Abstract
MicroRNAs (miRNA), a class of non-coding RNAs, are emerging as important modulators of neuronal development, structure and function. A connection has been established between abnormalities in miRNA expression and miRNA-mediated gene regulation and psychiatric and neurodevelopmental disorders as well as cognitive dysfunction. Establishment of this connection has been driven by progress in elucidating the genetic etiology of these phenotypes and has provided a context to interpret additional supporting evidence accumulating from parallel expression profiling studies in brains and peripheral blood of patients. Here we review relevant evidence that supports this connection and explore possible mechanisms that underlie the contribution of individual miRNAs and miRNA-related pathways to the pathogenesis and pathophysiology of these complex clinical phenotypes. The existing evidence provides useful hypotheses for further investigation as well as important clues for identifying novel therapeutic targets.
Keywords: microRNA; psychiatric disorder; schizophrenia; risk gene; 22q11.2; intellectual disability, autism; cognitive dysfunction
1. Introduction
Non-coding RNAs (ncRNAs), transcribed RNAs that are not further translated into proteins, play an important regulatory role in shaping protein production and are an integral part of the epigenetic network. One class of ncRNAs that has been extensively studied in recent years is miRNAs, which are about 22 nucleotides long (Bartel, 2004). miRNAs regulate gene expression primarily through post-transcriptional gene silencing by complementary binding to their target mRNAs (Lewis et al., 2003). The interaction of miRNAs with their target mRNAs is largely through 5’ seed region of the miRNA and one or more binding sites in the 3’UTR of the targets, though it is shown that the interaction can be mediated through other regions. This interaction directs miRNA-associated complexes to mediate translational repression and/or mRNA degradation (Prosser et al., 2011). Since the interaction of miRNAs and their mRNA targets are primarily determined by the short seed region encompassing only 6-8 nucleotides, one miRNA typically has multiple mRNA targets (Lewis et al., 2005) while several miRNAs can bind on the same mRNA target. Therefore, miRNAs can thus act in combinatorial or synergistic fashion by integrating different intracellular signals and/or coordinating several different signaling pathways at once (Krek et al., 2005). Furthermore, the production of miRNAs is regulated at various steps during biogenesis, both at transcription and post-transcriptional levels (Krol et al., 2010b). Importantly, miRNAs can incorporate into different RNA-binding protein complexes, which provide information for subcellular localization and control the accessibility of potential targets at different intracellular locations. Genuine interaction between a miRNA and its targets can be experimentally detected and validated by either indirect methods, such as luciferase assays or by more direct approaches such as high-throughput sequencing of RNA isolated by crosslinking and immunoprecipitation (HITS-CLIP) (Chi et al., 2009) and Tandem affinity purification of miRNA target mRNAs (TAP-Tar) (Brest et al., 2011) (reviewed by (Thomson et al., 2011). More recently a resource of mouse targeted miRNA knockout embryonic stem cells has been generated (Prosser et al., 2011). Studies utilizing such approaches and resources are poised provide a comprehensive understanding the role that miRNAs play in animal development and disease.
Overall, miRNA provide great control flexibility by integrating signals from different pathways under a variety of physiological conditions and, therefore, can have a great impact on neuronal function and communication (Cao et al., 2006). Along these lines, it is becoming increasingly clear that miRNAs have a profound impact on cognitive function and are involved in the etiology of several neuropsychiatric disorders, including schizophrenia, mental retardation (or intellectual disability) as well as autism and autism spectrum disorders. Here, we first summarize relevant evidence from human and animal model studies and then we discuss the contribution of some of the altered miRNAs to various neural processes that can potential impact psychiatric disease pathophysiology.
2. Altered miRNA expression and function in various neuropsychiatric disorders
Accumulating evidence from human and animal studies strongly suggests that alterations in miRNA regulation or function associate with the genetic architecture of neuropsychiatric disorders including schizophrenia, autism and various forms of intellectual dysfunction as summarized below.
2.1 Schizophrenia
Schizophrenia (SCZ) is one of the most common psychiatric disorders with a prevalence of ~1% in most of the populations studied worldwide (Xu et al., 2011). SCZ is a disabling disease, which is characterized by positive (psychotic) symptoms such as hallucinations, delusions, and disorganized behavior, negative symptoms such as social withdrawal and apathy, as well as increasingly recognized cognitive deficits (Arguello et al., 2010). Classical family, twin, and adoption studies estimating the recurrence risk to relatives have provided direct evidence for a genetic etiology. The risk of developing SCZ increases exponentially with the degree of genetic relatedness to a patient and reaches ~50% for a monozygotic twin (Sullivan et al., 2003).
miRNA profiling in postmortem brain tissues from individuals with SCZ has shown alterations in the levels of many miRNAs (Beveridge et al., 2009; Beveridge et al., 2010; Beveridge et al., 2008; Kim et al., 2010; Moreau et al., 2011; Perkins et al., 2007; Santarelli et al., 2011)(see also below). However miRNA dysregulation in the disease brain is not specific to SCZ and has been described in a variety of other psychiatric, neurodevelopmental and neurological disorders (Abu-Elneel et al., 2008; Kuhn et al., 2008; Talebizadeh et al., 2008). Given the important role that miRNA play in posttranscriptional gene regulation and their potential to regulate a large number of target genes, the majority of the observed changes likely reflect reactive changes due to the disease state or medication. Such changes cannot be interpreted as indicative of a role of miRNAs in the disease pathogenesis and pathophysiology. Given the strong genetic component of SCZ, conclusive evidence that miRNAs might be one of the important components of the etiology and pathophysiology of SCZ can only be obtained by analyzing the impact that well established mutations or proximal processes affected by them (Kvajo et al., 2010) have on the formation, steady-state levels and function of miRNAs. In that respect it is notable that the most important insight into the relationship between SCZ etiology and miRNAs come from recent studies on a mouse model of the 22q11.2 microdeletion (Stark et al., 2008), a well established and largest known genetic risk factor for schizophrenia (Karayiorgou et al., 2010; Xu et al., 2010)
The 22q11.2 microdeletion is a major recurrent de novo copy number variant (CNV) responsible for introducing new SCZ cases in the population (ISC, 2008; Karayiorgou et al., 1995; Stefansson et al., 2008; Xu et al., 2008a; Xu et al., 2009). A 1.5-Mb human 22q11.2 region has been shown to be the critical region for 22q11.2 microdeletion syndrome. Because this 1.5-Mb region is highly conserved in the syntenic region of mouse chromosome 16 and harbors nearly all orthologues of the human genes, a mouse model carrying the microdeletion (Df(16)A+/−) was generated to investigate the abnormalities at different levels (Drew et al., 2011). Df(16)A+/− mice exhibit a variety of structural, behavioral, and cognitive alterations that are correlated with neuroanatomical abnormalities and cognitive dysfunction found in individuals with 22q11.2 microdeletions. For example, disruption of prepulse inhibition (PPI), a measure of sensorimotor gating and preattentive processes, is observed in both Df(16)A+/− mice (Stark et al., 2008) and individuals with 22q11.2 microdeletions(Ornitz et al., 1986; Sobin et al., 2005). In addition aspects of cognitive dysfunction in 22q11.2 microdeletion carriers(Casey et al., 1995) (Lajiness-O’Neill et al., 2005; Shprintzen et al., 1978) are also observed in Df(16)A+/− mice, as demonstrated by decreased accuracy in delayed non-match to place (DNMP) task of spatial working memory and deficits in both cued and contextual fear conditioning (Stark et al., 2008). Morphological analysis reveals that the CA1 neurons of Df(16)A+/− animals have simplified dendritic trees and decreased spine density (Mukai et al., 2008), which may partially account for reduction in hippocampal volume (Campbell et al., 2006; Eliez et al., 2000; Simon et al., 2005) in individuals with 22q11.2 microdeletions. Moreover, altered neural synchrony between dorsal hippocampus (HPC) and medial prefrontal cortex (PFC), as compared to WT mice (Sigurdsson et al., 2010) is consistent with PFC-HPC coupling abnormalities observed in schizophrenia patients (Ford et al., 2002; Lawrie et al., 2002; Meyer-Lindenberg et al., 2005). Although additional comparative analysis is necessary, results so far outline a number of conserved anomalies in hippocampal and frontal circuitry in 22q11.2 microdeletion carriers and the Df(16)A+/− mouse model (Drew et al., 2011; Karayiorgou et al., 2010) and provide a reliable model to interrogate the effects of miRNA dysregulation on neural circuit structure and function in a disease context.
mRNA and miRNA profiling indicated that miRNA alterations represent a major changes in Df(16)A+/− mice. Dgcr8 gene, an important component of the miRNA biogenesis, is located within the 1.5-Mb microdeletion region (Stark et al., 2008). Stark et al. showed that the hemizygous deletion of the Dgcr8 gene is the cause of down-regulation (by ~20–70%) of 10-20% of all known mature miRNAs, including a number of miRNA clusters involved in neural development (see section 3.2) (Stark et al., 2008). In addition to Dgcr8, the 22q11.2 microdeletion and the equivalent mouse deficiency also remove one copy of a miRNA gene, miR-185, located within the minimal 1.5-Mb 22q11.2 critical region.
