Abstract
OBJECTIVE
Optimal vitamin D levels are associated with reduced cardiovascular and all-cause mortality. We investigated whether optimal 25-hydroxyvitamin D (25[OH]D) is protective in individuals with the metabolic syndrome.
RESEARCH DESIGN AND METHODS
The Ludwigshafen Risk and Cardiovascular Health (LURIC) study is a cohort study of subjects referred for coronary angiography between 1997 and 2000, from which 1,801 with the metabolic syndrome were investigated. Mortality was tracked for a median of 7.7 years. Multivariable survival analysis was used to estimate the association between 25(OH)D levels and mortality.
RESULTS
Most subjects (92%) had suboptimal levels of 25(OH)D (<75 nmol/L), with 22.2% being severely deficient (<25 nmol/L). During follow-up, 462 deaths were recorded, 267 (57.8%) of which were cardiovascular in origin. After full adjustment, including the metabolic syndrome components, those with optimal 25(OH)D levels showed a substantial reduction in all-cause (hazard ratio [HR] 0.25 [95% CI 0.13–0.46]) and cardiovascular disease mortality (0.33 [0.16–0.66]) compared with those with severe vitamin D deficiency. For specific cardiovascular disease mortality, there was a strong reduction for sudden death (0.15 [0.04–0.63]) and congestive heart failure (0.24 [0.06–1.04]), but not for myocardial infarction. The reduction in mortality was dose-dependent for each of these causes.
CONCLUSIONS
Optimal 25(OH)D levels substantially lowered all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome. These observations call for interventional studies that test whether vitamin D supplementation provides a useful adjunct in reducing mortality in these subjects.
The cluster of cardiovascular risk factors termed the metabolic syndrome is an important determinant of vascular disease (1–4), which is the major cause of morbidity and mortality worldwide. Available pharmacologic and lifestyle interventions have been shown to attenuate the hazard associated with the syndrome and its components (5,6). However, these treatment modalities still fail to normalize risk, and much research effort is therefore dedicated to exploring additional treatment approaches, for which targeting suboptimal vitamin D levels presents a potentially important option.
Studies in the U.S. and Europe show that most of the general population have 25-hydroxyvitamin D (25(OH)D) levels below the target level of 75 nmol/L (7,8), with levels being even lower in those with the metabolic syndrome (9). The high prevalence of a poor vitamin D status has gained much public health interest because of its association with cardiovascular disease conditions, including arterial hypertension, diabetes, and the metabolic syndrome. Moreover, prospective studies have shown that low 25(OH)D levels are associated with increased all-cause and cardiovascular mortality (10–13). Whether these associations are causal remains to be explored, but it is often stressed that vitamin D metabolites regulate a very wide range of genes with significance for overall and cardiovascular health (14), making causality a plausible hypothesis.
In light of nascent evidence for a protective effect of optimal vitamin D levels, it is perhaps surprising that no studies have specifically addressed whether vitamin D levels predict mortality and cardiovascular events in subjects with the metabolic syndrome. Such data are needed to assess the potential of supplementation studies in this increased-risk population. We therefore studied a large cohort of subjects referred for coronary angiography, focusing our analyses on 1,801 individuals who fulfilled the criteria for the metabolic syndrome.
RESEARCH DESIGN AND METHODS
Study population
The Ludwigshafen Risk and Cardiovascular Health (LURIC) study is a prospective cohort study designed to evaluate determinants of cardiovascular health (15–17). Between July 1997 and January 2000, 3,316 Caucasian subjects referred for coronary angiography were recruited at the Herzzentrum (Cardiac Center) Ludwigshafen in southwest Germany. Exclusion criteria were any acute illness other than acute coronary syndrome, any predominant noncardiac chronic disease, and a history of malignant neoplasm(s) within the past 5 years. The metabolic syndrome was identified in 1,801 subjects. Written informed consent was obtained from each participant, and the study was approved by the institutional review board at the Ärztekammer Rheinland-Pfalz (Medical Association of Rheinland-Pfalz).
