Abstract
The concentration of amyloid-β (Aβ) within the brain extracellular space is one determinant of whether the peptide will aggregate into toxic species that are important in Alzheimer's disease (AD) pathogenesis. Some types of synaptic activity can regulate Aβ levels. Here we demonstrate two distinct mechanisms that are simultaneously activated by NMDA receptors and regulate brain interstitial fluid (ISF) Aβ levels in opposite directions in the living mouse. Depending on the dose of NMDA administered locally to the brain, ISF Aβ levels either increase or decrease. Low doses of NMDA increase action potentials and synaptic transmission which leads to an elevation in synaptic Aβ generation. In contrast, high doses of NMDA activate signaling pathways that lead to ERK (extracellular-regulated kinase) activation, which reduces processing of APP into Aβ. This depression in Aβ via APP processing occurs despite dramatically elevated synaptic activity. Both of these synaptic mechanisms are simultaneously active, with the balance between them determining whether ISF Aβ levels will increase or decrease. NMDA receptor antagonists increase ISF Aβ levels, suggesting that basal activity at these receptors normally suppresses Aβ levels in vivo. This has implications for understanding normal Aβ metabolism as well as AD pathogenesis.
Introduction
Aggregation of amyloid-β (Aβ) as soluble oligomers and insoluble plaques is central to the pathogenesis of Alzheimer's disease (Hardy, 2006). The concentration of monomeric soluble Aβ is a critical factor determining whether and when Aβ will form these toxic multimers (Lomakin et al., 1997). Thus, understanding the mechanisms that regulate Aβ levels has implications for understanding disease pathogenesis, as well as developing anti-Aβ therapies. Amyloid plaques exist within the brain extracellular space. While the initial seed of a plaque may form intracellularly or extracellularly (Gouras et al., 2000; Meyer-Luehmann et al., 2003), it appears that soluble Aβ within the extracellular fluid, or interstitial fluid, contributes to plaque growth (Yan et al., 2009). Numerous studies have elucidated the mechanisms by which amyloid precursor protein (APP) is cleaved by β-secretase and γ-secretase to produce Aβ (for review, see Zhang et al., 2011); however, the mechanisms that regulate how much Aβ is produced are only partly understood.
Recent studies demonstrate that synaptic activity is a key regulator of Aβ generation (Kamenetz et al., 2003). Seizures in APP transgenic mice rapidly increase brain interstitial fluid (ISF) Aβ levels, whereas blocking neuronal activity with tetrodotoxin (TTX) reduces Aβ levels (Cirrito et al., 2005). Synaptic activity leads to elevated clathrin-mediated endocytosis at the presynaptic terminal, which increases APP internalization and subsequent Aβ generation by β-secretase and γ-secretase within endosomes (Cirrito et al., 2008). A portion of the synaptically generated Aβ is then secreted at the axon terminal (Wei et al., 2010), which is capable of aggregating and forming Aβ plaques (Harris et al., 2010). The above studies are consistent with a synaptic mechanism that directly leads to Aβ generation and release from neurons.
Second messenger signaling pathways that are induced by neurotransmitter receptors can alter APP processing and therefore alter Aβ production. For instance, muscarinic acetylcholine receptors activate protein kinase C, which increases α-secretase cleavage of APP, thus reducing Aβ levels in animal models and humans (Nitsch et al., 2000; Hock et al., 2003; Caccamo et al., 2006; Davis et al., 2010). NMDA receptors (NMDA-Rs) can also modulate Aβ levels. In addition to NMDA-Rs inducing synaptic plasticity, they also cause nonamyloidogenic processing of APP (Fazeli et al., 1994). Activation of these receptors increases α-secretase localization at synapses and reduces Aβ production (Gordon-Krajcer et al., 2002; Marcello et al., 2007). Depending on the culture system used, some studies have found NMDA-Rs to either increase or decrease Aβ levels (Lesné et al., 2005; Hoey et al., 2009).
Our studies in living mice demonstrate that NMDA-Rs can either increase or decrease ISF Aβ levels depending on the level of receptor activation. Low doses of NMDA elevate ISF Aβ levels by increasing synaptic transmission and subsequent Aβ generation, whereas high doses of NMDA depress Aβ by activating synaptic-dependent signaling cascades that reduce amyloidogenic processing of APP. The balance between these mechanisms within a single cell and within neuronal networks will determine whether NMDA-Rs increase or decrease Aβ in vivo.
Materials and Methods
Animals.
All experimental procedures involving animals were performed in accordance with guidelines established by the Animal Studies Committee at Washington University. We bred APP/PS1ΔE9 (PS1APP) hemizygous mice (The Jackson Laboratory) (Savonenko et al., 2003) to wild-type C3H/B6 mice, and then aged the PS1APP+/− offspring to 2.5–3.5 months for use in experiments. Animals were screened for the PS1APP transgenes by PCR from tail DNA. Three-month-old C57BL6 mice were ordered directly from The Jackson Laboratory for use in experiments. An equal number of males and females were used in each experiment.
Compounds.
All pharmacological agents were purchased from Sigma-Aldrich unless otherwise noted. The NMDA-receptor antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) was diluted into saline and administered intraperitoneally at 20 mg/kg. FR180204, PD98059, and ifenprodil were purchased from Tocris Bioscience.
In vivo microdialysis.
In vivo microdialysis to assess brain ISF Aβ in the hippocampus of awake, freely moving PS1APP mice was performed similarly to previously described (Cirrito et al., 2003, 2005, 2008). This technique samples soluble molecules within the extracellular fluid that are smaller than 30 kDa, the molecular-weight cutoff of the probe membrane. Aβ capable of entering the probe, likely to be predominantly monomeric Aβ, has been dubbed “exchangeable Aβ” or “eAβ” (Cirrito et al., 2003).
Under isoflurane volatile anesthetic, guide cannula (BR-style, Bioanalytical Systems) were cemented into the left hippocampus (bregma −3.1 mm, 2.5 mm lateral to midline, and 1.2 mm below dura at a 12° angle). Two-millimeter microdialysis probes were inserted through the guides so the membrane was contained entirely within the hippocampus (BR-2, 30 kDa MWCO membrane, Bioanalytical Systems). Microdialysis perfusion buffer was artificial CSF (in mm: 1.3 CaCl2, 1.2 MgSO4, 3 KCl, 0.4 KH2PO4, 25 NaHCO3, and 122 NaCl, pH 7.35) containing 4% bovine serum albumin that was filtered through a 0.1 μm membrane. For studies infusing BAPTA-AM via reverse microdialysis, the microdialysis perfusion buffer had calcium replaced with magnesium. Flow rate was a constant 1.0 μl/min. Samples were collected every 60–90 min with a refrigerated fraction collector into polypropylene tubes and assessed for Aβx–40 or Aβx–42 by sandwich ELISA at the completion of each experiment. Basal levels of ISF Aβ were defined as the mean concentration of Aβ over 6 h preceding drug treatment. Once basal ISF Aβ levels were established, PS1APP mice were treated with a variety of compounds by reverse microdialysis or by intraperitoneal injection. For each animal, all ISF Aβ levels were normalized to the basal Aβ concentration for that mouse. Experiments took place under constant light conditions. Mouse ISF Aβ levels fluctuate in a diurnal rhythm if mice are allowed to recover for 2 weeks after microdialysis cannula placement and exposed to 2 weeks of a 12 h/12 h light/dark cycle before assessment of ISF Aβ (Kang et al., 2009). However, under constant light conditions without entraining mice to a 12 h/12 h light/dark cycle after microdialysis cannula placement, ISF Aβ levels fluctuate hourly by only 10–15% over a 3 d period.