Compelling evidence for miRNA dysregulation due to 22q11.2 microdeletions provides a useful etiological context to interpret results obtained from ongoing studies monitoring miRNA expression changes in the brains and peripheral blood cells in schizophrenia. For example, two recent studies provide supporting evidence suggesting that miRNAs dysregulated as a result of the 22q11.2 microdeletion may have a more general role in SCZ pathogenesis (Figure 1). Moreau MP et al. tested 435 miRNAs and 18 small nucleolar RNAs in the Brodmann area 9 of the prefrontal cortex using real-time quantitative PCR. After controlling for confounding variables such as sample storage time, brain pH, alcohol at time of death, and postmortem interval, 19% of analyzed miRNAs exhibited altered expression associated with diagnosis of SCZ or bipolar disorder and both conditions were associated with reduced miRNA expression levels (Moreau et al., 2011). Interestingly, when the 24 misexpressed miRNAs with posterior probabilities of a nonzero diagnostic effect > 95% were compared with 22 identified in the mouse model of 22q11.2 microdeletion, 8 of them overlapped. Another line of evidence came from an expression profiling study of miRNAs in peripheral blood mononuclear cells of 112 patients with SCZ and 76 non-psychiatric controls (Gardiner et al., 2011). Gardiner et al. showed that a cluster of 17 of the most substantially downregulated miRNAs were located within an imprinted region (DLK1-DIO3) on chromosome 14 (14q32). These miRNAs account for 53% of the 30 miRNAs that lie within this locus and are expressed in the peripheral blood mononuclear cells (Gardiner et al., 2011). Notably, the expression levels of many miRNAs within this cluster including miR-134 were also down-regulated in Df(16)A+/− and Dgcr8+/− mice. It is interesting to note that many of these convergent miRNAs have been suggested to be synaptically enriched (Lugli et al., 2008) (Figure 1).
Figure 1. Convergent downregulation of miRNAs in schizophrenia patients and Df(16)A+/- mice.
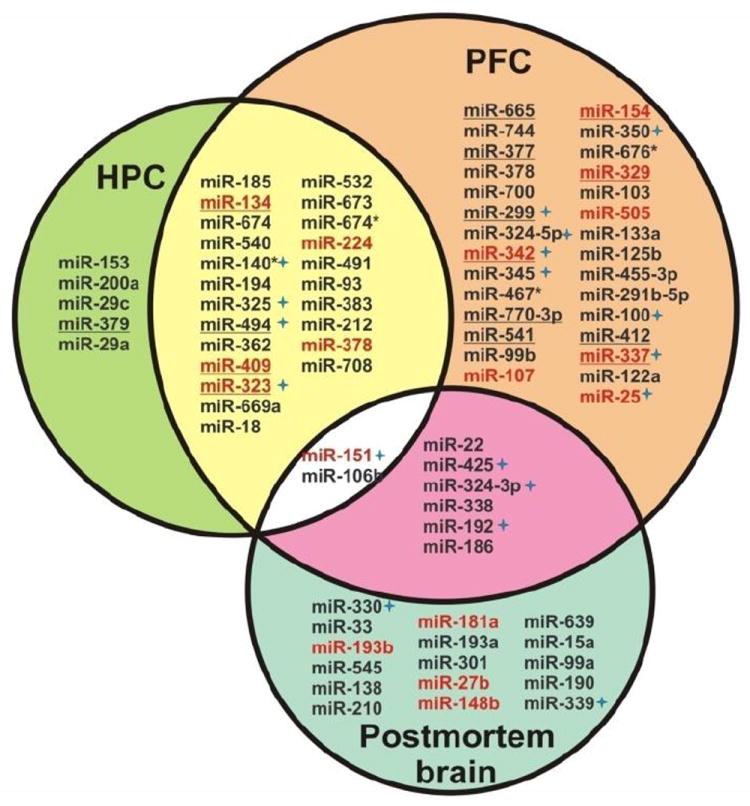
In Df(16)A+/− mice, expression of 30 miRNAs in HPC and 60 miRNAs in PFC is reduced due to hemizygosity of Dgcr8 gene. Among them 25 miRNAs are downregulated in both HPC and PFC. In a study of postmortem brain samples from patients with SCZ or bipolar disorder (BP), Moreau et al. identified 24 dysregulated miRNAs (having >95% posterior probability of nonzero effect of psychiatric diagnosis). Among those, 8 are down-regulated in the PFC of Df(16)A+/− mice, including miR-151 and miR-106b that are downregulated in the HPC as well (Moreau et al 2011). In addition, miRNA profiling in PBMC of patients with SCZ found 83 miRNAs downregulated with a false discovery rate (FDR) < 5% (Gardiner at al., 2011). Interestingly, 15 miRNAs, including miR-134, are transcribed and possibly co-regulated from the maternally expressed DLK1-DIO3 locus on chromosome 14q32 (a homologous locus on mouse chromosome 12qF1), suggesting that fine control of miRNA expression from this locus may be critical for normal brain development and function. MiRNAs identified as downregulated in both the Gardiner et al study and the Df(16)A+/− mice are shown in RED. Overlapping miRNAs located in the DLK1-DIO3 locus are underlined. It is important to note that many of these convergent miRNAs are synaptically enriched, suggesting they may function at synaptic sites. Those miRNAs with a synaptic enrichment ratio (synaptic fraction/total homogenate) > 2 are marked with a blue star (Lugli et al 2008).
Although the observed fold change of individual miRNAs in studies of the Df(16)A+/− mouse model (Stark et al., 2008) as well as in human postmortem studies (Beveridge et al., 2009; Beveridge et al., 2010; Beveridge et al., 2008; Gardiner et al., 2011; Kim et al., 2010; Moreau et al., 2011; Perkins et al., 2007; Santarelli et al., 2011) is generally small, it is conceivable (although not yet unequivocally established) that even relatively small changes in specific miRNA levels can disrupt the regulation of the target protein expression. A recent study provided support to this notion by demonstrating that protein synthesis of a given target gene is nonlinearly sensitive to a threshold of its mRNA level, that is, protein production is severely repressed when its mRNA level is below the threshold and is proportion to the mRNA level when above the threshold. It is miRNAs that fine tune this threshold level of target mRNAs (Mukherji et al., 2011). Notably, many of the miRNAs found altered in the aforementioned expression profiling studies are not among the most highly expressed miRNAs in the brain, suggesting that instead of stoichiometrically saturating their target mRNAs under physiological conditions, they may “coexist” with them and fine tune their threshold levels. Transition of target mRNA levels around the threshold may be sensitive to even modest fluctuation in the levels of such miRNAs (especially for targets that have multiple binding sites for the dysregulated miRNAs) thus having considerable impact on target protein production. It has been recently shown that alterations in the levels of at least some miRNAs have a low magnitude but widespread impact on proteome, consistent with the notion that individual miRNAs can act as “rheostats” to adjust the fine-scale control of global protein output (Baek et al., 2008; Selbach et al., 2008). In that context, modest dysregulation of many miRNAs, which may interact additively or cooperatively, could, in principle, have an even more prevalent impact on proteome. Although more studies are required to examine if such effects are at play in individuals with 22q11.2 microdeletions, an alternative, but not mutually exclusive scenario is that 22q11.2 microdeletions, by partially disabling miRNA machinery, may act by creating a sensitized genetic background where the generally modest changes in the levels of a subset of miRNAs may act by unmasking additional deleterious mutations in these miRNA that affect their expression or activity but remain “dormant” in a wild type genetic background (Brenner et al., 2010).