A detailed questionnaire was used to collect a range of demographic characteristics, including lifestyle factors such as alcohol consumption, smoking, and physical activity. Daily physical activity was recorded using a nonvalidated 11-point scale ranging from bedridden to extremely active. Key points on the scale were 1, bed rest; 2, mostly supine, 3, not very active, 6, usual office work; 9, heavy work or sports; and 11, extremely sportive.
Laboratory analyses
A fasting venous blood sample was obtained in the morning before coronary angiography from supine subjects. Selected variables were measured after samples were snap frozen and stored at –80°C. A summary of analytic methods relevant to this study has been previously reported (15). Serum levels of 25(OH)D were assayed weekly using a radioimmunoassay (DiaSorin SA, Antony, France) with intra-assay and interassay coefficients of variation of 8.6% and 9.2%, respectively (15,16). We have also validated this radioimmune assay using liquid chromatography coupled to tandem mass spectrometry, which correlated significantly (r = 0.88, P < 0.001), with intra-assay and interassay coefficients of variation of less than 10%.
Diagnoses
Serum vitamin D status was categorized based on 25(OH)D levels as being optimal (≥75 nmol/L), or having insufficiency (50–74.99 nmol/L), moderate deficiency (25–49.99 nmol/L), or severe deficiency (<25 nmol/L), as recommended by the Endocrine Society (18,19). For reference, other organizations may use other criteria, such as the Institute of Medicine, that recommend levels >50 nmol/L (20). Metabolic syndrome in adults was defined using the consensus statement from the International Diabetes Federation Task Force on Epidemiology and Prevention; the National Heart, Lung, and Blood Institute; the American Heart Association; the World Heart Federation; the International Atherosclerosis Society; and the International Association for the Study of Obesity (21). Specifically, it was identified in those having 3 or more of the following (1): blood pressure ≥130/85 mmHg or receiving antihypertensive medication (2); fasting glucose ≥5.6 mmol/L or receiving drug treatment for diabetes (3); triglycerides ≥1.7 mmol/L or receiving specific drug treatment (4); HDL cholesterol <1.03 mmol/L in men or <1.30 mmol/L in women or receiving specific drug treatment (5); or waist circumference >102 cm in men or >88 cm in women (21).
Follow-up
Follow-up procedures (median 7.7 years) have been described in detail elsewhere (15–17). Briefly, information on mortality was obtained from local registries. We used death certificates to classify the deceased into those who died of cardiovascular versus noncardiovascular causes. This classification was done independently by two experienced clinicians who were blinded to the study participants, except for information that was required to classify the cause of death. In the event of disagreement or uncertainty, a classification decision was made by one of the LURIC study principal investigators (W.M.).
Statistical analysis
Subjects were stratified according to widely used cutoffs for the definition of vitamin D status based on 25(OH)D levels (18). Baseline demographics of the subjects categorized by vitamin D status are described as percentages for categoric data and as, depending on their distribution, continuous data are presented as means ± SD or as geometric means with 95% CIs. Comparisons between groups were performed using the χ2 test for categoric data and ANOVA for continuous data. Kaplan-Meier curves, followed by the log-rank test, were used to evaluate differences in overall and cardiovascular mortality for the 25(OH)D groups. Hazard ratios (HR) with 95% CIs for the mortality categories were calculated using Cox proportional hazards regression models, which enabled adjustment for potential confounding parameters. In these analyses, model 1 describes the crude association; model 2 was adjusted for age, sex, smoking, alcohol and physical activity; and model 3 was further adjusted for BMI, waist circumference, diastolic blood pressure, type 2 diabetes, total cholesterol, cystatin C, C-reactive protein, the New York Heart Association (NYHA) functional classification, cardiovascular medication, and month of blood sampling (seasonality). HRs for 25(OH)D categories were calculated using the severe vitamin D deficiency group as the reference. Assumptions underlying the Cox proportional hazards regression model were evaluated by log minus log survival and partial (Schönfeld) residuals versus survival time plots, and were found valid. All statistical tests were two-sided, and statistical significance was defined as P < 0.05. All data were analyzed using SPSS 18.0 software (SPSS Inc, Chicago, IL).