Compounds delivered to the hippocampus via reverse microdialysis were diluted in microdialysis perfusion buffer, which consisted of artificial CSF with 4% bovine serum albumin. Several compounds were delivered to the brain using reverse microdialysis. With this approach, determining the absolute concentrations of a compound within the brain is difficult because of differences in efflux from the probe, diffusion within the brain, and compound pharmacokinetics; therefore, only the starting concentration of each compound is reported.
For the NMDA dose escalation study (Fig. 1A), each dose of compound was administered for 3 h in ascending fashion. One group of mice received doses from 0.25–10 μm and another group received doses ranging from 5 to 250 μm NMDA. When delivered in this fashion, we grossly estimate that the dose achieved within the brain is 50% of the actual concentration within the microdialysis perfusion buffer.
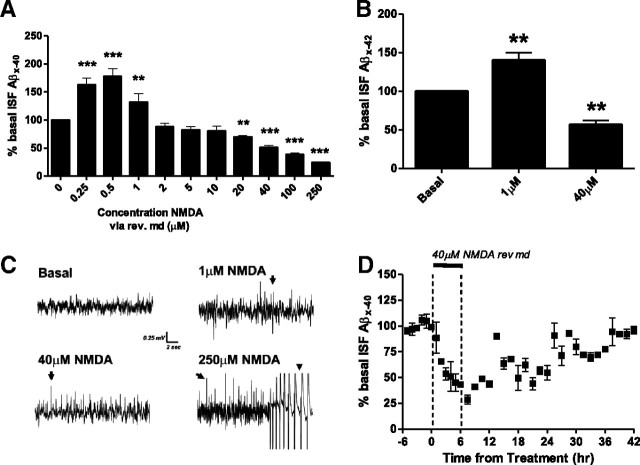
Figure 1.
Differential dose-dependent effect of NMDA-R signaling on ISF Aβ levels in vivo. A, Direct NMDA infusion into the brain via reverse microdialysis caused a differential dose-dependent change in ISF Aβ levels. Mean Aβ concentrations during hours 2–3 of a total 3 h treatment at each dose. At NMDA doses of 0.25–1 μm, there were significant increases in ISF Aβ40 with a 32 ± 15.2% elevation at 1 μm compared to basal levels (p < 0.01). At NMDA doses of 20–250 μm, there was a dose-dependent decrease in ISF Aβ40 levels. Forty micromolar NMDA reduced Aβ40 levels by 49.3 ± 4.3% with a maximal depression of 76.2 ± 1.8% at 250 μm NMDA. n = 6–10 per dose. B, A subset of mice also had Aβ42 levels assessed during NMDA infusion. One and forty micromolar NMDA altered Aβ42 levels by increasing 40.0 ± 9.9% (p < 0.01) and decreasing 43.3 ± 5.7% (p < 0.01), respectively (n = 6 per group). C, Representative EEG trace from a PS1APP+/− administered escalating doses of NMDA by reverse microdialysis. EEG activity recorded within the hippocampus showed synchronous spikes in activity at 1 and 40 μm NMDA (arrows) and intermittent epileptiform activity at 250 μm NMDA (arrowhead). D, Forty micromolar NMDA was administered to the hippocampus for 6 h and then removed from the microdialysis perfusion buffer. During the next 30 h of sampling, ISF Aβ levels gradually returned to baseline levels; 94.3 ± 13.5% compared to basal levels in each mouse (n = 5). **p < 0.01, ***p < 0.001.
Murine ISF Aβ40 levels in wild type were assessed using a similar protocol as PS1APP mice, except longer sample times of 6 h at a 0.5 μl/min flow rate were necessary to concentrate ISF samples. For each mouse, the basal concentration of Aβ was established over a single 6 h collection followed by two 6 h collections during treatment with either 1 μm NMDA, 40 μm NMDA, or CPP.
EEG recording.
To record EEG activity, bipolar recording electrodes (Teflon-coated, platinum–iridium wire, 0.0055 inch coated OD, A-M Systems) were attached to the shaft of the microdialysis guide cannula with Elmer's Super-Fast Epoxy Resin (Elmer's). Electrodes extended ∼1 mm from the tip of the guide, so that the electrode tip was located at the center of the 2 mm microdialysis membrane. EEG activity was assessed using a P511K A.C. Preamplifier (Grass Instruments), digitized with a DigiData 1440A Data Acquisition System (Molecular Devices), and recorded digitally using pClamp 10.2 (Molecular Devices). EEG traces are representative data from one animal undergoing each drug treatment so that traces can be directly compared between treatments. The frequency of synchronous spikes in EEG activity were analyzed using pClamp 10.2. Positive-going activity that exceeded three times the SD of the amplitude under basal conditions were marked as synchronous spikes in activity.
Aβ ELISAs.
ISF Aβ levels were assessed using sandwich ELISAs as described previously (Kim et al., 2009). Briefly, a mouse anti-Aβ40 antibody (mHJ2) or mouse anti-Aβ42 antibody (mHJ7.4) was used to capture and a biotinylated central domain antibody (mHJ5.1) was used to detect, followed by streptavidin-poly-HRP-40 (Fitzgerald Industries). All ELISA assays were developed using Super Slow ELISA TMB (Sigma) and absorbance read on a BioTek Epoch plate reader at 650 nm. These assays equally recognize human and murine Aβ so the same assays were used for samples generated in PS1APP human APP transgenic mice and wild-type mice. The standard curves for each assay, however, used either human or mouse Aβ peptide.
Western blots.
Three-month-old PS1APP mice treated with vehicle or CPP by intraperitoneal injection and 8 h later were killed by cardiac perfusion with chilled saline with 0.3% heparin and the hippocampus microdissected and snap frozen on dry ice. Hippocampal tissue was homogenized by sonication in 10:1 volume:wet weight of 150 mm NaCl, 50 mm Tris, pH 7.4, 0.5% deoxycholic acid, 0.1% SDS, 1% Triton X-100, 2.5 mm EDTA, and protease inhibitors. Western blotting for full-length APP was performed using 4–12% Bis-Tris NuPAGE gels (Invitrogen) under reducing conditions with 15 μg of protein loaded per lane. Nitrocellulose blots were probed with rabbit anti-APP directed against the C terminus of APP (Invitrogen), followed by donkey anti-rabbit antibody reabsorbed against mouse IgG and conjugated to peroxidase (Santa Cruz Biotechnology). Gels were stripped and reprobed with rabbit anti-tubulin (Sigma) as a loading control protein. Bands were detected with Lumigen-TMA6 (GE Healthcare) and captured digitally using the Kodak ImageStation 440CF. Densitometry was performed using the Kodak 1D Image Analysis software and each band normalized to tubulin signal in each lane.
ERK pathway multiplex assay.
Hippocampal tissue was isolated from CPP (20 mg/kg, i.p.) and vehicle-treated PS1APP mice (8 h treatment) were then lysed in the EpiTag Cell Lysis Kit (Novagen) by mechanical homogenization. ERK2 total protein and phosphorylated forms of MEK1/2 and ERK2 were measured together using the WideScreen EpiTag ERK Pathway Panel I (Novagen) with concentrations calibrated against the EpiTag ERK Pathway Standards Mix. Bead fluorescence was quantified using the xMAP Luminex platform.
Secretase assays.
Enzymatic activity of α-secretase and β-secretase was measured using FRET-based cleavage assays (R&D Systems). Hippocampal tissue was isolated from CPP (20 mg/kg, i.p.) and vehicle-treated PS1APP mice (8 h treatment) were then lysed in Cell Extraction Buffer (Novagen) by mechanical homogenization. Cell extracts were incubated with secretase-specific peptides conjugated to the reporter molecules EDANS and DABCYL for 15–30 min. EDANS fluorescence was read on a Synergy 2 microtiter plate reader (BioTek).
Statistics.