Although targets of the Dgcr8-dependent miRNA dysregulation have not been reported yet, the functional consequences of alterations in miRNA biogenesis have been studied in some detail (Figure 2). Behavioral tests showed that Dgcr8-deficient mice show impaired acquisition of the spatial working memory-dependent task (the T-maze delayed nonmatch to place task) as seen in Df(16)A+/− mice suggestive of altered function of the frontal regions of the mouse neocortex and/or their interaction with the hippocampus. Interestingly, unlike Df(16)A+/− mice, Dgcr8-deficient mice have normal associative memory. Thus, Dgcr8 deficiency and the ensuing abnormality of miRNA biogenesis appears to contribute to some but not all of the cognitive phenotypes observed in the Df(16)A+/− mice. Pinpointing the affected miRNAs and their targets will facilitate the identification of the neural substrates underlying these phenotypes. Notably, cognitive deficits, in particular working memory deficits, have become increasingly recognized as key components of SCZ and may reflect a more general disruption of neural networks that underlie both sensory perception and cognition. Working memory is thought to be primarily modulated by the prefrontal cortex (PFC) and to depend on persistent and recurrent neuronal excitation even in the absence of continued sensory stimulation. In that respect, the cognitive profile of Dgcr8 deficiency may reflect a bottom up impact from defects in neuronal connections and/or synaptic transmission or plasticity to cortical networks. Consistent with this notion, electrophysiology test on prefrontal pyramidal neurons of Dgcr8+/− mutant mice showed that layer 5 (L5) pyramidal neurons from heterozygous mutant mice showed a higher level of short-term synaptic depression (STD) and less potentiation following physiologically relevant persistent high-frequency stimulation, while intrinsic membrane properties and basal synaptic transmission upon activation of superficial layer afferents are normal. These synaptic phenotypes implicate a deficit at the presynaptic level in prefrontal pyramidal neurons of Dgcr8+/− mutant mice. On the contrary, unlike the robust deficits observed in the PFC basic synaptic transmission and plasticity at the CA3/CA1 synapse of Dgcr8+/− mice appeared normal suggesting that the effects of Dgcr8 deficiency on synaptic plasticity are not manifested ubiquitously in the brain. Dgcr8 deficiency caused only modest morphological changes in both PFC and hippocampus (Fenelon et al., 2011; Stark et al., 2008). These included changes in the density of layer 2/4 (L2/4) neurons, a modest but significant decrease in the size of spines of basal dendrites of cortical L5 and hippocampal CA1 pyramidal neurons as well as a modest decrease in the complexity of peripheral basal dendritic branches in CA1 pyramidal neurons. Using an independent Dgcr8+/− mouse model Schofield CM et al. showed that layer V pyramidal neurons in the medial prefrontal cortex of Dgcr8-deficient mice have decreased complexity of basal dendrites, and electrical properties were altered including decrease of frequency but not amplitude of miniature excitatory postsynaptic currents (mEPSC) and spontaneous excitatory postsynaptic Currents (sEPSC) of L5 pyramidal cells in slices from P25-30 mice (Schofield et al., 2011). The reason for the discrepancy between this and the Fenelon et al study regarding basal excitatory transmission and dendritic structures is not clear. Nevertheless, both studies suggested the possible involvement of miRNA dysregulation in neuronal electrophysiological properties which warrants further investigation.
Figure 2. Alterations in cognitive performance and synaptic plasticity due to 22q11.2 associated miRNA dysregulation.
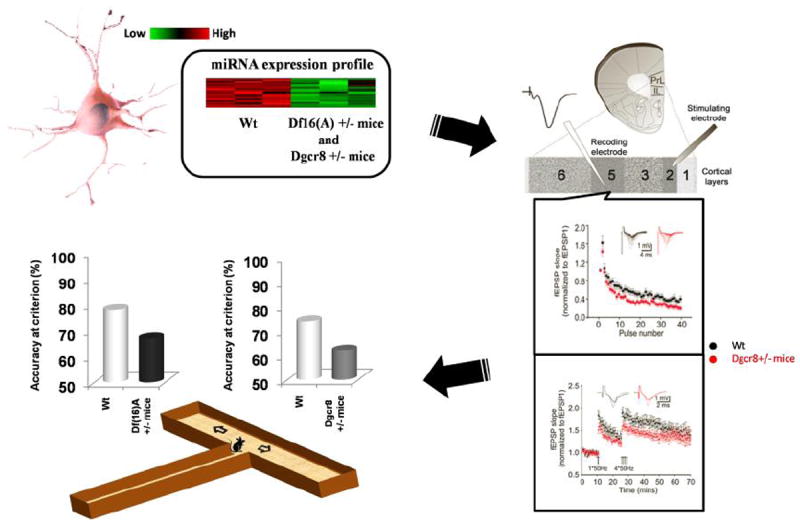
Both Df(16)A+/− and Dgcr8+/− mice show deficits in the T-maze delayed nonmatch to place task. Animals lacking a single copy of Dgcr8 (Dgcr8+/− mice) show similar spatial WM deficits and offer a simpler genetic model for identifying at least some potential neural substrates. Interestingly, mPFC layer 5 neurons in Dgcr8+/− mutants show greater synaptic depression, less synaptic summation and reduced synaptic potentiation upon stimulation of their superficial afferents.
Recent human genetic studies have started providing suggestive evidence that the contribution of miRNAs and related processing enzymes to the genetic etiology of SCZ may extend beyond the 22q11.2 SCZ susceptibility locus. First of all, several large-scale genome-wide scans for structural variants associated with SCZ have identified a number of variants within the genes that control the miRNA biogenesis pathway. For example, Xu et al identified a de novo duplication encompassing the DICER1 gene in a genome-wide scan for de novo CNVs in sporadic SCZ (Xu et al., 2008a). CYFIP1, another gene within a recurrent CNV regions in a SCZ cohort on 15q11.2(Stefansson et al., 2008), binds two components of miRNA mediated translational control machinery, the Fragile X Mental Retardation Protein (FMRP) and the translation initiation factor eIF4E (Jin et al., 2004; Napoli et al., 2008). In addition, genome-wide scans for CNVs have also identified a number of structural variants enriched in patients with SCZ that contain miRNAs. For example, hsa-miR-211 and hsa-miR-484 are within CNVs at 15q13.1 and 16p13.11 identified by several genome scans in SCZ samples (Ingason et al., 2009; ISC, 2008; Kirov et al., 2009; Kirov et al., 2008; Stefansson et al., 2008). Furthermore, Hansen et al. conducted an association study of 101 brain expressed miRNA loci in a Danish and Norwegian SCZ cohort using a case-control design. They found suggestive evidence that two miRNA loci, miR-206 and miR-198 were associated with SCZ in the Danish and Norwegian sample, respectively (Hansen et al., 2007). More recently, a large sample genome-wide association study reported a strong association between SCZ and a genetic variant in the vicinity of the miR-137 gene locus at chromosome 1p21.3 as well as weaker associations with a number of predicted miR-137 targets (Ripke et al., 2011). The effect of the linked variant on the expression of miR-137 remains unknown and, provided that the reported association is not a false finding, it is expected to be rather modest. In addition, although supporting evidence for some of the predicted targets has been obtained using in vitro assays (Kwon et al., 2011) whether predicted targets represent genuine targets in vivo and more importantly whether they are responsive to the expected modest changes in miR-137 expression remains to be determined. This is an important issue given the rather poor correct prediction rate of available programs (Rajewsky, 2006) and the fact that suppression of downstream targets is miRNA-concentration dependent (Mukherji et al., 2011). As we noted elsewhere (Rodriguez-Murillo et al., 2011) interpreting results from GWAS should be done with care and in the absence of a link between miR-137 common variants and the function or expression of this miRNA the possibility that the positive correlation from GWAS reflect disease risk confered by a neighboring gene/locus cannot yet be excluded.