RESULTS
The metabolic syndrome was identified in 1,811 subjects (54.6%) referred for coronary angiography, for whom serum levels of 25(OH)D were available in 1,801 (99.4%). Study participants had to demonstrate clinical stability, except for acute coronary syndrome.
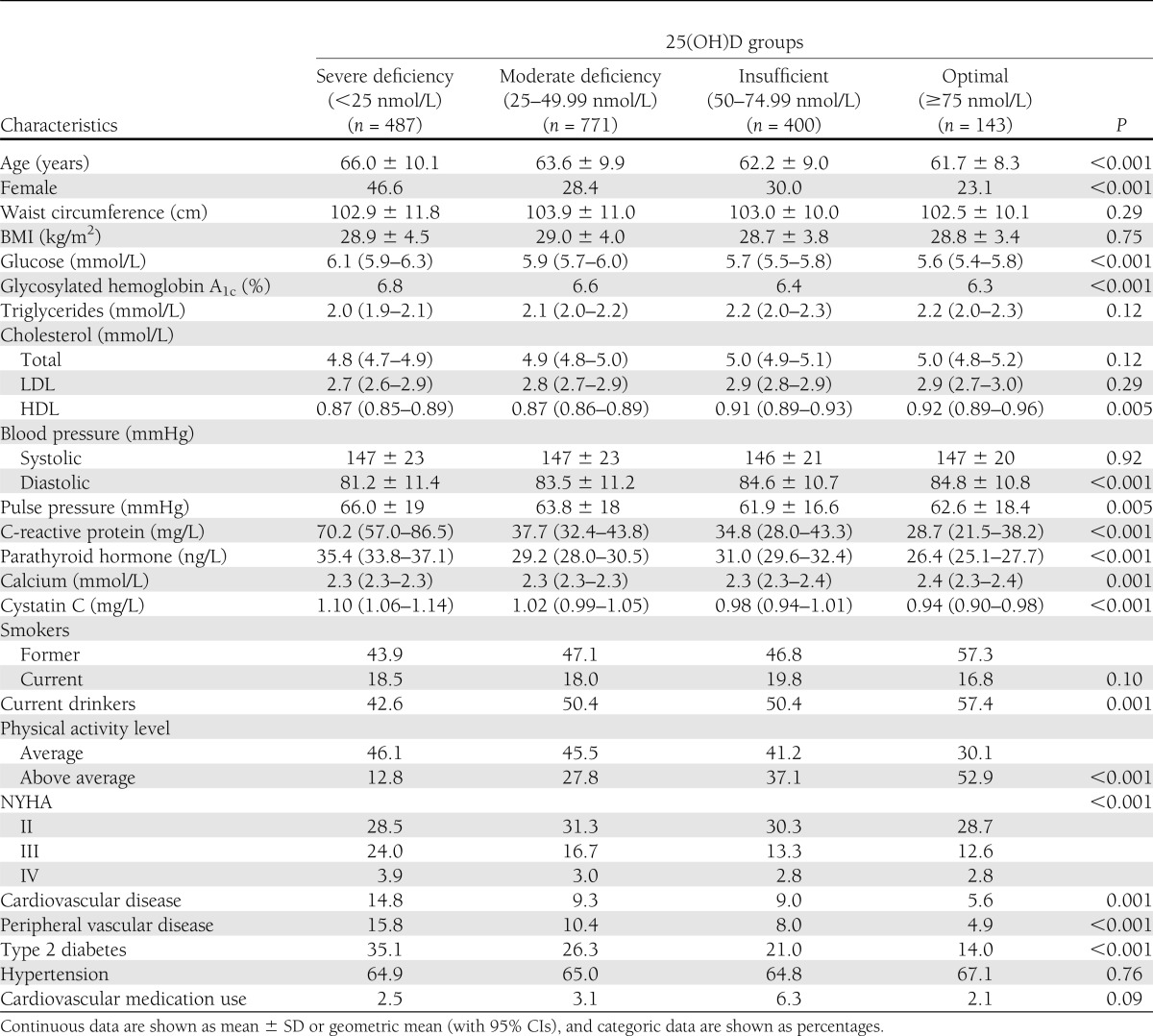
In the 1,801 subjects with the metabolic syndrome and complete 25(OH)D data, 92% had suboptimal (<75 nmol/L) 25(OH)D levels, with 27.0% having insufficiency (50–74.99 nmol/L), 42.8% moderate deficiency (25–49.99 nmol/L), and 22.2% severe deficiency (<25 nmol/L). As reported in Table 1, 25(OH)D levels had an inverse relationship with fasting glucose and the prevalence of diabetes, whereas HDL cholesterol levels increased with increasing 25(OH)D levels. Surprisingly, there was also a small positive association with diastolic blood pressure, but no association was observed with systolic blood pressure or prevalence of hypertension, although pulse pressure did decrease with increasing 25(OH)D levels. The decrease in the proportion of subjects at NYHA functional classes III and IV suggested lower rates of heart failure in those with increased 25(OH)D levels (Table 1). A number of lifestyle factors were positively associated with 25(OH)D levels, including alcohol consumption and physical activity (Table 1).
