. Radiology. 1993;189:15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra V, Sung E, Mewes K, Bakay RA, Abosch A, Gross RE. Safety of magnetic resonance imaging of deep brain stimulator systems: a serial imaging and clinical retrospective study. Clinical article. J Neurosurg. 2010;112:497–502. doi: 10.3171/2009.7.JNS09572. [DOI] [PubMed] [Google Scholar]
- 6.Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 7.Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35:502–506. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- 8.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 9.Gorny KR, Bernstein MA, Felmlee JP, Ward HA, McGee KP, Lanners DM, et al. Calorimetric calibration of head coil SAR estimates displayed on a clinical MR scanner. Phys Med Biol. 2008;53:2565–2576. doi: 10.1088/0031-9155/53/10/008. [DOI] [PubMed] [Google Scholar]
- 10.Greene P. Deep-brain stimulation for generalized dystonia. N Engl J Med. 2005;352:498–500. doi: 10.1056/NEJMe048333. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JM, Tkach J, Phillips M, Baker K, Shellock FG, Rezai AR. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson’s disease: case report. Neurosurgery. 2005;57:E1063. doi: 10.1227/01.neu.0000180810.16964.3e. [DOI] [PubMed] [Google Scholar]
- 12.Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004;35:1989–1994. doi: 10.1161/01.STR.0000133341.74387.96. [DOI] [PubMed] [Google Scholar]
- 13.Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973;29:158–161. doi: 10.1001/archneur.1973.00490270040005. [DOI] [PubMed] [Google Scholar]
- 14.Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 15.Larson PS, Richardson RM, Starr PA, Martin AJ. Magnetic resonance imaging of implanted deep brain stimulators: experience in a large series. Stereotact Funct Neurosurg. 2008;86:92–100. doi: 10.1159/000112430. [DOI] [PubMed] [Google Scholar]
- 16.Linfante I, Llinas RH, Caplan LR, Warach S. MRI features of intracerebral hemorrhage within 2 hours from symptom onset. Stroke. 1999;30:2263–2267. doi: 10.1161/01.str.30.11.2263. [DOI] [PubMed] [Google Scholar]
- 17.Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 2007;61:1–13. doi: 10.1227/01.neu.0000279719.75403.f7. [DOI] [PubMed] [Google Scholar]
- 18.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Medtronic. MRI Guidelines for Medtronic Deep Brain Stimulation Systems. Minneapolis: Medtronic Inc; 2007. [Accessed May 24, 2011]. ( http://professional.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/dbs-2007-mri.pdf) [Google Scholar]
- 20.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21(6):E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 21.Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18:163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel J, Fuss G, Backens M, Reith W, Magnus T, Becker G, et al. Transient dystonia following magnetic resonance imaging in a patient with deep brain stimulation electrodes for the treatment of Parkinson disease. Case report. J Neurosurg. 2003;99:772–774. doi: 10.3171/jns.2003.99.4.0772. [DOI] [PubMed] [Google Scholar]
- 23.Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. Clinical article. J Neurosurg. 2010;112:479–490. doi: 10.3171/2009.6.JNS081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taber KH, Hayman LA, Herrick RC, Kirkpatrick JB. Importance of clot structure in gradient-echo magnetic resonance imaging of hematoma. J Magn Reson Imaging. 1996;6:878–883. doi: 10.1002/jmri.1880060607. [DOI] [PubMed] [Google Scholar]
- 25.Tagliati M, Jankovic J, Pagan F, Susatia F, Isaias IU, Okun MS. Safety of MRI in patients with implanted deep brain stimulation devices. Neuroimage. 2009;47(Suppl 2):T53–T57. doi: 10.1016/j.neuroimage.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 26.US Federal Food and Drug Administration. [Accessed May 24, 2011];FDA Public Health Notification: MRI-caused injuries in patients with implated neurological stimulators. 2005 May 10; ( http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062125.htm)
- 27.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]