Data in figures are presented as mean ± SEM. All statistical analysis was performed using Prism version 5.04 for Windows (GraphPad). Statistical analysis to compare the mean values for multiple groups was performed using a one-way ANOVA with correction for multiple comparisons. Comparison of only two groups was performed using a two-tailed unpaired t test. Analysis of effects of drug versus vehicle treatment over time was done with a repeated-measures two-way ANOVA with correction for multiple comparisons. Values were accepted as significant if p ≤ 0.05.
Results
Differential dose-dependent effect of NMDA-R signaling on ISF Aβ levels in vivo
Aβ is produced primarily within neurons and secreted into the brain extracellular fluid, or ISF. We used a brain microdialysis technique to specifically measure dynamic changes in ISF Aβ levels over time in living mice (Cirrito et al., 2003, 2008). This technique permits serial sampling of ISF Aβ up to every hour for several days while the mice are awake and have freedom of movement. Agents can be administered to these mice either by intraperitoneal injection or can be administered directly to the brain by adding the compound to the microdialysis probe perfusion buffer, a technique called reverse microdialysis. Compounds within the perfusion buffer diffuse out of the probe and into the brain, simultaneous with Aβ diffusion into the probe, allowing for a continuous application of drug with minimal tissue disruption and while bypassing the blood–brain barrier.
Three- to four-month-old PS1APP hemizygous transgenic mice were implanted with hippocampal microdialysis probes (Cirrito et al., 2003). Mice at this age do not yet contain Aβ deposits, thus allowing us to study normal brain Aβ metabolism without complicating factors, such as Aβ aggregates, which could alter the equilibrium and metabolism of extracellular Aβ. The basal ISF Aβ concentration for each mouse was established over a 6 h period followed by treatment with a dose escalation of NMDA by reverse microdialysis. The concentration of NMDA within the microdialysis probe was gradually stepped from 0.25 to 250 μm with exposure to each dose lasting 3 h. Administration of 0.25 to 1 μm NMDA significantly elevated ISF Aβ40 levels by 30–75% over basal levels with the peak increase at 0.5 μm NMDA (Fig. 1A). Doses between 2 and 10 μm NMDA had no effect on ISF Aβ40 levels, whereas doses of 20 μm and above caused a significant depression in ISF Aβ levels. For instance, 40 μm NMDA caused an ∼50% decrease in ISF Aβ. Levels of Aβ42 were affected in a similar way as Aβ40 by both the low and high doses of NMDA (Fig. 1B).
To confirm that NMDA-Rs underlie the dose-dependent regulation of Aβ metabolism (Fig. 1A), we performed a similar dose–response study using homoquinolinic acid (HQ), another selective agonist for NMDA-Rs (Grimwood et al., 2002). Similar to NMDA, low doses of HQ (0.25–1 μm) increased ISF Aβ levels, whereas high doses of HQ (5–20 μm) depressed Aβ levels significantly. The peak increase in ISF Aβ occurred at 0.25 μm HQ (increased by 50.0 ± 2.7% of basal levels). Infusion of 20 μm HQ reduced ISF Aβ by 54.5 ± 2.3% of basal levels (n = 5 per group).
Interestingly, other types of glutamate receptors can also modulate ISF Aβ levels. Metabotropic glutamate receptor 2/3 agonists suppress ISF Aβ levels (Cirrito et al., 2005), whereas antagonists increase Aβ (Cirrito et al., 2008). Baseline AMPA receptor (AMPA-R) activity increases ISF Aβ levels in vivo. Infusion of an AMPA-R antagonist, NBQX (10 μm by reverse microdialysis), into the hippocampus suppressed ISF Aβ levels by 21.7 ± 1.5% compared to vehicle-treated mice (p = 0.0002). These receptors are responsible for much of the excitatory neurotransmission within the brain, so blocking AMPA-Rs reduces synaptic activity, which likely reduces synaptic Aβ generation (Cirrito et al., 2005). Whether similar or distinct mechanisms are used by NMDA-Rs and other types of glutamate receptors to modulate ISF Aβ levels are unknown.
Our original hypothesis was that this excitatory neurotransmitter would depolarize neurons, increase synaptic activity, and consequently elevate brain ISF Aβ levels. In our studies, low doses of NMDA resulted in effects consistent with this hypothesis; low doses of NMDA infused directly into the brain elevated synaptic transmission and Aβ generation within hours. Surprisingly, however, as we gradually increased the dose of NMDA, there was a dose-dependent decrease in ISF Aβ levels that was contrary to the initial hypothesis.
Aβ is reduced despite dramatic elevations in neuronal activity
Neurotransmitter receptors can have paradoxical effects on neural networks, especially in the case of receptors that desensitize following intense activation or in the case of excitatory receptors on inhibitory interneurons. To determine how various doses of NMDA affected neuronal activity, we assessed extracellular field potentials, or depth EEG, within the hippocampus of mice being administered NMDA via reverse microdialysis (Fig. 1C). The EEG recording electrodes were attached directly to the microdialysis guide cannula to record electrical activity at the same location that NMDA was administered and Aβ was collected (Cirrito et al., 2005). Under basal conditions, there were synchronous spikes in EEG activity at a frequency of 0.03 ± 0.007 Hz (mean ± SEM; n = 5). One micromolar NMDA had a small, but significant, effect on recorded EEG; new synchronous spikes in activity occurred with a frequency of 0.1 ± 0.013 Hz (p < 0.05). Increasing the dose to 40 μm NMDA had a more dramatic effect on EEG activity with spikes in activity occurring at 0.23 ± 0.03 Hz (p < 0.01). This elevated activity, however, was subthreshold for epileptiform activity. At doses of 250 μm NMDA, EEG activity was dramatically higher with spikes in activity occurring at 1.7 ± 0.13 Hz (p < 0.0001) and epileptiform activity occurring several times during a 3 h administration though this activity did not generalize to result in a behavioral manifestation. Very low doses of NMDA elevated ISF Aβ levels, which is consistent with our previous publications of a presynaptic generation of Aβ that is tightly coupled to synaptic transmission (Cirrito et al., 2005, 2008). The reduction in Aβ by higher doses of NMDA, however, did not appear to be due to this synaptic generation of Aβ given that even in the presence of hippocampal seizures, ISF Aβ levels were still dramatically reduced.
The EC50 of NMDA at NMDA-Rs is 35 μm in vitro (Patneau and Mayer, 1990). Delivered by reverse microdialysis in vivo, NMDA concentrations of 0.25–1 μm (or lower within the parenchyma) should activate only a very small percentage of receptors [estimated 1% bound at 1 μm NMDA based on Patneau and Mayer (1990)]. Given that almost all neurons within the brain express NMDA-Rs, this small percentage is likely a large number of receptors that are activated. In support of this, 1 μm NMDA delivered by reverse microdialysis increases neuronal activity as detected by increased synchronous spikes in EEG recordings (Fig. 1C).
Reductions in ISF Aβ are not due to neuronal injury
Glutamate receptor overstimulation is known to cause excitotoxic injury to neurons (Olney and Sharpe, 1969). We postulated that the decrease in ISF Aβ could be due to cell death as opposed to a true change in Aβ metabolism. We infused 40 μm NMDA into the hippocampus for 6 h, which caused a rapid decrease in ISF Aβ (Fig. 1D). At the end of 6 h of treatment, NMDA was removed from the microdialysis perfusion buffer and ISF Aβ levels were measured for an additional 36 h. During this washout period, ISF Aβ levels gradually returned to baseline levels (96% of baseline at 30 h from the beginning of the washout period). The complete recovery of Aβ levels strongly suggests that the depression in Aβ by 40 μm NMDA was not caused by an excitotoxic lesion. In addition, FluoroJade B staining around the microdialysis probe, to detect degenerating cells, did not show increased reactivity in mice administered 40 μm NMDA (data not shown). Studies described later will demonstrate that the depression in Aβ by 40 μm NMDA can be blocked with several agents, further suggesting the depression in Aβ is caused by altered cellular metabolism as opposed to neuronal injury. This is also consistent with other publications that have delivered similar concentrations of NMDA to the brain via reverse microdialysis and detected physiological alterations as a consequence (Duva et al., 2001).