Table 1.
Baseline characteristics in those with the metabolic syndrome according to 25(OH)D concentrations
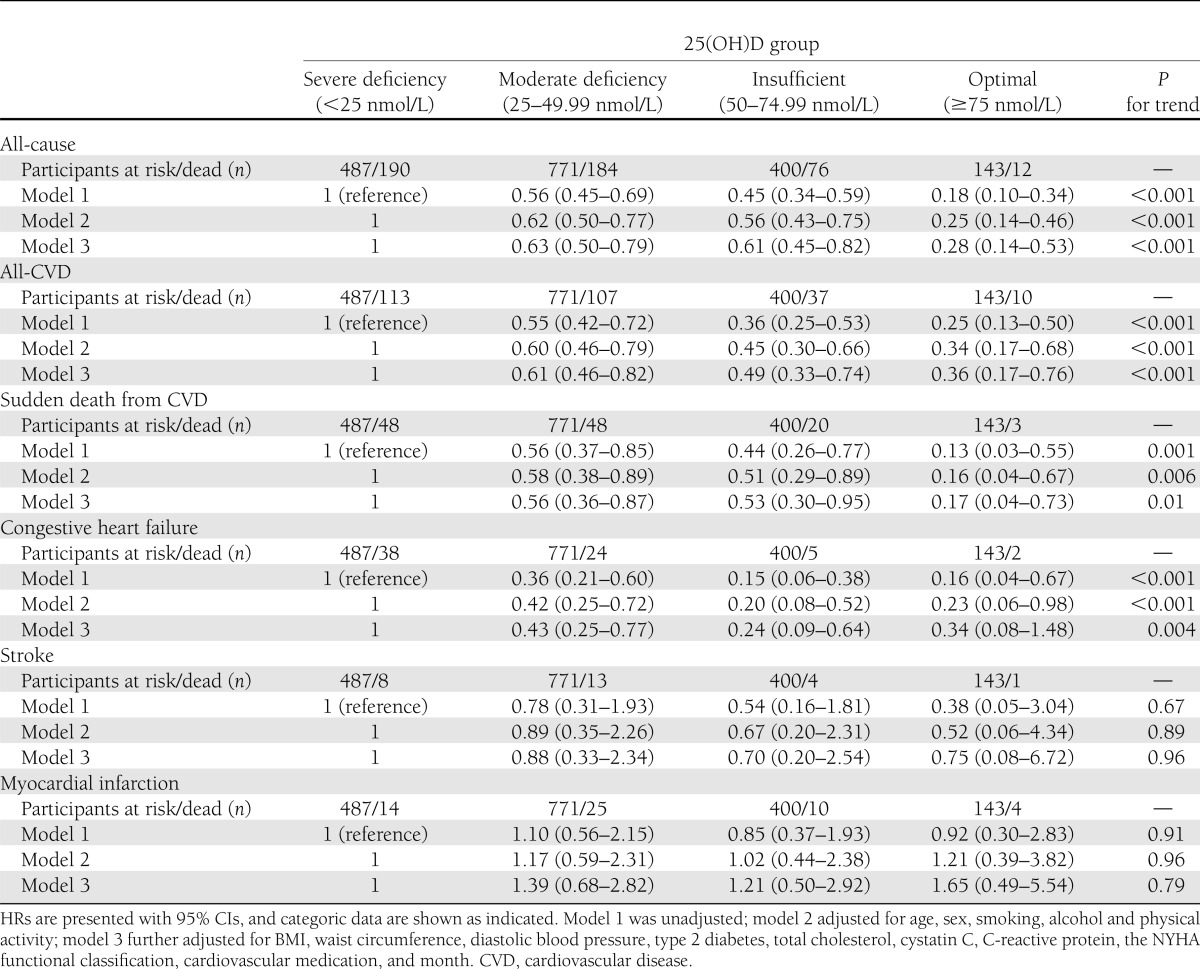
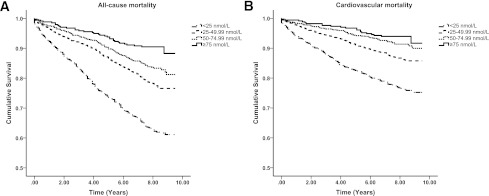
Across the 12,514 person-years of follow-up, 462 deaths were recorded, of which 267 (57.8%) were cardiovascular in origin (Table 2). Figure 1 presents the Kaplan-Meier plots for all-cause and cardiovascular mortality according to the four 25(OH)D groups. There was a clear dose-dependent reduction in all-cause mortality (P < 0.001 for trend; Table 2), showing a 75% reduction (HR 0.25 [95% CI 0.13–0.46]) in those with optimal 25(OH)D levels, relative to those with severe deficiency, after full adjustment. For cardiovascular disease, a comparable reduction in mortality was observed (P < 0.001 for trend; Table 2).
Table 2.
HRs for all-cause and cardiovascular mortality in those with the metabolic syndrome according to 25(OH)D concentrations
Figure 1.
Kaplan-Meier plots for all-cause (left) and cardiovascular mortality (right) according to 25(OH)D groups in those with the metabolic syndrome. Log-rank analysis indicated a significant difference between all 25(OH)D groups (P < 0.001).
Diabetes is associated with a strong reduction in 25(OH)D levels and is a major predictor of mortality. However, the removal of 965 subjects with type 2 diabetes from this analysis only modestly reduced the observed effect sizes. For example, after removal of those with type 2 diabetes, we found a 64% reduction for all-cause mortality in subjects with optimal 25(OH)D levels (HR 0.36 [95% CI 0.20–0.64), relative to those with severe deficiency (P < 0.001 for trend).