As mentioned above, miRNA expression-profiling studies have also observed significant changes in miRNA levels in postmortem brains of individuals with SCZ (Beveridge et al., 2009; Beveridge et al., 2010; Beveridge et al., 2008; Kim et al., 2010; Moreau et al., 2011; Perkins et al., 2007; Santarelli et al., 2011). In the first study of this kind, Perkins et al. studied the expression pattern of 264 human miRNAs using postmortem prefrontal cortex samples from 13 patients with SCZ and two with schizoaffective disorder, as well as 21 psychiatrically unaffected controls. They showed that fifteen miRNAs were significant decreased and one was upregulated in SCZ patients as compared with controls (Perkins et al., 2007). Although there are discrepancies among the various miRNA expression profiling studies, in aggregate and in the context of accumulating evidence from human genetic studies, they tend to support the view that altered miRNA levels could be a significant factor in the dysregulation of cortical gene expression in SCZ at least at the mRNA level. In the same context, the observations that the expression levels of some miRNAs are sensitive to antipsychotics or psychotomimetics drugs can also be interpreted as supportive (but not conclusive) evidence of involvement of miRNA related regulation in SCZ etiology. For instance, three miRNAs, miR-128a, miR-128b and miR-199a were up-regulated in response to haloperidol treatment in rats as compared to untreated controls (Perkins et al., 2007). In an independent study, miR-219 expression level was reduced in the prefrontal cortex of mice in response to dizocilpine, a selective NMDA receptor antagonist. This dizocilpine-induced effect on miR-219 could be attenuated by pretreating the mice with the antipsychotic drugs haloperidol and clozapine (Kocerha et al., 2009).
2.2 Autism spectrum disorders
Autism spectrum disorders (ASDs) are a heterogeneous group of neurodevelopmental disorders with impairment in social interaction and repetitive and stereotyped behaviors (as defined in DSM-IV, American Psychiatric Association, 1994). Symptoms start at age three or earlier. The prevalence of ASDs in general population is about 1%. Family and twins studies indicate a strong genetic component (Bailey et al., 1995; Folstein and Rutter, 1977; Greenberg et al., 2001; Steffenburg et al., 1989).
Several recent studies started to explore the possibility of whether dysregulation of miRNAs plays a role in ASDs. Several human genetic studies provided some potential connections between miRNA abnormalities and ASD phenotypes due to chromosome structural mutations. One such an example is the 22q11.2 microduplications. In contrast to the enrichment of 22q11.2 microdeletion (but not microduplication) in SCZ cohort (Brunet et al., 2008), a higher frequency of 22q11.2 microduplication (but not microdeletion) was observed in unrelated ASD cases according to the results of several genome-wide CNV screenings (Glessner et al., 2009; Marshall et al., 2008). Because the expression level of DGCR8 gene is up-regulation in the 22q11.2 microduplication, miRNA biogenesis process is likely to be affected. In addition, miR-185 gene within in the 22q11.2 duplication is also likely altered (BX, MK, JAG unpublished). Similarly, hsa-miR-211, another microRNA gene, is located within a recurrent genomic imbalance region at 15q13.2-q13.3 that has been associated with ASDs, intellectual disability, epilepsy, and/or electroencephalogram (EEG) abnormalities (Miller et al., 2009). A number of expression profiling studies examined miRNA dysregulation in ASD patient samples. Talebizadeh et al. checked the expression profile of 470 miRNAs of the lymphoblastoid cell line samples from 6 ASD patients and 6 matched controls using microarrays. Nine miRNAs were shown to be differential expressed in the ASD samples as compared to controls (Talebizadeh et al., 2008). In an independent study Abu-Elneel et al probed the expression of 466 miRNAs of the postmortem cerebellar cortex samples from 13 ASD patients and 13 non-autistic controls using multiplex quantitative PCR method. They found that 28 out of 277 miRNAs that could be reliably detected were differentially expressed in at least one of the ASD samples as compared to the mean value observed in non-autistic controls (Abu-Elneel et al., 2008). Three miRNAs, miR-23a, miR-134 and miR-146b overlapped between these two studies. More recently, Sarachana et al compared miRNA expression in lymphoblastoid cells from three pairs of monozygotic twins discordant for diagnosis of ASD, a normal sibling for two of the twin pairs, two pairs of autistic and unaffected siblings, and a pair of normal monozygotic twins. 43 miRNAs were found as significantly changed between autistic and nonautistic individuals. Two miRNAs (miR-23a and miR-106b) overlapped with the ones reported by Abu-Elneel et al (Abu-Elneel et al., 2008; Sarachana et al., 2010). Ghahramani Seno et al used a discordant sibling pair design to study mRNA and miRNA expression profile in lymphoblastoid cells of 20 severe autism patients and 22 unaffected siblings. They identified a subgroup of samples with similar expression pattern using cluster analysis and determined that 12 miRNAs were differentially expressed in this subset of ASD samples (Ghahramani Seno et al., 2011). Although initial genetic studies at the genomic level suggest that miRNA alterations could contribute to the genetic heterogeneity and phenotypic variation of ASDs, miRNA gene profiling studies have not yet produced a convergent picture and additional larger scale systematic investigation will be necessary.
2.3 Rett syndrome
Rett syndrome (RTT) is a neurodevelopmental disorder with an incidence of 1:10,000–15,000 (Hagberg, 1985). RTT occurs almost exclusively in girls and ninety-nine percent of affected girls are sporadic cases. Patients with classic RTT have an apparently normal development before 6–18 months of age, then gradually exhibit developmental stagnation, stereotypical movements, microcephaly, seizures, autistic features and intellectual disability (Hagberg et al., 1983). Detailed anatomic examination reveals that RTT patients have a smaller brain and the size and dendritic arborization of individual neurons are also reduced (Armstrong et al., 1995; Leonard and Bower, 1998; Sirianni et al., 1998).
Mutations in the gene encoding methyl-CpG binding protein 2 (MeCP2) have been associated with many RTT cases and are thought to be the main cause of RTT (Amir et al., 1999). Evidence that miRNAs might involve in the etiology and clinical expression of RTT came from the finding that miR-132 controls the expression a Mecp2 isoform in primary cortical neurons through its 3’UTR (Klein et al., 2007). This finding, combined with the observations that miR-132 is a miRNA that regulates neuronal morphogenesis in responding to extrinsic trophic cues such as BDNF and that lack of Mecp2 decreases BDNF levels in mouse models of RTT, suggested that miR-132 might exert homeostatic control over Mecp2 translation (Klein et al., 2007; Vo et al., 2005). More recently, Hansen et al generated a transgenic mouse strain where miR-132 is over-expressed in forebrain neurons. miR-132 transgenic mice displayed reduced Mecp2 levels, a significant increase of dendritic spine density in hippocampal neurons as well as deficits in a novel object test (Hansen et al., 2010). Interestingly, Mecp2 appears to also control miRNAs as well as their downstream targets. Nomura et al reported that Mecp2 regulated the expression of another brain specific imprinted miRNA, miR-184, by binding to its promoter region (Nomura et al., 2008). When cultured cortical neurons are depolarized, Mecp2 is released from the promoter binding site of the paternal allele leading to up-regulation of paternal allele-specific expression of miR-184. It should be noted, however, that the authors observed a down-regulation of miR-184 expression in the Mecp2-deficient mouse brain. In addition, no morphological consequence was identified when miR-184 was overexpressed in the cultured cortical neurons (Nomura et al., 2008). More recently, two miRNA expression profiling studies of a Mecp2 knockout mouse model further demonstrated a broader alteration of miRNA expression in response to lack of Mecp2. Urdinguio et al used miRNA microarrays to investigate the miRNA expression profiles of Mecp2 knockout mice, a mouse model of Rett syndrome. They reported 65 out of 245 miRNAs altered in their expression with more than 70% of them downregulated (Urdinguio et al., 2010). Wu et al used massively parallel sequencing methods to identify miRNAs altered in cerebella of Mecp2-null mice before and after the onset of severe neurological symptoms. They found that ~17% of all known mature miRNAs were considerably dysregulated (>1.5-fold) in cerebella of knockout mice before the onset of severe neurological symptoms. A further analysis revealed that many up-regulated mature miRNAs belong to the miRNA clusters within the Dlk1-Gtl2 imprinted domain (Wu et al., 2010). Interestingly, dysregulation of miRNAs within this genomic region was also reported in SCZ samples albeit in opposite direction (see section 2.1). Transcription of miRNAs within this cluster has been shown to be regulated by neuronal activity and has been implicated in regulation of dendritic morphology (Fiore et al., 2009). Overall, Mecp2 seems to be an important component of a miRNA-modulated regulatory network: miRNAs such as miR-132 control Mecp2 level and, in turn, Mecp2-regulated miRNAs may serve as critical mechanistic links to the downstream phenotypes. Therefore, deficits in Mecp2 expression may lead to the disruption of miRNA regulatory machinery, which may contribute to clinical phenotypes observed in Rett syndrome.