NMDA-R antagonists increase ISF Aβ levels
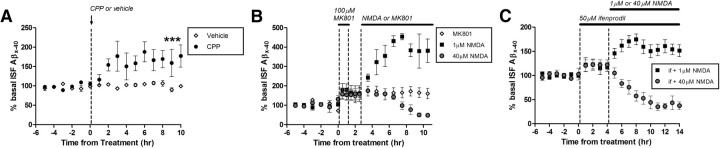
To determine how endogenous NMDA-R signaling alters Aβ levels, we administered CPP, a selective NMDA-R antagonist, systemically to PS1APP transgenic mice. CPP (20 mg/kg i.p.) caused a significant 75% increase in ISF Aβ levels over 3 h, which remained high until at least 10 h after treatment compared to vehicle-treated mice (Fig. 2A). This demonstrates that basal NMDA-R signaling within the hippocampus normally suppresses Aβ levels. Since CPP does not cause excitotoxicity, this is also further evidence that modulation of Aβ levels is a physiological response to NMDA-R signaling and not due to degenerating neurons.
Figure 2.
NMDA-R antagonists increase ISF Aβ levels. A, NMDA-R antagonist CPP (20 mg/kg i.p.) increased ISF Aβ levels by 77.4 ± 28.7% compared to vehicle-treated (saline) PS1APP mice at 10 h after treatment (p < 0.05, n = 6 per group). B, MK801 (100 μm by reverse microdialysis), a use-dependent NMDA-R antagonist, was infused into the hippocampus for 60 min followed by a 90 min washout and reapplication of MK801, 1 μm NMDA, or 40 μm NMDA for 8 h (n = 5 per group). MK801 alone significantly increased ISF Aβ40 by 60.7 ± 16.6% (p < 0.05) compared to basal levels. One micromolar NMDA increased ISF Aβ by 281.7 ± 60% (p < 0.01), whereas 40 μm NMDA reduced Aβ by 49.6 ± 7.1% (p < 0.05) compared to basal levels. C, Infusion of the NR2B subunit-selective antagonist, ifenprodil (50 μm), alone increased ISF Aβ40 by 19.3 ± 3.3% (p < 0.05) compared to basal levels. Cotreatment of ifenprodil and either 1 μm NMDA or 40 μm NMDA increased Aβ by 51.6 ± 12.2% (p < 0.05) and decreased Aβ by 62.5 ± 7.9% (p < 0.0001; n = 5–6 per group), respectively. Data are presented as mean ± SEM. ***p < 0.001.
Paradoxically, low doses of exogenous NMDA have the opposite effect on Aβ than endogenous NMDA signaling has, suggesting that a separate population of receptors underlie the two phenomenon. To remove the basally active NMDA-Rs for the experimental system, we infused MK801, a noncompetitive use-dependent NMDA-R antagonist, into the hippocampus. This compound will only block NMDA-Rs that have open channels when the drug is present (Huettner and Bean, 1988; Ivanov et al., 2006). Similar to CPP, MK801 increased ISF Aβ levels by 60% (Fig. 2B). After 60 min of administration, MK801 was removed from the microdialysis perfusion buffer to wash out existing compound from the brain for 90 min. One cohort of PS1APP mice had MK801 reapplied after the washout period. In these mice, ISF Aβ levels did not have an additional change, suggesting that during the washout phase no new NMDA-Rs were recruited to regulate Aβ metabolism. A second and third cohort of mice were administered either 1 or 40 μm NMDA after the washout period, which caused an increase in Aβ by 280% and a decrease by 50%, respectively (Fig. 2B). This suggests that exogenous application of NMDA activates a separate set of receptors, likely on separate neurons, from those used under basal conditions to regulate Aβ metabolism.
A functional NMDA-R consists of two NR1 subunits (out of eight known subunits) and two NR2 subunits (out of four known subunits). The combination of subunits within each receptor impacts the receptor's properties, such as kinetics and localization. For instance, NR2B-containing receptors can be localized axonally and at extrasynaptic sites (Köhr, 2006). To determine whether NR2B receptors modulate Aβ metabolism, we infused ifenprodil, a NR2B-selective inhibitor, into the hippocampus. Ifenprodil alone increased ISF Aβ levels by 20% (Fig. 2C). Pretreatment with ifenprodil marginally affected the change in Aβ metabolism by either low- or high-dose NMDA (Fig. 2C). This suggests that NR2B-containing receptors likely account for a relatively small portion of synaptic-dependent Aβ metabolism.
NMDA-R modulation alters endogenous murine Aβ levels
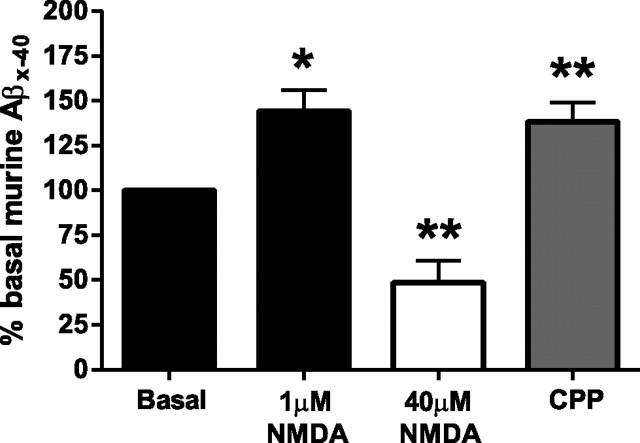
As with any transgenic animal model, a concern is that an observation could be due to overexpression of a protein as opposed to a normal cellular process. Using microdialysis, we established basal ISF murine Aβ levels in the hippocampus of young wild-type C57BL6 mice and then administered either 1 μm NMDA or 40 μm NMDA for 4 h by reverse microdialysis. A low dose of NMDA caused a significant 45% increase in ISF Aβ, whereas a high dose caused a 50% reduction in Aβ (Fig. 3). Similarly, CPP caused a 50% increase in ISF murine Aβ levels in C57BL6 mice (Fig. 3). These data demonstrate that NMDA-Rs modulate physiological Aβ metabolism.
Figure 3.
Effect of NMDA-R modulation on endogenous murine Aβ levels. ISF Aβ levels in wild-type C57BL6 mice increased by 44.2 ± 11.7% (*p < 0.05) and decreased by 51.4 ± 12.3% (p < 0.01) compared to basal levels in response to 1 and 40 μm NMDA delivered into the hippocampus by reverse microdialysis (n = 5–6 per group). CPP (20 mg/kg, i.p.) elevated Aβ levels by 38.3 ± 10.7% (**p < 0.01) compared to basal levels in each mouse (n = 5). Data presented as mean ± SEM.