We also performed a sensitivity analysis to rule out that 25(OH)D is a marker of end-stage disease in this population by excluding subjects who died within 2 years of follow-up, leaving 1,561 subjects. The results remained essentially unaltered: comparing optimal versus severe deficiency yielded HRs of 0.16 (95% CI 0.06–0.39) for all-cause mortality and 0.24 (0.09–0.67) for cardiovascular disease–specific mortality. We further removed 268 subjects with a glomerular filtration rate of <60 mL/min/1.73 m2, which represents a loss of more than half the adult level of normal kidney function (22). Likewise, we found the current study results remained essentially unchanged (data not shown).
A total of 35 subjects (2.0%) reported regularly taking vitamin supplements, which usually contained B complex vitamins or vitamin D3. Because mean 25(OH)D levels were only modestly higher in users of vitamin D preparations (51.4 ± 31.2 nmol/L) compared with the remaining cohort (40.7 ± 24.7 nmol/L; P = 0.048), while 1,25-dihydroxyvitamin D levels, as well age and parathyroid hormone levels, did not differ significantly (data not shown), these subjects were included in the present analysis. Sensitivity analyses excluding those subjects did not affect the risk estimates (data not shown).
Despite a limited number of subjects available for analyses of cause-specific mortality, we observed a 85% reduced sudden death in those with optimal 25(OH)D levels (HR 0.15 [95% CI 0.04–0.63]) contrasted with severe deficiency (P = 0.004 for trend, Table 2). Mortality due to congestive heart failure similarly showed a significant trend (P = 0.001). Vitamin D status was not significantly associated with fatal myocardial infarction. Risk of fatal strokes was also not significantly related to 25(OH)D levels, but interpretation of this analysis is limited by the very low number of events (n = 25).