2.4 Fragile X syndrome
Fragile X syndrome (FXS) is the most common inherited form of mental retardation, affecting about 1:4000 males and 1:8000 females (Turner et al., 1996). It results in a spectrum of cognitive and behavioral manifestation including deficits in speech and language skills similar to the ones seen in ASD patients (Merenstein et al., 1996). FXS is caused by the repeat expansion of a single trinucleotide gene sequence (CGG) in the 5’UTR of FMRP, which leads to the failure of FMRP gene expression (Penagarikano et al., 2007). FMRP is a RNA binding protein and is thought to act through its translational repression effect. It has been reported that mutations in FMRP affect neuronal morphology as well as electrophysiological properties of neurons such as synaptic plasticity and long term potentiation (Bolduc et al., 2008; Dictenberg et al., 2008; Huber et al., 2002; Jin et al., 2004; Zhang et al., 2001).
FMRP protein was found to associate with Argonaute-2 (Ago2) and Dicer, both of which are critical components of miRNA pathway (Caudy et al., 2002; Ishizuka et al., 2002; Jin et al., 2004). In addition, several studies indicated that the maturation and function of some miRNAs is partially FMRP dependent. Xu et al. showed that ectopic expression of miR-124a precursors in vivo decreased dendritic branching of sensory neurons in Drosophila. This effect was partially rescued by the inactivation of dFMR1. They further showed that pre-miR-124a (precursor of miR-124a) levels were increased while the level of the mature form was reduced in dFMR1 mutants (Xu et al., 2008b). More recently, Edbauer et al. showed that several miRNAs, including miR-125b and miR-132, are associated with FMRP in the mouse brain (Edbauer et al., 2010). Alterations of miR-125b and miR-132 expression resulted in spine morphology changes and FMRP was required for the effect of miR-125b and miR-132 on the spine morphology. Furthermore, the expression of the NMDA receptor subunit NR2A was regulated by FMRP partially through miR-125b (Edbauer et al., 2010). This finding is consistent with the several previous results indicating that loss of FMRP alters NMDA receptor function in mice (Pfeiffer and Huber, 2007; Pilpel et al., 2009). Muddashetty et al showed that miR-125a could reversibly control PSD-95 expression, which in turn, alters the dendritic spine morphology. FMRP phosphorylation status in response to mGluR signaling controls the binding affinity of AGO2-miR-125a complex to PSD-95 mRNA. These studies indicated that FMRP gene, at least in part, executes its function via miRNA–modulated regulatory networks.
2.5 Tourette’s syndrome
Tourette’s syndrome (TS) is a neurodevelopmental condition characterized by chronic vocal and motor tics and associated with behavioral abnormalities. TS has a prevalence of 1% in general population and 3/4 of the patients are male (Staley et al., 1997). The age of onset of the disease ranges from 2 to 14 years old with a peak age of tic onset at 6–7 years of age (Kerbeshian et al., 2009; Robinson, 2010). TS is often comorbid with other neuropsychiatric disorders such as attention deficit/hyperactivity disorder and obsessive-compulsive disorder (Cavanna et al., 2009). Although a strong genetic component is suggested based on family, segregation and twin studies, gene identification via linkage and association studies have been largely unsuccessful indicating it is a complex disease (O’Rourke et al., 2009).
A potential link between miRNA and TS was first proposed by Abelson et al based on identification of a sequence variant (var321) in the 3’UTR of Slit and Trk-like1 (SLITRK1) gene (Abelson et al., 2005). The variant was identified when the authors screened the sequence surrounding the breakpoint of a de novo chromosomal inversion of a TS patient. Mutational screening of the resident SLITRK1 gene in 174 unrelated TS patients revealed that two patients (but none of the 2,148 controls) carried a sequence variant (var321) in the 3’UTR of SLITRK1, which affects the binding of a miRNA, hsa-miR-189. They further showed that miR-189 has a modest dose-dependent effect on SLITRK1 expression in an in vitro luciferase assay system. In situ hybridization experiments indicated that the expression of miR-189 and SLITRK1 mRNA are overlapping in many neuroanatomical circuits of postnatal mouse and fetal human brains that are most commonly implicated in TS (Abelson et al., 2005). Finally, over-expression of SLITRK1 in cortical neuronal cultures was shown to promote dendritic growth (Abelson et al., 2005). The association between var321 variant and TS phenotype was followed up in several independent datasets (Chou et al., 2007; Deng et al., 2006; Keen-Kim et al., 2006; Scharf et al., 2008; Verkerk et al., 1991; Wendland et al., 2006; Zimprich et al., 2008). Although var321 was detected in some of these studies, it failed to cosegregate with TS phenotype. Because var321 is a very rare variant in the general population (minor allele frequency of 0.1%), additional studies with even larger samples in homogeneous populations are needed to clarify if the original finding was a false positive i.e. due to population stratification, a common problem with the case/control design or the follow-up replication studies were underpowered.
2.6 Down syndrome
The Down syndrome (DS) is characterized by mild to moderate mental retardation and its prevalence is estimated to be 1/800 (Carothers et al., 1999). The syndrome is caused by an extra whole or part of chromosome 21, which has a severe impact on the development of nervous system leading to impaired maturation of neurons including atrophic dendritic structure, decreased neuronal numbers and abnormal neuronal differentiation in the brain of DS patient. Some patients show early appearance of senile plaques (Mrak and Griffin, 2004; Wisniewski et al., 1985). A potential connection between miRNAs and DS phenotypes was recently explored (Kuhn et al., 2008). Five miRNA genes (miR-99a, let-7c, miR-125b-2, miR-155, and miR-802) located on human chromosome 21 were found up-regulated in the fetal brain tissue of DS patients compared to age- and sex-matched controls (Kuhn et al., 2008). The same group further demonstrated that a common target of miR-155 and miR-802 is Mecp2 (see section 2.3). In brain samples from human and mouse models, the expression of Mecp2 and its downstream targets, Creb1 and Mef2c, were all altered. In a DS mouse model, the expression level of Mecp2, Creb1 and Mef2c was restored when endogenous miR-155 or miR-802 were knocked down by intra-ventricular injections of corresponding antagomirs (Kuhn et al., 2010). These results suggest that over-expression of the miRNAs on chromosome 21 may repress the expression of Mecp2, which in turn contributes, at least in part, to the neural deficits observed in the brains of DS individuals. An important unresolved issue in these studies is that none of analyzed miRNAs are located within the Down syndrome critical region, which was previously identified to be associated with many of the DS phenotypes (Delabar et al., 1993). Therefore, how these miRNAs contribute to the DS phenotypes, especially intellectual disability requires further analysis.