Action potentials are only required for NMDA-dependent elevations in ISF Aβ
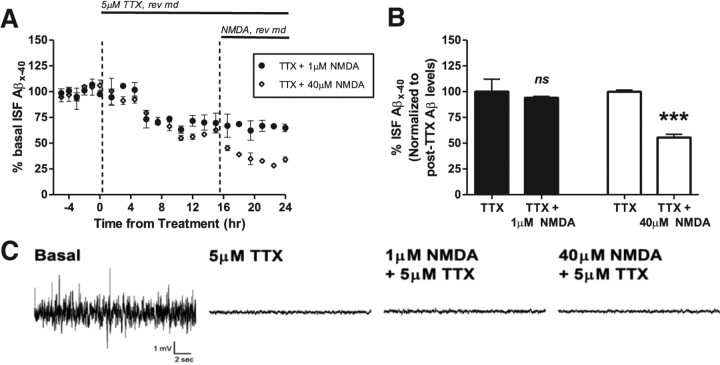
To determine whether NMDA-dependent changes to Aβ were primarily due to depolarization/action potentials or to signaling cascades activated by these receptors, we pretreated mice with TTX for 15 h and then coinfused TTX with either 1 μm NMDA or 40 μm NMDA into the hippocampus for 8 h (Fig. 4A). In the presence of TTX, infusion of NMDA will activate NMDA-Rs normally; however, the neuronal response is limited to local action of NMDA-Rs. TTX alone reduced Aβ levels by 40% similar to previous reports (Kamenetz et al., 2003; Cirrito et al., 2005). TTX completely blocked the increase in ISF Aβ levels caused by 1 μm NMDA (Fig. 4B); however, 40 μm NMDA still caused a significant 45% reduction in ISF Aβ levels compared to TTX treatment alone. As expected, even though NMDA-Rs were activated, TTX blocked the downstream neuronal activity caused by both concentrations of NMDA as measured by hippocampal EEG (Fig. 4C). This suggests that low doses of NMDA require action potentials to increase Aβ generation (Cirrito et al., 2005, 2008), but in contrast, reduction of Aβ by high doses of NMDA do not.
Figure 4.
Presynaptic versus postsynaptic mechanisms that regulate Aβ metabolism. A, TTX (5 μm) was delivered continuously into the hippocampus by reverse microdialysis for 16 h followed by coinfusion with either 1 or 40 μm NMDA. B, In this setting, 1 μm NMDA had no effect on ISF Aβ levels, whereas 40 μm NMDA reduced ISF Aβ levels by 44.4 ± 3.1% compared to levels during hour 16 of TTX-treatment alone (***p < 0.001). (n = 5–6 per group). C, Representative EEG traces during a dose escalation of NMDA during TTX treatment. TTX (5 μm) was delivered for 16 h, followed by 3 h each of 1 μm and then 40 μm NMDA (n = 3 mice). Data presented as mean ± SEM.
Calcium is necessary for NMDA-dependent changes of ISF Aβ
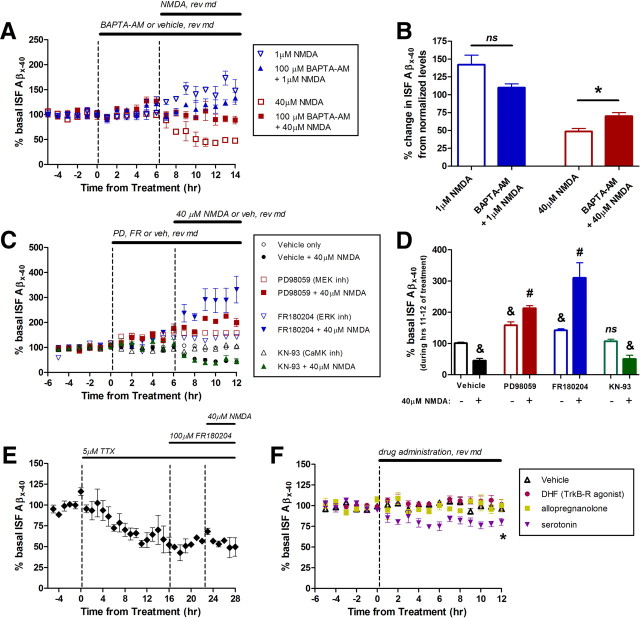
NMDA-Rs are cation channels that are primarily permeable to sodium and calcium influx. Given that calcium can have a multitude of effects within a cell, we determined whether calcium entry was responsible for the NMDA-dependent changes in ISF Aβ. Basal ISF Aβ levels were determined in PS1APP mice followed by administration of vehicle or BAPTA-AM, a calcium chelator, by reverse microdialysis for 6 h (Fig. 5A). BAPTA-AM alone increased ISF Aβ levels by 27% by the end of treatment, suggesting that some basal calcium signaling normally lowers ISF Aβ. Beginning at hour 7, BAPTA-AM was coadministered with either 1 μm NMDA or 40 μm NMDA for an additional 8 h. One micromolar NMDA only increased ISF Aβ levels by 10% in the presence of BAPTA-AM when normalized to BAPTA-AM treatment alone (Fig. 5B). This increase could be driven by residual presynaptic cycling supported by any unchelated calcium that remains. BAPTA-AM also reduced the effect of 40 μm NMDA. Forty micromolar NMDA only reduced ISF Aβ by 30% in the presence of the calcium chelator when normalized to pretreatment levels. Not surprisingly, these data suggest that calcium is at least partially responsible for both the elevation and depression of ISF Aβ by NMDA. In vivo, it is unlikely that all free calcium is buffered by BAPTA-AM; therefore, it is still possible that NMDA entirely requires calcium to modulate Aβ levels.
Figure 5.
The ERK signaling cascade is required for NMDA-R-dependent reductions in Aβ generation. A, BAPTA-AM (100 μm) was infused into the hippocampus by reverse microdialysis to chelate calcium. BAPTA-AM alone increased ISF Aβ levels by 27.1 ± 8.3% (p < 0.05, n = 5). B, At hour 14 from initial treatment, 1 μm NMDA coinfused with BAPTA-AM did not change ISF Aβ levels compared to BAPTA-AM alone (n = 4) and 40 μm NMDA with BAPTA-AM only reduced Aβ levels by 30.0 ± 4.9% compared to BAPTA-AM alone (p < 0.001, n = 5). C, MEK inhibitor (PD98059, 100 μm) and ERK inhibitor (FR180204, 100 μm) infused into the hippocampus increased ISF Aβ levels by 58.6 ± 19.0% (p < 0.01) and 42.4 ± 8.1% (p < 0.01), respectively, by 12 h after administration (n = 5–6 per group). D, Instead of decreasing Aβ, 40 μm NMDA further increased ISF Aβ levels when in the presence of either of these inhibitors (112.9 ± 8.5% and 210.5 ± 47.8%, respectively). KN-93, a CaMK inhibitor, did not alter ISF Aβ levels alone or in the presence of NMDA. Neither the MEK nor ERK inhibitor altered the effect of 1 μm NMDA on ISF Aβ levels (data not shown). &Significantly different from vehicle treatment. #Significantly different from each inhibitor alone. E, TTX was infused into the hippocampus for 16 h, followed coinfusion of FR180204 for 6 h, then addition of 40 μm NMDA for 6 h. In this setting, ISF Aβ levels did not change compared to Aβ levels during TTX and FR180204 alone (n = 4). F, PS1APP mice were treated with a Trk-B receptor agonist (DHF, 20 μm) or allopregnanolone (10 μm) by reverse microdialysis but did not alter ISF Aβ levels. Serotonin (1 mm) infusion reduced ISF Aβ by 19.5 ± 4.1% (p < 0.05; n = 5 per group). Data are presented as mean ± SEM. *p < 0.05.
ERK signaling is required for NMDA-dependent depression of ISF Aβ levels
Calcium influx through NMDA-Rs can lead to activation of several second messenger signaling pathways, including the extracellular regulated kinase (ERK) pathway (Bading and Greenberg, 1991; Kurino et al., 1995; Xia et al., 1996; Ivanov et al., 2006). ERK is a mitogen-activated protein kinase (MAPK) that is activated by a wide variety of extracellular ligands (Boulton and Cobb, 1991). In this signaling cascade, an extracellular ligand leads to activation of Raf kinase, which results in sequential phosphorylation of MEK and then ERK, which is the active form of this kinase. Phosphorylated ERK (pERK) acts within the cytoplasm to phosphorylate other proteins and alter their function or it translocates to the nucleus to alter gene transcription.
