Abstract
OBJECTIVE
Glycemic control in type 2 diabetes generally worsens over time, requiring intensification of therapy. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial provided the opportunity to observe glycemic control in a real-world setting. We assessed the adequacy of metformin, sulfonylureas, and insulin to maintain glycemic control and their effects on weight.
RESEARCH DESIGN AND METHODS
Diabetes control was measured at baseline and yearly for a median of 5 years in the 4,900 patients from the nonintervention arm of this study allocated to placebo.
RESULTS
Median HbA1c was 6.9% at baseline and increased by an average of 0.22% over 5 years (P < 0.001). Median weight was 86.3 kg at baseline and decreased by 0.4 kg over 5 years (P = 0.002). Baseline therapy was lifestyle measures only in 27%, oral agents without insulin in 59%, and insulin in 14% (7% also taking oral agents). Over 5 years, insulin use increased to 32% (21% also taking oral agents). Use of oral agents remained similar at 56%. Only 2% of patients at baseline and 4% after 5 years were taking oral agents other than metformin or sulfonylureas. Initiation of insulin therapy in 855 patients produced a sustained reduction of HbA1c from a median of 8.2 to 7.7%, with a weight gain of 4.6 kg over 5 years.
CONCLUSIONS
With intensification of traditional therapies, glycemic control deteriorated very little over 5 years in a large cohort of type 2 diabetes. However, the requirement for insulin therapy doubled, at the expense of significant weight gain and risk of hypoglycemia.
When type 2 diabetes develops, pharmacologic intervention is usually required. Progression means that this therapeutic intervention needs to be adjusted over time (1). Insulin resistance and β-cell dysfunction, the metabolic defects that characterize type 2 diabetes, occur early in its pathogenesis (2) and lead to increased hepatic glucose production and impairment of insulin-mediated glucose disposal. A continuing decline in β-cell function underlies the worsening hyperglycemia that generally occurs in type 2 diabetes (3,4), inducing the need for progressive intensification of hypoglycemic therapy (5,6). Conventional management often includes multiple oral hypoglycemic agents, particularly metformin and sulfonylureas, and then insulin, usually only when the oral treatments are no longer effective, because insulin therapy can lead to hypoglycemia and weight gain (7).
In the UK Prospective Diabetes Study (UKPDS), glycemic control progressively deteriorated over time, despite intensive therapy including insulin (8). This outcome is supported by findings from the U.S. National Health and Nutrition Examination Survey, which showed in clinical practice from 1988 to 2000 that combined therapy with oral hypoglycemic agents and insulin increased in adults with type 2 diabetes but failed to control glycemia adequately (5).
The main focus of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial was whether fenofibrate could reduce cardiovascular disease risk in type 2 diabetes (9). There was no protocol for management of glycemic control, which was managed by the patients’ usual doctors. The study therefore incidentally allowed us to assess the outcomes of real-world diabetes care, mainly in primary care, from 63 different areas in Australia, New Zealand, and Finland. The aim of the current analysis was to track the history of type 2 diabetes and to investigate the progression of type 2 diabetes and consequent changes in treatment. Because few patients were treated with agents for glycemic control other than metformin, sulfonylureas, and insulin, this study addresses the question of the adequacy of standard, established therapy to maintain satisfactory glycemic control. The effects of intensified therapy on weight gain were also analyzed.
RESEARCH DESIGN AND METHODS
Subjects
The FIELD study was a randomized, double-blind, placebo-controlled trial of fenofibrate therapy (9). We recruited patients aged 50–75 years with type 2 diabetes according to World Health Organization criteria from 63 centers in Australia, New Zealand, and Finland between February 1998 and November 2000. Of 13,900 patients screened, 9,795 were randomized to comicronized fenofibrate (200 mg daily) or placebo. Reasons for exclusion during the run-in period before randomization are shown in Supplementary Fig. 1.