Because exposure to sunlight is a major potential confounder, although all analyses were adjusted for seasonality, we also performed a sensitivity analysis stratifying the study into those measured during the “summer” (May to October) and “winter” (November to April). The associations were similar to those from the overall analysis, with HRs in the “summer” of 0.62 (95% CI 0.45–0.87), 0.62 (0.41–0.93), and 0.32 (0.15–0.68) for moderate deficiency, insufficient, and optimal, respectively, compared with those with severe deficiency. Likewise, for the “winter” period, the HRs were 0.63 (0.46–0.87), 0.56 (0.34–0.91), and 0.17 (0.04–0.69), respectively. The observations also remained similar for all-cardiovascular disease mortality (data not shown).
CONCLUSIONS
To the best of our knowledge, this is the first study to show that optimal levels of 25(OH)D substantially reduce the risk of all-cause and cardiovascular mortality in subjects with the metabolic syndrome, a population well-known to exhibit excess mortality (1–4). We observed a 75% and 69% reduction in all-cause and cardiovascular disease mortality, respectively, in those with optimal levels compared with those with severe 25(OH)D deficiency. The analyses further showed that these associations were not driven by diabetes, a factor known to be associated with reduced 25(OH)D levels. The findings presented here strengthen a limited longitudinal literature describing the association between vitamin D and mortality (10–12,16) and extend our prior observations derived from the LURIC cohort (17).
Although answers regarding the causal role of vitamin D need to come from randomized controlled trials, it is promising to note that most criteria usually taken as support for causality were met: these include temporality, strength, consistency, biologic gradient, coherence, and plausibility. Indeed, numerous mechanisms have been identified by which vitamin D metabolites may affect metabolic homeostasis and atherogenesis. For example, vitamin D effects include regulation of glycemic homeostasis, as observed in the current study; whereby reduced vitamin D levels are associated with lower glucose levels and/or reduced insulin secretion and increased insulin resistance (18,23–26).
Not all studies have identified the association with vitamin D and insulin action, including in subjects with the metabolic syndrome (27). However, vitamin D can also influence the effects of glycemia independently of these mechanisms by blunting the effect of advanced glycation end products on endothelial cells (28), which may contribute to the increased arterial stiffness and endothelial dysfunction observed in individuals deficient in vitamin D (29). Vitamin D can also exert protective effects on the vessel walls by inhibition of macrophage to foam cell formation (30) and via its anti-inflammatory effects (31,32). The latter is likewise consistent with the current data, which showed an inverse association of 25(OH)D levels with C-reactive protein.
Vitamin D suppresses atherosclerotic disease and attenuates thrombogenesis (32,33). In the current study, however, we did not find an association with acute myocardial infarction or stroke; the latter might have resulted from the limited number of events. Vitamin D sufficiency has been associated with an downregulation of the renin-angiotensin-aldosterone system, thus potentially attenuating the development of hypertension (18), although only pulse pressure was inversely associated with 25(OH)D levels in the current study. The action of vitamin D on the renin-angiotensin-aldosterone system (18), along with its suppression of cardiac hypertrophy (34) and hypercontractility (35,36), likely contributes to the reduction in sudden cardiac death and heart failure mortality.
Surprisingly, 25(OH)D levels were not associated with the measures of adiposity, namely waist circumference and BMI (37). However, this may be an artifact of selecting those with the metabolic syndrome, for which central adiposity is a component and who are thus overall generally more obese. Likewise, in these more ill subjects (e.g., those with heart failure), issues of wasting may also potentially be present that interfere with the association. In the overall population of 3,316 subjects, when including those with and without the metabolic syndrome, we did observe a significant inverse association between BMI and 25(OH)D levels (16).