In PS1APP hemizygous mice, we blocked the ERK signaling pathway by infusing agents that inhibit either MEK (PD98059) or ERK (FR180204) through the microdialysis probe. These inhibitors alone increased ISF Aβ levels by 58% and 42%, respectively (Fig. 5C). Interestingly, however, in the presence of either inhibitor, 40 μm NMDA no longer decreased ISF Aβ levels but instead dramatically increased ISF Aβ levels by twofold and threefold compared to basal levels in each mouse (Fig. 5D). Inhibitors of two different points of the ERK signaling cascade block the effect of 40 μm NMDA, strongly suggesting that the ERK signaling cascade is required for the NMDA-dependent reduction in ISF Aβ. In contrast, an inhibitor of calcium/calmodulin kinase (CaMK), KN-93, did not block the reduction in ISF Aβ by 40 μm NMDA. Neither the MEK nor ERK inhibitor affected the increase in ISF Aβ levels by 1 μm NMDA (data not shown), demonstrating that ERK signaling is only required for depression of ISF Aβ but not action potential-mediated synaptic Aβ generation.
The remarkable elevation in Aβ by coadministration of ERK pathway inhibitors and 40 μm NMDA was likely due to synaptic mechanisms that generate Aβ as a consequence of synaptic transmission (Cirrito et al., 2005). To test this hypothesis directly, we pretreated PS1APP mice with TTX and then FR180204 to block action potentials and ERK signaling, then cotreated with 40 μm NMDA. In this setting, ISF Aβ levels did not change compared to pretreatment levels (Fig. 5E), suggesting that blocking the ERK signaling that reduces APP processing into Aβ as well as blocking synaptic Aβ generation is sufficient to prevent NMDA from altering ISF Aβ levels at all.
Only select ERK activators regulate Aβ metabolism
Numerous receptors and signaling pathways can converge to activate ERK, which in turn can have a multitude of downstream transcriptional or protein targets. ERK activation and activity, however, is highly regulated and can have very precise biological responses in large part due to cellular context, scaffold proteins, and compartmentalization where it is activated (for review, see Pouysségur et al., 2002). We determined the extent that other ERK activators would impact Aβ metabolism. Using reverse microdialysis, we infused a Trk-B receptor agonist [7,8-dihydroxyflavone (DHF)], a glucocorticoid receptor ligand (allopregnanolone), or a serotonin receptor ligand (serotonin) into the brain (Fig. 5F). In addition to the many effects these pathways have within a neuron, ERK is also activated (Hetman et al., 1999; Basta-Kaim et al., 2007; Cowen, 2007). Interestingly, neither activation of Trk-B receptors nor glucocorticoid receptors altered ISF Aβ levels. Infusion of serotonin however significantly reduced ISF Aβ levels by 20% (Fig. 5F). The particular cellular cues, cofactors, and scaffold proteins that underlie this downstream substrate specificity are still unknown.
NMDA-R signaling alters secretase cleavage of APP
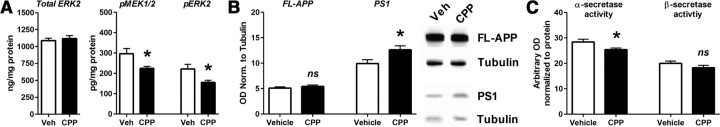
We sought to determine the mechanisms by which NMDA-R signaling alters Aβ levels. To alter NMDA-R signaling uniformly throughout the brain, PS1APP were administered vehicle or the NMDA-R antagonist CPP by intraperitoneal injection. Eight hours after treatment, tissue was harvested and processed for a Luminex bead-based assay to measure various components of the ERK cascade. CPP did not alter total ERK2 (Fig. 6A) or ERK1 (data not shown) protein levels; however, the phosphorylated active forms of both the ERK2 and MEK1/2 were significantly reduced (Fig. 6A). Phospho-ERK1 protein levels were not detectable with this assay. Full-length APP levels were unaffected by CPP administration (Fig. 6B); however, APP C-terminal fragment (CTF)-α was significantly reduced by 15.2 ± 2.1% compared to vehicle-treated mice (p < 0.05; n = 8 per group), while APP CTF-β was unaffected.
Figure 6.
ERK signaling alters processing of APP. CPP (20 mg/kg, i.p.) or vehicle (saline) was delivered to PS1APP+/− mice and brains removed 8 h later for biochemical analysis (n = 8 mice per group). A, ERK2 total protein levels did not change following CPP treatment; however, phospho-MEK1/2 and phospho-ERK2 protein levels were reduced by 24.2 ± 9.6% and 29.0 ± 10.7%, respectively (p < 0.05). B, Full-length APP protein levels, as assessed by SDS-PAGE/Western blot, were not altered by CPP treatment. However, total presenilin-1 protein levels were significantly increased by 26.7 ± 1.9% (p < 0.05). C, α-Secretase enzymatic activity was decreased by 8.9 ± 1.3% compared to vehicle-treated mice (p < 0.05), whereas β-secretase activity was unaltered. D, Data are presented as mean ± SEM. *p < 0.05.
ERK signaling has been shown to increase α-secretase activity (Kojro et al., 2006) and decrease γ-secretase activity (Kim et al., 2006; Tung et al., 2008), both of which are consistent with the depression in ISF Aβ caused by 40 μm NMDA. We measured enzymatic activity of α-secretase and β-secretase in brain homogenates from the CPP and vehicle-treated PS1APP mice using fluorometric cleavage assays. Eight hours after CPP treatment, α-secretase activity was significantly reduced, whereas β-secretase activity was unaltered within the hippocampus (Fig. 6C); enzymatic activity was consistent with our observed changes in APP-CTFs. Whether ERK causes a posttranslational or transcriptional effect to alter α-secretase activity in vivo is still unclear. Interestingly, presenilin-1 (PS1) protein levels were elevated as assessed by Western blot (Fig. 6B). Both of these changes in APP processing are consistent with an elevation in ISF Aβ levels following NMDA-R antagonist treatment.
Discussion
The relationship between synaptic activity and regulation of Aβ levels is complex. A single neurotransmitter receptor, such as NMDA-R, can simultaneously activate multiple mechanisms within a cell to differentially regulate Aβ generation. Calcium influx through these receptors depolarizes and excites neurons leading to synaptic transmission, which is closely linked to synaptic Aβ generation. Calcium also acts as a second messenger. When sufficient calcium levels are reached through high levels of NMDA-R activity, the ERK signaling cascade is activated, which depresses APP processing into Aβ, likely via enhancing α-secretase cleavage activity. Our studies administering NMDA to the hippocampus demonstrate that this neurotransmitter system can have dramatic effects on Aβ levels with up to a 75% reduction at the highest doses of NMDA. Given that NMDA-R antagonists, in both APP transgenic and wild-type mice, elevate Aβ levels, it appears that basal signaling through these receptors contributes to normal suppression of Aβ generation.