3. Potential mechanistic connections between miRNA dysregulation and neuropsychiatric disorders
Although genetic and miRNA expression profiling studies described above provide strong evidence that miRNAs are involved in various psychiatric and neurodevelopmental disorders, the details on how miRNA dysregulation contributes to specific clinical pictures remains to be elucidated. A collective role of miRNAs in modulating normal neural morphology and function as well as various behavioral phenotypes is supported by many recent studies (see recent reviews in (Fineberg et al., 2009; Siegel et al., 2011)) and the impact of altered expression of individual miRNAs has been assessed using a variety of strategies. Below we discuss a few relevant examples that offer some potential mechanistic insight that might illuminate the connection between miRNA dysfunction and neuropsychiatric disorders.
3.1 Insights from Global Disruption of miRNA biogenesis and action
The majority of miRNAs identified so far are transcribed by RNA polymerase II as long primary transcripts called pri-miRNA. Pri-miRNAs are then processed into stem-loop precursor miRNAs (pre-miRNAs) by the microprocessor (a complex containing type-III RNase Drosha and its partner protein Dgcr8) in the nucleus. Pre-miRNAs are then exported to the cytoplasm and further cleaved into mature miRNA duplexes by Dicer, another type-III RNase. The final mature miRNAs have one strand incorporated into the ribosome induced silencing complex (RISC) with the help of Dicer and several other RNA binding proteins including Ago2, Pact and Trbp. The miRNA-associated RISC binds to the target mRNA to inhibit its translation or cause the degradation of the target mRNA (Kim, 2005). Disruptions of the components in miRNA biogenesis pathway have been shown to have a critical impact on neuronal survival, development, differentiation and function in the central nervous system. For example, knockout of the Dicer gene led to severe defects in neural tube morphogenesis arising from abnormal neuronal differentiation in zebra fish and embryonic lethal in mouse (Giraldez et al., 2005; Murchison et al., 2005). Conditional knockout of Dicer in mouse further demonstrated that morphogenesis of the neurons in the cortex and hippocampus was disrupted (Davis et al., 2008) and postnatal progressive neuron death observed in the cerebellum and forebrain (Kim et al., 2007; Schaefer et al., 2007). Mutation of dAgo1, one of the Argonaute proteins that facilitate the loading of miRNAs into the RISC, results in global developmental defects in Drosophila including the most prominent malformation of the nervous system (Kataoka et al., 2001). Similarly Ago2-null mice have severe defects in neural tube formation and die early in development (Liu et al., 2004). Deficiency of Dgcr8 gene, which is disrupted by the 22q11.2 microdeletion, was the first example of a clinically relevant disruption of a component of miRNA biogenesis pathway. Similar to the situation of Dicer knockout, homozygous Dgcr8 knockout mice die at embryonic day 6.5 (Stark et al., 2008; Wang et al., 2007), while Dgcr8 heterozygous mice (Dgcr8+/−) show partially impaired miRNA biogenesis and result in a number of neuronal and behavioral deficits similar to what have been observed in human disease conditions (Fenelon et al., 2011; Schofield et al., 2011; Stark et al., 2008) (see section 2.1). Recently, the development of high throughput sequencing technologies and advanced bioinformatics tools, afforded the identification of many small RNAs with miRNA capabilities and characteristics. Interestingly, recent studies showed that these putative miRNAs are generated by bypassing one or more key steps of the classic pathway of miRNA biogenesis described above (Yang and Lai, 2011). It will be interesting to examine whether any components of these alternative pathways contribute to the various neuropsychiatric disorders.
3.2 Insights from Disruption of individual miRNAs
3.2.1 Individual miRNAs modulate dendritic complexity and spine morphology in neurons
Alterations in dendritic complexity and spine morphology of neurons have been reported frequently in various psychiatric and neurodevelopmental disorders. Understanding the molecular underpinnings of these changes may provide insight into the etiologies of these conditions and may reveal new drug targets. miR-134 is the first miRNA shown to contribute to dendritic complexity and spine morphology of neurons. Overexpression of miR-134 significantly decreased spine volume while overexpression of a 2′-O-methylated anti-miR-134 oligonucleotide increased spine width (Schratt et al., 2006). Schratt et al proposed that BDNF treatment can relieve miR-134-dependent translation inhibition of its target, Limk1 (a kinase that regulates actin and microtubule polymerization), which in turn resulted in higher Limk1 protein level and dendritic spine morphology changes. Further investigation indicated that Myocyte enhancing factor 2 (Mef2) was necessary and sufficient to induce expression of miR-134 in response to external stimuli, such as neurotrophic factors and neuronal activity. High level of miR-134 inhibited translation of Pumilio2, a translational repressor and promoted neurite outgrowth (Fiore et al., 2009).
miR-132 is another extensively studied microRNA that has been shown to modulate neuronal morphology. Vo et al identified miR-132, as a target of the transcription factor cAMP-response element binding protein (CREB) through a genome-wide screen. miR-132 was enriched in neurons and tightly associated with a CREB element and was highly responsive to neurotrophin signaling. Overexpression of this miRNA in primary cortical neurons dramatically increased neurite outgrowth. Conversely, inhibition of miR-132 blunted neurite outgrowth under basal conditions and blocked the response to BDNF (Vo et al., 2005). Magill et al further demonstrated that ablation of the miR-212/132 locus dramatically reduced dendritic length, branching, and spine density in newborn hippocampal neurons in young adult mice. Because miR-132 was shown to be the predominantly active product of the miR-212/132 locus in hippocampal neurons, the authors concluded that miR-132 was required for normal dendrite maturation in newborn neurons in the adult hippocampus through CREB mediated signaling (Magill et al., 2010). In an independent study, Hansen et al employed a transgenic mouse strain that expresses miR-132 in forebrain neurons. Morphometric analysis of hippocampal neurons indicated a dramatic increase in dendritic spine density in miR-132 overexpressing mice (Hansen et al., 2010). Edbauer et al further demonstrated that miR-132 and miR-125b interacts with FMRP in mouse brain and regulated dendritic spine morphology of hippocampal neurons in opposite directions. Down-regulation of FMRP gene affected the impact of these miRNAs on spine morphology (Edbauer et al., 2010).
Overall, these studies suggest that a group of miRNAs play multiple regulatory roles in controlling neuronal morphology in response to external stimuli, such as neurotrophic factors and neuronal activity. Given that miR-134 is altered in the brain of the 22q11.2 microdeletion model (Stark et al 2008) and alteration of miR-132 expression is associated with FMRP expression (Edbauer et al., 2010), it would be tempting to speculate that the mechanisms suggested above might be part of the pathophysiological processes underlying the corresponding clinical conditions. Along these lines, observations from independent studies suggest that miRNAs may regulate dendritic morphology in concert with other disease-related signaling pathways. For example, two independent studies demonstrated that miR-138 controls the depalmitoylating enzyme lysophospholipase1 (Lypla1)/acyl protein thioesterase 1 (APT1), which modulates neuronal protein palmitoylation process (Banerjee et al., 2009; Siegel et al., 2009). In that respect, it is noteworthy, that in the case of the 22q11.2 microdeletion combined deficits of both miRNA biogenesis and neuronal palmitoylation (due to hemizygous deletion of ZDHHC8 gene that encodes for a palmitoyltranferase) contribute to the changes in dendritic morphology and spine morphogenesis (Mukai et al., 2008; Stark et al., 2008).