A baseline positive association was observed between alcohol intake with 25(OH)D levels. Short-term studies have suggested that alcohol intake does not affect vitamin D metabolism (38); however, consumption at moderate levels might have assisted in the reduction in cardiovascular disease mortality (39). Overall alcohol consumption per se is usually associated with an overall excess of deaths (39), so is unlikely to be a significant determinant of the apparent protective effect on mortality associated with the 25(OH)D levels, particularly given that we adjusted for alcohol consumption in the analyses.
Several limitations of our study should be noted. We assessed 25(OH)D only at one time point, and this may not reflect long-term vitamin D status. However, despite significant seasonal variation, 25(OH)D levels are reported to track over time in a comparable manner similar to other established risk factors such as blood pressure and lipids (40). A single measure also does not consider the significant seasonal variations in 25(OH)D levels. However, we adjusted the analyses for seasonality by incorporating the timing of the sampling, which should partly address this issue. We also performed sensitivity analyses stratified by season, for which the results consistently showed a clear protective effect for all-cause and all-cardiovascular mortality.
Significantly, momentary 25(OH)D was found to be a powerful long-term predictor: the Kaplan-Meier plots showed a consistent linear relationship with mortality, continuing for more than 8 years after a single assessment of 25(OH)D. Inclusion of only Caucasian subjects limits the generalizability of the study but does help to minimize potential confounding resulting from the use of an ethnically diverse population; for example, skin pigmentation and cultural aspects, such as style of dress, could differentially influence the magnitude of photosynthesis at different times of the year (8).
Likewise, because these subjects with the metabolic syndrome had undergone elective angiography due to cardiovascular symptoms or events, they are thus not representative of the general population with the metabolic syndrome and are likely to display an elevated mortality risk. Although, we performed multivariable adjustments, we cannot rule out that unmeasured or unknown confounders could have influenced our results.
A possible alternative explanation of our results is that those with the highest risk for mortality had limited sunlight exposure due to existing health-related issues, thereby confounding the association between increased risk and reduced vitamin D levels. Related, those with greater physical activity levels would likely be healthier, and if the activities were performed outdoors, would also have increased ultraviolet B exposure. This would in turn affect 25(OH)D levels. Moreover, increased physical activity may also be associated with other beneficial lifestyle practices that reduce morbidity and mortality. We addressed these potential confounding issues in several ways: we adjusted our Cox regression analyses for parameters that are related to outdoor activities and sun light exposure, including NYHA functional class, physical activity, cardiovascular medication, renal function, and month of blood sampling. These adjustments had only modest effects. Similarly, sensitivity analyses that excluded those who died within the first 2 years of follow-up only minimally attenuated the observed effects. Considering the magnitude of the observed benefit of higher 25(OH)D levels and the very modest effect-size attenuation seen after comprehensive statistical and sample adjustments, it seems fair to conclude that residual confounding is a unlikely hypothesis.
In summary, 25(OH)D levels were dose-dependently associated with a robust reduction in all-cause and cardiovascular mortality in subjects with the metabolic syndrome. We hope these findings will spur interventional randomized, controlled trials to confirm the effects of vitamin D on mortality and, if positive, help establish recommendations for supplementation in these subjects.
Acknowledgments
LURIC has received funding through the 6th Framework Program (integrated project Bloodomics, grant LSHM-CT-2004-503485) and the 7th Framework Program (integrated project Atheroremo, Grant Agreement number 201668) of the European Union. B.ó.H. is funded by a Biotechnology and Biological Sciences Research Council studentship grant to G.N.T. and J.A.B.