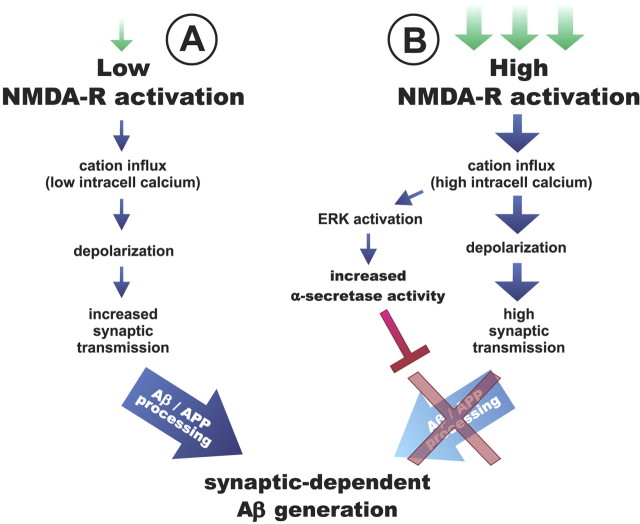
Model of NMDA-dependent mechanisms that regulate Aβ generation
Low doses of exogenous NMDA depolarize neurons, elevate synaptic transmission, and increase Aβ generation and secretion (Fig. 7A). Recruitment of NMDA-Rs and neurons that are not active under basal conditions appear to be responsible for this increase in Aβ. While this can occur via a presynaptic mechanism that requires clathrin-mediated endocytosis (Cirrito et al., 2008), other areas of a neuron may also contribute to this increase in generation. In contrast, high doses of NMDA activate the ERK signaling pathway, which downregulates APP processing into Aβ despite increased synaptic activity (Fig. 7B). This depression in Aβ appears to be in part due to enhanced α-secretase enzymatic activity. We propose that at low levels of NMDA-R activation, very little ERK is activated; thus, the balance is shifted so that synaptic transmission drives Aβ generation. Strong NMDA-R activation, however, recruits ERK, which reduces APP processing into Aβ. The balance between enhancing Aβ generation and depressing APP processing determines the total amount of Aβ that a neuron will produce at a given time. Midrange doses of NMDA (Fig. 1A) (2–10 μm) had no net change in ISF Aβ levels; this likely represents an equal balance of these competing pathways. Under normal conditions, basal NMDA-R signaling is sufficient to drive ERK activation and suppress AP processing into Aβ. This likely occurs in a subpopulation of neurons where either baseline NMDA-R activity is high or at a set of NMDA-Rs that are particularly capable to activating ERK.
Figure 7.
Model of synaptic mechanisms that regulate Aβ metabolism. A, Synaptic Aβ generation: NMDA infusion into the hippocampus increases synaptic activity and elevates Aβ generation and then secretion. APP processing progresses normally here, but at an increased rate. B, Synaptic modulation of APP processing: high levels of NMDA-R activation cause calcium influx into the neuron, which activates the ERK signaling cascade. ERK enhances α-secretase cleavage of APP, thus reducing APP processing into Aβ. The balance between synaptic generation and APP processing determines whether overall ISF Aβ levels increase or decrease, respectively.
Neuronal depolarization and synaptic transmission lead to APP endocytosis and Aβ generation and then secretion (Cirrito et al., 2008). This appears to be a normal metabolic pathway for Aβ generation. Increasing synaptic activity in a cell may simply drive more APP through this pathway and therefore generate more Aβ. In contrast, changing APP processing via secretase activity alters the ability of a cell to produce Aβ. APP is primarily cleaved by α-secretase within the secretory pathway (Lammich et al., 1999), which is upstream of endocytic events that lead to Aβ generation (Koo and Squazzo, 1994). Thus, in the presence of 40 μm NMDA, elevated α-secretase activity cleaves APP, which reduces the substrate available for later Aβ generation within endosomes (Fig. 1A). When ERK signaling is inhibited, however, 40 μm NMDA has no effect on the APP substrate; thus, a dramatic increase in synaptic Aβ generation is unmasked (Fig. 5C).
Basal NMDA-R and ERK activity lowers ISF Aβ levels
Administration of NMDA-R antagonists or inhibitors of the ERK signaling cascade block steady-state activity and increase ISF Aβ levels. This suggests that there is a physiologic suppression of Aβ levels by NMDA-Rs and by ERK in vivo. NR2B-containing receptors appear to account for only a small portion of the basal suppression in Aβ since ifenprodil (NR2B-selective) only increases Aβ by 20%, whereas broader antagonists such as MK801 and CPP and increase Aβ by 60–75%.
NMDA receptors, ERK, and Aβ metabolism
Two very similar studies have assessed the role of NMDA-Rs in Aβ metabolism in neuronal culture and produced conflicting evidence. Lesné et al. (2005) determined that NMDA increased Aβ production and Aβ levels in primary neuronal cultures by reducing α-secretase activity. In contrast, Hoey et al. (2009) found that NMDA reduced Aβ generation in culture. This reduction in Aβ was associated with activated ERK and nonamyloidogenic processing of APP. Ironically, our in vivo data are consistent with both of these studies. We propose that the difference between these studies may be the degree of ERK activation or another component of that signaling cascade. NMDA-R expression or activity could shift the balance between the Aβ generation and APP processing mechanisms depending on the dose of NMDA used and the developmental state in each culture model.
It appears that NMDA-Rs, specifically during prolonged activation of extrasynaptic receptors, can induce changes in APP transcription shifting from APP695 transcripts to more amyloidogenic APPs that contain a Kunitz protease inhibitor (APP-KPI) domain (Bordji et al., 2010). While transcriptional changes may occur in some systems, it is unlikely to be playing a role in our study given that the PS1APP mice we used overexpress an APP695 transgene that is not affected by normal APP transcriptional regulation. Our administration of NMDA in these studies was relatively short-lived (hours), however, so it is possible that prolonged dosing would have recruited changes in transcription.
Calcium entry through NMDA-Rs can act as a second messenger. Influx of calcium triggers a variety of signaling pathways, including protein kinase C (PKC), protein kinase A (PKA), and calcium/calmodulin-dependent kinase II (CaMKII), all of which can activate ERK and may regulate some facet of Aβ metabolism (Chandler et al., 2001; Kawasaki et al., 2004). Alternatively, for NMDA-Rs that contain a NR2B subunit, calcium can cause dissociation of factors physically associated with the receptor, which leads to direct activation of the ERK signaling cascade (Krapivinsky et al., 2003; Kim et al., 2005). Importantly, inhibition of NR2B-containing receptors only marginally affected Aβ metabolism in our studies, suggesting that NR2B plays a relatively minor role in regulation of Aβ metabolism. The subcellular localization of NMDA-Rs or their subunit composition may still play critical roles in regulating Aβ levels, however, in that these factors can influence when and how ERK activity is regulated in neurons (Kim et al., 2005; Ivanov et al., 2006).
ERK signaling and Aβ production
Activated pERK is elevated in the brains and CSF of AD patients (Perry et al., 1999; Klafki et al., 2009). These studies propose that ERK activation is a consequence of AD-related changes, including oxidative stress and inflammation; however, the possibility cannot be discounted that ERK activity also contributes to AD progression. Several studies demonstrate that ERK can modulate APP processing, suggesting that this signaling pathway may be a direct factor in AD processes. Decreasing β-secretase or γ-secretase cleavage or increasing α-secretase cleavage of APP all can reduce Aβ generation. Similar to our study, ERK has been found to increase α-secretase cleavage of APP (Kojro et al., 2006) as well as reduce γ-secretase activity. Inhibition of ERK increases γ-secretase activity in vitro and in vivo (Kim et al., 2006). Activated pERK phosphorylates a component of the γ-secretase complex, nicastrin, which appears to reduce cleavage activity (Kim et al., 2006; Tung et al., 2008). While our data show that PS1 protein levels are elevated following NMDA-R antagonism (Fig. 6D), given that PS1 is not the rate-limiting component of the complex, it is unclear whether this would elevate γ-secretase activity in vivo. These alterations in α-secretase and γ-secretase both could result in reduced APP processing into Aβ; however, whether ERK also induces other changes that impact Aβ metabolism is unknown.