3.2.2 Individual miRNAs modulate neurogenesis, neuronal proliferation, migration and integration
Neuropsychiatric disorders such as SCZ, ASD and mental retardation have been associated with a lot of neurodevelopmental abnormalities from neurogenesis, neuronal proliferation to neuron migration and integration (Hsieh and Eisch, 2010; Wegiel et al., 2010; Yang et al., 2011). Recent studies demonstrated that microRNAs takes part in many aspects of these neurodevelopment processes. Therefore, the regulatory mechanisms involve in individual miRNAs might provide important insights of these pathogenic processes. miR-124a is one of the most abundant miRNAs in mammalian brain and mainly expressed in differentiating and mature neurons, accounting for 25%–48% of all mouse brain miRNAs (Deo et al., 2006; Lagos-Quintana et al., 2002). Ectopic expression of miR-124a in HeLa cells leads to a shift of expression profile from non-neuronal pattern to neuronal-like pattern (Lim et al., 2005) indicating this miRNA might regulate the development of neuronal identity of the cells. Several studies have shown that miR-124a promote neuronal progenitor differentiation by de-repression of RE1-Silencing Transcription Factor (REST) and downregulation of target mRNAs such as PTBP1, a RNA-binding protein that globally represses alternative pre-mRNA splicing in non-neuronal cells (Conaco et al., 2006; Makeyev et al., 2007; Wu and Xie, 2006)More recently, Cheng et al. showed that miR-124 regulated neurogenesis in the subventricular zone stem cell niche by repressing the SRY-box transcription factor Sox9 in the adult mammalian brain (Cheng et al., 2009). Yu et al. showed that miR-124 controls neurite outgrowth in differentiating mouse P19 cells and mouse primary cortical neurons (Yu et al., 2008). In addition to it roles in neuronal development, Rajasethupathy et al. provided evidence that miR-124 also plays an important role in serotonin mediated long-term plasticity of synapses in the mature nervous system of Aplysia californica (Rajasethupathy et al., 2009).
miR-9 is another miRNA abundantly expressed in vertebrate brains (Griffiths-Jones, 2006; Lagos-Quintana et al., 2002). Several studies have suggested that miR-9/miR-9* targets Nr2e1, REST, Corepressor of REST (CoREST), and BAF53a to suppress progenitor proliferation and promotes neural differentiation (Packer et al., 2008; Yoo et al., 2009; Zhao et al., 2009). miR-9 also promotes proliferation of human embryonic stem cell-derived neural progenitors by targeting STMN1 gene (Delaloy et al., 2010). Mutant mice lacking miR-9-2 and miR-9-3 (referred to as miR-9-2/3 double mutants) designed to examine the miR-9/miR-9* function in telencephalic development, exhibited dysregulation of pallial and subpallial progenitor proliferation/differentiation. They also exhibited multiple defects in telencephalic structures (Shibata et al., 2011).
Luikart et al provided evidence that the expression pattern of miR-132 is consistent with an effect on integration of newborn neurons in dentate gyrus into mature circuits. When a retroviral vector containing a “sponge” that consists of multiple miR-132 binding sites was introduced into the newborn neurons and miR-132 expression was down-regulated, the integration of newborn neurons into the excitatory synaptic circuitry of the adult brain was disrupted. (Luikart et al., 2011). Interestingly, this mouse line also exhibited a decrease in the expression of MeCP2 and impairment in novel object recognition memory (Hansen et al., 2010). More recently, Gaughwin et al. showed that miR-134 can modulate cortical development in a stage-specific fashion. Through interaction with Doublecortin and/or Chordin-like 1, miR-134 promoted cell proliferation and counteracted Chrdl-1–induced apoptosis and Dcx-induced differentiation in neural progenitors. miR-134 also affected neuronal migration in vitro and in vivo in a Dcx-dependent manner. When overexpressed in differentiating cortical neurons, miR-134, led to subtle alterations in neurites including reduction of number, length and overall complexity of processes. Exogenous BMP-4 treatment can significantly reverse the effect of miR-134 overexpression. The author concluded that miR-134 might be a modulator of exogenous BMP-4 signals on neurite outgrowth in a noggin-reversible manner (Gaughwin et al., 2011).
3.2.3 Individual miRNAs modulate neuronal electrophysiological properties in response to neuronal activity
miRNA expression is modulated by neuronal activity (Krol et al., 2010a) and studies from Dgcr8 mutant mice have demonstrated that miRNAs alterations in neuropsychiatric diseases conditions lead to the changes of the electrical and synaptic properties of neurons (Fenelon et al., 2011; Schofield et al., 2011). Lambert et al tested the effects of miR-132 expression on synaptic function and their results indicated that the some properties in short term synaptic plasticity of cultured mouse hippocampal neurons was altered when miR-132 was overexpressed including the increase of paired-pulse ratio and decreases synaptic depression. However, the presynaptic vesicular release properties such as the initial probability of neurotransmitter release, the size of the readily releasable pool of synaptic vesicles and the rate of refilling of vesicle pool were unchanged (Lambert et al., 2010). Therefore, the impact of individual miRNA expression on neuronal electrophysiological properties warrants further investigation.
4. Conclusions
The evidence reviewed here strongly suggests that miRNAs play an important role in the pathogenesis and pathophysiology of psychiatric and neurodevelopmental disorders as well as cognitive dysfunction. Although the exact mode of action of individual miRNAs affected in various psychiatric conditions remains largely unclear, our understanding is rapidly improving by the convergence of findings from various recent studies, including ones involving carefully designed animal models (Kvajo et al., 2011). A comprehensive understanding of the roles of miRNAs will be important for determining whether miRNAs-related pathways could serve as novel targets for drug development for these devastating conditions.
Acknowledgments
Work in the authors’ laboratory is supported by grants from NIMH, McKnight Foundation, March of Dimes, Lieber Center for Schizophrenia Research, Simons Foundation and NARSAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JF, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310:317–20. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Abu-Elneel K, et al. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9:153–61. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Arguello PA, et al. Development of animal models for schizophrenia. Dis Model Mech. 2010;3:22–6. doi: 10.1242/dmm.003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D, et al. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195–201. doi: 10.1097/00005072-199503000-00006. [DOI] [PubMed] [Google Scholar]
- Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Banerjee S, et al. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64:871–84. doi: 10.1016/j.neuron.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, et al. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, et al. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15:1176–89. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–68. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Bolduc FV, et al. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–5. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner JL, et al. Loss of individual microRNAs causes mutant phenotypes in sensitized genetic backgrounds in C. elegans. Curr Biol. 2010;20:1321–5. doi: 10.1016/j.cub.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brest P, et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat Genet. 2011;43:242–5. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- Brunet A, et al. Failure to detect the 22q11.2 duplication syndrome rearrangement among patients with schizophrenia. Behav Brain Funct. 2008;4:10. doi: 10.1186/1744-9081-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LE, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–28. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Cao X, et al. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- Carothers AD, et al. International variation in reported livebirth prevalence rates of Down syndrome, adjusted for maternal age. J Med Genet. 1999;36:386–93. [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Caudy AA, et al. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–6. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, et al. The behavioral spectrum of Gilles de la Tourette syndrome. J Neuropsychiatry Clin Neurosci. 2009;21:13–23. doi: 10.1176/jnp.2009.21.1.13. [DOI] [PubMed] [Google Scholar]
- Cheng LC, et al. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, et al. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–86. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou IC, et al. Association of the Slit and Trk-like 1 gene in Taiwanese patients with Tourette syndrome. Pediatr Neurol. 2007;37:404–6. doi: 10.1016/j.pediatrneurol.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Conaco C, et al. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TH, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–30. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delabar JM, et al. Molecular mapping of twenty-four features of Down syndrome on chromosome 21. Eur J Hum Genet. 1993;1:114–24. doi: 10.1159/000472398. [DOI] [PubMed] [Google Scholar]
- Delaloy C, et al. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6:323–35. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, et al. Examination of the SLITRK1 gene in Caucasian patients with Tourette syndrome. Acta Neurol Scand. 2006;114:400–2. doi: 10.1111/j.1600-0404.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- Deo M, et al. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235:2538–48. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- Dictenberg JB, et al. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–39. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, et al. The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2011;29:259–81. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–84. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliez S, et al. Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry. 2000;157:409–15. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Fenelon K, et al. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:4447–52. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg SK, et al. MicroRNAs potentiate neural development. Neuron. 2009;64:303–9. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Fiore R, et al. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Ford JM, et al. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–92. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Gardiner E, et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughwin P, et al. Stage-specific modulation of cortical neuronal development by Mmu-miR-134. Cereb Cortex. 2011;21:1857–69. doi: 10.1093/cercor/bhq262. [DOI] [PubMed] [Google Scholar]
- Ghahramani Seno MM, et al. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011;1380:85–97. doi: 10.1016/j.brainres.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–8. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- Glessner JT, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–73. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, et al. Excess of twins among affected sibling pairs with autism: implications for the etiology of autism. Am J Hum Genet. 2001;69:1062–7. doi: 10.1086/324191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006;342:129–38. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Rett syndrome: Swedish approach to analysis of prevalence and cause. Brain Dev. 1985;7:276–80. [PubMed] [Google Scholar]
- Hagberg B, et al. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–9. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hansen KF, et al. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T, et al. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS ONE. 2007;2:e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiol Dis. 2010;39:73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingason A, et al. Copy number variations of chromosome 16p13.1 region associated with schizophrenia. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISC. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–41. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka A, et al. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–7. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci U S A. 1995;92:7612–6. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, et al. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, et al. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells. 2001;6:313–25. doi: 10.1046/j.1365-2443.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- Keen-Kim D, et al. Overrepresentation of rare variants in a specific ethnic group may confuse interpretation of association analyses. Hum Mol Genet. 2006;15:3324–8. doi: 10.1093/hmg/ddl408. [DOI] [PubMed] [Google Scholar]