Participants were followed up every 4 months in the first year and then every 6 months for an average of 5 years for the development of cardiovascular events until the study closed in 2005. All had an initial plasma total cholesterol concentration of 3.0–6.5 mmol/L, plus total cholesterol-to-HDL cholesterol ratio of 4.0 or higher, or plasma triglyceride of 1.0–5.0 mmol/L, and did not require lipid-modifying therapy. The study excluded those with renal impairment (plasma creatinine >130 μmol/L), chronic liver disease, or symptomatic gallbladder disease, or those who had a cardiovascular event within the 3 months before recruitment.
Many participants were recruited from community-based sources through national diabetes registers, but hospital-treated patients were also recruited. There was no requirement for a prior hospital admission related to diabetes. Diabetes care remained in the hands of patients’ usual doctors, mostly in the primary care setting, rather than the doctors involved in the trial. There was no protocol for diabetes management. The 4,900 patients in the nonintervention arm allocated to placebo were the subjects of this study to obviate any potential confounding effect of fenofibrate therapy on glycemia.
All patients gave written informed consent to participate in the FIELD study, which was undertaken in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by local and national ethics committees.
Protocol
Body weight and blood pressure were measured at each visit, and concomitant medication was recorded. Plasma glucose and HbA1c were measured at baseline on two occasions, then annually, and at study end. Insulin levels were measured at baseline, 2 years, 5 years, and at study end. All adverse events, including cardiovascular and microvascular complications as well as major hypoglycemic episodes, were recorded at each visit.
Statistical analyses
Normally distributed variables are displayed as means and standard deviations, and non-normally distributed variables as medians and interquartile ranges. Where appropriate, we used χ2 tests to compare categoric variables, one-way ANOVA or t tests (paired or unpaired as appropriate) for normally distributed continuous variables, and Kruskal–Wallis or Wilcoxon rank-sum tests for nonnormally distributed continuous variables. Analyses were not adjusted for multiple comparisons. Although the overall type I error might be inflated and appropriate caution should be taken in interpreting the results, testing a global null hypothesis was not the primary interest in this analysis (10). The averages of the measurements at visit 1 (16 weeks before randomization) and visit 3 (6 weeks before randomization) were used as baseline values.