Use of ERK signaling as a treatment strategy for AD
NMDA-R agonists are unlikely to be a viable chronic therapy for AD given the role of these receptors in neuronal transmission, synaptic plasticity, and excitotoxicity. However, it is ultimately ERK signaling that is responsible for suppression of Aβ levels; NMDA-R activation is only one means by which this can occur. It is possible that activation of ERK via other means could be used to depress Aβ levels therapeutically. Calcium influx through NMDA-Rs has been shown to activate ERK (Bading and Greenberg, 1991; Krapivinsky et al., 2003). The cellular context in which ERK is activated dictates the downstream effects of this kinase; thus, not all ERK activators may lower Aβ levels to a similar extent (Pouysségur et al., 2002). Dopamine, metabotropic glutamate, and serotonin neurotransmitter receptors have also been linked to ERK activation [for review, see Sweatt (2001) and Cowen (2007)], as well as a wide range of other molecules, such as growth factors, integrins, and neurosteroids. In this study, only a subset of these receptors lead to depressed Aβ levels. There are currently several drugs, including some approved by the FDA for use in humans, that target receptors that may be capable of activating ERK in vivo. Such drugs may contribute to disease risk by altering Aβ levels over a lifetime. Compounds that activate ERK within the brain may also be candidate preventative or therapeutic agents capable of lowering Aβ and reducing AD plaque pathogenesis or growth.
Footnotes
We thank the National Institutes of Health/NIA (K01 AG029524 and P50 AG568127 to J.R.C.), the Shmerler family (J.R.C.), the Charles F. and Joanne Knight Alzheimer's Disease Research Center at Washington University (J.R.C.), the McDonnell Center for Cell and Molecular Biology (J.R.C.), Cure Alzheimer's Fund (D.M.H.), and Elison Medical Foundation (D.M.H.).
The authors declare no competing financial interests.
References
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Leśkiewicz M, Tetich M, Otczyk M, Kubera M, Lasoń W. Effects of neurosteroids on glucocorticoid receptor-mediated gene transcription in LMCAT cells—a possible interaction with psychotropic drugs. Eur Neuropsychopharmacol. 2007;17:37–45. doi: 10.1016/j.euroneuro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Bordji K, Becerril-Ortega J, Nicole O, Buisson A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-β production. J Neurosci. 2010;30:15927–15942. doi: 10.1523/JNEUROSCI.3021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton TG, Cobb MH. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991;2:357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-Methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen DS. Serotonin and neuronal growth factors—a convergence of signaling pathways. J Neurochem. 2007;101:1161–1171. doi: 10.1111/j.1471-4159.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- Davis AA, Fritz JJ, Wess J, Lah JJ, Levey AI. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J Neurosci. 2010;30:4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duva MA, Tomkins EM, Moranda LM, Kaplan R, Sukhaseum A, Jimenez A, Stanley BG. Reverse microdialysis of N-methyl-D-aspartic acid into the lateral hypothalamus of rats: effects on feeding and other behaviors. Brain Res. 2001;921:122–132. doi: 10.1016/s0006-8993(01)03108-0. [DOI] [PubMed] [Google Scholar]
- Fazeli MS, Breen K, Errington ML, Bliss TV. Increase in extracellular NCAM and amyloid precursor protein following induction of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci Lett. 1994;169:77–80. doi: 10.1016/0304-3940(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Gordon-Krajcer W, Salińska E, Lazarewicz JW. N-Methyl-d-aspartate receptor-mediated processing of beta-amyloid precursor protein in rat hippocampal slices: in vitro—superfusion study. Folia Neuropathol. 2002;40:13–17. [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood S, Wafford KA, Macaulay A, Hutson PH. N-Methyl-D-aspartate receptor subtype-selectivity of homoquinolinate: an electrophysiological and radioligand binding study using both native and recombinant receptors. J Neurochem. 2002;82:794–800. doi: 10.1046/j.1471-4159.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, Kim D, Hamto P, Lo I, Yu GQ, Palop JJ, Masliah E, Mucke L. Transsynaptic progression of amyloid-beta-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68:428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Hock C, Maddalena A, Raschig A, Müller-Spahn F, Eschweiler G, Hager K, Heuser I, Hampel H, Müller-Thomsen T, Oertel W, Wienrich M, Signorell A, Gonzalez-Agosti C, Nitsch RM. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer's disease. Amyloid. 2003;10:1–6. doi: 10.3109/13506120308995249. [DOI] [PubMed] [Google Scholar]
- Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates α-secretase amyloid precursor protein processing and inhibits amyloid-β production. J Neurosci. 2009;29:4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, Mason SM, Paul SM, Holtzman DM. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron. 2009;64:632–644. doi: 10.1016/j.neuron.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kim SK, Park HJ, Hong HS, Baik EJ, Jung MW, Mook-Jung I. ERK1/2 is an endogenous negative regulator of the gamma-secretase activity. FASEB J. 2006;20:157–159. doi: 10.1096/fj.05-4055fje. [DOI] [PubMed] [Google Scholar]
- Klafki HW, Lewczuk P, Kamrowski-Kruck H, Maler JM, Müller K, Peters O, Heuser I, Jessen F, Popp J, Frölich L, Wolf S, Prinz B, Luckhaus C, Schröder J, Pantel J, Gertz HJ, Kölsch H, Müller BW, Esselmann H, Bibl M, et al. Measurement of ERK 1/2 in CSF from patients with neuropsychiatric disorders and evidence for the presence of the activated form. J Alzheimers Dis. 2009;18:613–622. doi: 10.3233/JAD-2009-1167. [DOI] [PubMed] [Google Scholar]
- Köhr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Kojro E, Postina R, Buro C, Meiringer C, Gehrig-Burger K, Fahrenholz F. The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 2006;20:512–514. doi: 10.1096/fj.05-4812fje. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Kurino M, Fukunaga K, Ushio Y, Miyamoto E. Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem. 1995;65:1282–1289. doi: 10.1046/j.1471-4159.1995.65031282.x. [DOI] [PubMed] [Google Scholar]
- Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits α-secretase and promotes neuronal amyloid-β production. J Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin A, Teplow DB, Kirschner DA, Benedek GB. Kinetic theory of fibrillogenesis of amyloid beta-protein. Proc Natl Acad Sci U S A. 1997;94:7942–7947. doi: 10.1073/pnas.94.15.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello E, Gardoni F, Mauceri D, Romorini S, Jeromin A, Epis R, Borroni B, Cattabeni F, Sala C, Padovani A, Di Luca M. Synapse-associated protein-97 mediates α-secretase ADAM10 trafficking and promotes its activity. J Neurosci. 2007;27:1682–1691. doi: 10.1523/JNEUROSCI.3439-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Stalder M, Herzig MC, Kaeser SA, Kohler E, Pfeifer M, Boncristiano S, Mathews PM, Mercken M, Abramowski D, Staufenbiel M, Jucker M. Extracellular amyloid formation and associated pathology in neural grafts. Nat Neurosci. 2003;6:370–377. doi: 10.1038/nn1022. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 2000;48:913–918. [PubMed] [Google Scholar]
- Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166:386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-d-aspartate and quisqualate receptors. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X, Raina AK, Holbrook N, Siedlak SL, Harris PL, Smith MA. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport. 1999;10:2411–2415. doi: 10.1097/00001756-199908020-00035. [DOI] [PubMed] [Google Scholar]
- Pouysségur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Xu GM, Price DL, Borchelt DR, Markowska AL. Normal cognitive behavior in two distinct congenic lines of transgenic mice hyperexpressing mutant APP SWE. Neurobiol Dis. 2003;12:194–211. doi: 10.1016/s0969-9961(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tung YT, Hsu WM, Wang BJ, Wu SY, Yen CT, Hu MK, Liao YF. Sodium selenite inhibits gamma-secretase activity through activation of ERK. Neurosci Lett. 2008;440:38–43. doi: 10.1016/j.neulet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci. 2009;29:10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]