- Kerbeshian J, et al. Tourette syndrome and comorbid early-onset schizophrenia. J Psychosom Res. 2009;67:515–23. doi: 10.1016/j.jpsychores.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Kim AH, et al. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res. 2010;124:183–91. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–4. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–85. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- Kirov G, et al. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18:1497–503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, et al. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet. 2008;17:458–65. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- Klein ME, et al. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–4. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Kocerha J, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009;106:3507–12. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Krol J, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010a;141:618–31. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Krol J, et al. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010b;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Kuhn DE, et al. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts. Biochem Biophys Res Commun. 2008;370:473–7. doi: 10.1016/j.bbrc.2008.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuhn DE, et al. Chromosome 21-derived microRNAs provide an etiological basis for aberrant protein expression in human Down syndrome brains. J Biol Chem. 2010;285:1529–43. doi: 10.1074/jbc.M109.033407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kvajo M, et al. Molecules, signaling, and schizophrenia. Curr Top Behav Neurosci. 2010;4:629–56. doi: 10.1007/7854_2010_41. [DOI] [PubMed] [Google Scholar]
- Kvajo M, et al. Avoiding mouse traps in schizophrenia genetics: lessons and promises from current and emerging mouse models. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E, et al. Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.170. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Lajiness-O’Neill RR, et al. Memory and learning in children with 22q11.2 deletion syndrome: evidence for ventral and dorsal stream disruption? Child Neuropsychol. 2005;11:55–71. doi: 10.1080/09297040590911202. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, et al. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS ONE. 2010;5:e15182. doi: 10.1371/journal.pone.0015182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, et al. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–11. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Leonard H, Bower C. Is the girl with Rett syndrome normal at birth? Dev Med Child Neurol. 1998;40:115–21. [PubMed] [Google Scholar]
- Lewis BP, et al. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewis BP, et al. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–41. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Lugli G, et al. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem. 2008;106:650–61. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, et al. miR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLoS ONE. 2011;6:e19077. doi: 10.1371/journal.pone.0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill ST, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–7. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, et al. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–88. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein SA, et al. Molecular-clinical correlations in males with an expanded FMR1 mutation. Am J Med Genet. 1996;64:388–94. doi: 10.1002/(SICI)1096-8628(19960809)64:2<388::AID-AJMG31>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–86. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Miller DT, et al. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet. 2009;46:242–8. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau MP, et al. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69:188–93. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Trisomy 21 and the brain. J Neuropathol Exp Neurol. 2004;63:679–85. doi: 10.1093/jnen/63.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–10. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji S, et al. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43:854–9. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, et al. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–40. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–54. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Nomura T, et al. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum Mol Genet. 2008;17:1192–9. doi: 10.1093/hmg/ddn011. [DOI] [PubMed] [Google Scholar]
- O’Rourke JA, et al. The genetics of Tourette syndrome: a review. J Psychosom Res. 2009;67:533–45. doi: 10.1016/j.jpsychores.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz EM, et al. Maturation of startle modulation. Psychophysiology. 1986;23:624–34. doi: 10.1111/j.1469-8986.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Packer AN, et al. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci. 2008;28:14341–6. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, et al. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–29. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Perkins DO, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–30. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilpel Y, et al. Synaptic ionotropic glutamate receptors and plasticity are developmentally altered in the CA1 field of Fmr1 knockout mice. J Physiol. 2009;587:787–804. doi: 10.1113/jphysiol.2008.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, et al. A resource of vectors and ES cells for targeted deletion of microRNAs in mice. Nat Biotechnol. 2011;29:840–5. doi: 10.1038/nbt.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, et al. Characterization of small RNAs in aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–17. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- Ripke S, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011 doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PN. Whole-exome sequencing for finding de novo mutations in sporadic mental retardation. Genome Biol. 2010;11:144. doi: 10.1186/gb-2010-11-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Murillo L, et al. The Genetic Architecture of Schizophrenia: New Mutations and Emerging Paradigms. Annu Rev Med. 2011 doi: 10.1146/annurev-med-072010-091100. [DOI] [PubMed] [Google Scholar]
- Santarelli DM, et al. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–7. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Sarachana T, et al. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2:23. doi: 10.1186/gm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–8. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JM, et al. Lack of association between SLITRK1var321 and Tourette syndrome in a large family-based sample. Neurology. 2008;70:1495–6. doi: 10.1212/01.wnl.0000296833.25484.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CM, et al. Monoallelic deletion of the microRNA biogenesis gene Dgcr8 produces deficits in the development of excitatory synaptic transmission in the prefrontal cortex. Neural Dev. 2011;6:11. doi: 10.1186/1749-8104-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–9. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Selbach M, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Shibata M, et al. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011;31:3407–22. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ, et al. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Siegel G, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–16. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G, et al. microRNAs in neurons: manifold regulatory roles at the synapse. Curr Opin Genet Dev. 2011;21:491–7. doi: 10.1016/j.gde.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, et al. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–7. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, et al. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 2005;25:169–80. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Sirianni N, et al. Rett syndrome: confirmation of X-linked dominant inheritance, and localization of the gene to Xq28. Am J Hum Genet. 1998;63:1552–8. doi: 10.1086/302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, et al. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–9. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley D, et al. Tourette disorder: a cross-cultural review. Compr Psychiatry. 1997;38:6–16. doi: 10.1016/s0010-440x(97)90047-x. [DOI] [PubMed] [Google Scholar]
- Stark KL, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–60. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stefansson H, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenburg S, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–16. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, et al. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Talebizadeh Z, et al. Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Res. 2008;1:307. doi: 10.1002/aur.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DW, et al. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–53. doi: 10.1093/nar/gkr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, et al. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–7. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Urdinguio RG, et al. Disrupted microRNA expression caused by Mecp2 loss in a mouse model of Rett syndrome. Epigenetics. 2010;5:656–63. doi: 10.4161/epi.5.7.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vo N, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–31. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119:755–70. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, et al. Functional SLITRK1 var321, varCDfs and SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. Mol Psychiatry. 2006;11:802–4. doi: 10.1038/sj.mp.4001848. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, et al. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–82. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wu H, et al. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2010;107:18161–6. doi: 10.1073/pnas.1005595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7:R85. doi: 10.1186/gb-2006-7-9-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, et al. MicroRNAs in psychiatric and neurodevelopmental disorders. Brain Res. 2010;1338:78–88. doi: 10.1016/j.brainres.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet. 2011;43:864–8. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, et al. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008a;40:880–5. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Xu B, et al. Elucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scans. Proc Natl Acad Sci U S A. 2009;106:16746–51. doi: 10.1073/pnas.0908584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XL, et al. The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J Neurosci. 2008b;28:11883–9. doi: 10.1523/JNEUROSCI.4114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Lai EC. Alternative miRNA Biogenesis Pathways and the Interpretation of Core miRNA Pathway Mutants. Mol Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry. 2011;69:63–70. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, et al. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–6. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, et al. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314:2618–33. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zhao C, et al. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–71. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, et al. Sequence analysis of the complete SLITRK1 gene in Austrian patients with Tourette’s disorder. Psychiatr Genet. 2008;18:308–9. doi: 10.1097/YPG.0b013e3283060f6f. [DOI] [PubMed] [Google Scholar]