Sponsors had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
G.N.T., B.ó.H., J.A.B., S.P., and A.L. analyzed and interpreted data and participated in drafting and critical revision of the manuscript. J.E.F., M.E.K., and T.B.G. participated in drafting and critical revision of the manuscript. B.O.B. and W.M. participated in drafting and critical revision of the manuscript, contributed to the conception, design, and acquisition of data, and analyzed and interpreted data. B.O.B. and W.M. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the LURIC study team either temporarily or permanently involved in subject recruitment and sample and data handling; the laboratory staff at the Ludwigshafen General Hospital and the Universities of Freiburg, Ulm, and Graz; and the German registration offices and local public health departments for their assistance.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Thomas GN, Phillips AC, Carroll D, Gale CR, Batty GD. The metabolic syndrome adds utility to the prediction of mortality over its components: The Vietnam Experience Study. Atherosclerosis 2010;210:256–261 [DOI] [PubMed] [Google Scholar]
- 2.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, San Antonio Heart Study National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation 2004;110:1251–1257 [DOI] [PubMed] [Google Scholar]
- 3.Thomas GN, Schooling CM, McGhee SM, et al. Hong Kong Cardiovascular Risk Factor Prevalence Study Steering Committee Metabolic syndrome increases all-cause and vascular mortality: the Hong Kong Cardiovascular Risk Factor Study. Clin Endocrinol (Oxf) 2007;66:666–671 [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005;28:1769–1778 [DOI] [PubMed] [Google Scholar]
- 5.Thomas GN, Jiang CQ, Taheri S, et al. A systematic review of lifestyle modification and glucose intolerance in the prevention of type 2 diabetes. Curr Diabetes Rev 2010;6:378–387 [DOI] [PubMed] [Google Scholar]
- 6.Mehta A. Management of cardiovascular risk associated with insulin resistance, diabetes, and the metabolic syndrome. Postgrad Med 2010;122:61–70 [DOI] [PubMed] [Google Scholar]
- 7.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med 2009;169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol 2007;103:620–625 [DOI] [PubMed] [Google Scholar]
- 9.Kim MK, Il Kang M, Won Oh K, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 2010;73:330–338 [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc 2009;57:1595–1603 [DOI] [PubMed] [Google Scholar]
- 12.Parker J, Hashmi O, Dutton D, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas 2010;65:225–236 [DOI] [PubMed] [Google Scholar]
- 13.Pilz S, Dobnig H, Nijpels G, et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–672 [DOI] [PubMed] [Google Scholar]
- 14.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkelmann BR, März W, Boehm BO, et al. LURIC Study Group (LUdwigshafen RIsk and Cardiovascular Health) Rationale and design of the LURIC study—a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2001;2(Suppl. 1):S1–S73 [DOI] [PubMed] [Google Scholar]
- 16.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 17.Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008;93:3927–3935 [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Clinical Practice Guideline. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 20.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti KGMM, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 23.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–825 [DOI] [PubMed] [Google Scholar]
- 24.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 1986;29:142–145 [DOI] [PubMed] [Google Scholar]
- 25.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract 1995;27:181–188 [DOI] [PubMed] [Google Scholar]
- 26.Riachy R, Vandewalle B, Moerman E, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis 2006;11:151–159 [DOI] [PubMed] [Google Scholar]
- 27.Gulseth HL, Gjelstad IM, Tierney AC, et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care 2010;33:923–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talmor Y, Golan E, Benchetrit S, et al. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am J Physiol Renal Physiol 2008;294:F1059–F1064 [DOI] [PubMed] [Google Scholar]
- 29.Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 2010;58:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebsamen MC, Sun J, Norman AW, Liao JK. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res 2002;91:17–24 [DOI] [PubMed] [Google Scholar]
- 31.Liu LC, Voors AA, van Veldhuisen DJ, et al. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail 2011;13:619–625 [DOI] [PubMed] [Google Scholar]
- 32.Andress DL. Nonclassical aspects of differential vitamin D receptor activation: implications for survival in patients with chronic kidney disease. Drugs 2007;67:1999–2012 [DOI] [PubMed] [Google Scholar]
- 33.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 2006;29:722–724 [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996;97:1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006;41:350–359 [DOI] [PubMed] [Google Scholar]
- 36.Pilz S, Tomaschitz A, Drechsler C, Dekker JM, März W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res 2010;54:1103–1113 [DOI] [PubMed] [Google Scholar]
- 37.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laitinen K, Lamberg-Allardt C, Tunninen R, Karonen SL, Ylikahri R, Välimäki M. Effects of 3 weeks’ moderate alcohol intake on bone and mineral metabolism in normal men. Bone Miner 1991;13:139–151 [DOI] [PubMed] [Google Scholar]
- 39.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 2010;171:903–908 [DOI] [PubMed] [Google Scholar]