A measure of insulin sensitivity (homeostasis model assessment 2 [HOMA2]-%S) was calculated according the HOMA Calculator (v2.2.2, 12 December 2007; www.dtu.ox.ac.uk) on the basis of the updated HOMA2 model (11). Multivariate logistic regression was performed separately for the groups who were on lifestyle measures only or oral therapy to assess the independent effect of HbA1c, BMI, and HOMA-S on the probability of progression by the end of the study.
Analyses were repeated separately by country and tertiles of clinic recruitment to assess the robustness of the conclusions across providers. All analyses used SAS 9.1 software (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics
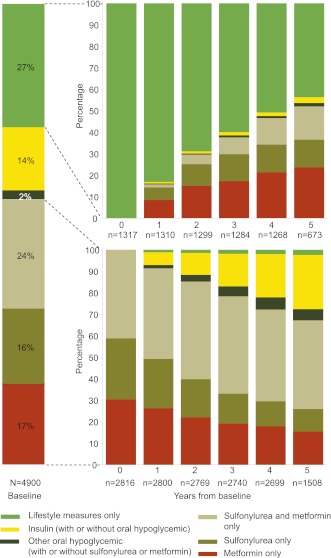
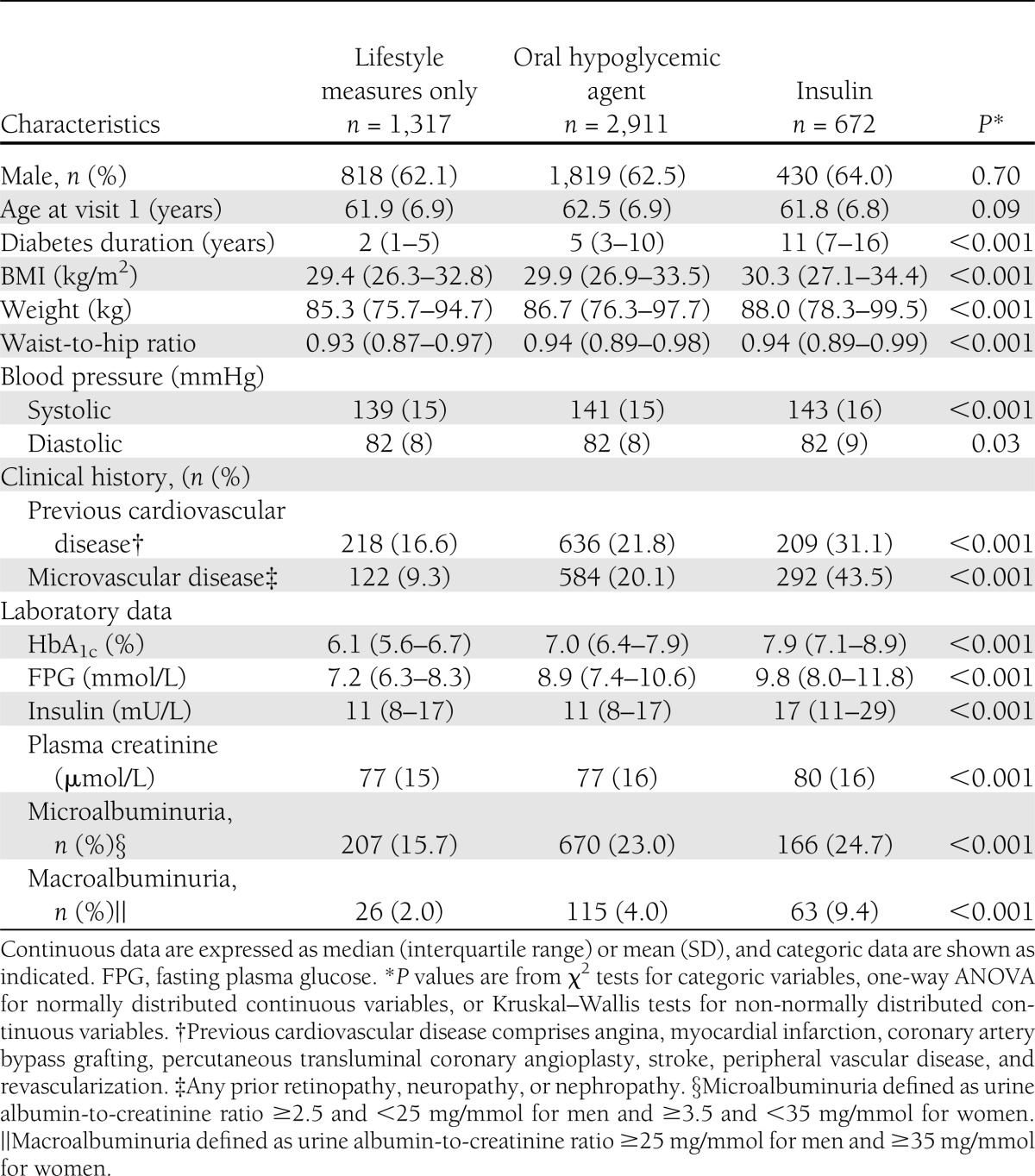
At entry into the trial, most patients were using lifestyle measures only (27%) or metformin and sulfonylureas separately or combined (59%) to treat their diabetes (Fig. 1). Metformin and sulfonylureas in combination were more often used than each of these therapies alone. Only 14% were using insulin, alone (6.2%) or combined with oral hypoglycemic agents (7.5%). Baseline HbA1c, fasting glucose, body weight, and BMI were highest in patients receiving insulin treatment (Table 1).
Figure 1.
Proportions of patients in the FIELD study receiving different treatments for diabetes at baseline (left column), over time for patients on lifestyle measures only at baseline (upper right columns), and for patients on metformin or a sulfonylurea or both at baseline (lower right columns). Other oral hypoglycemic agents included acarbose, guar gum, pioglitazone, and rosiglitazone.
Table 1.
Baseline characteristics of placebo patients (n = 4,900) in the FIELD study by diabetes treatment
Those taking insulin had the highest prevalence of previous cardiovascular disease and microvascular disease, consistent with the longer duration and relative severity of their diabetes, even though they were no older than those treated with lifestyle measures or oral hypoglycemic agents. This difference is exemplified by their higher prevalence of hypertension and use of cardiovascular medications—aspirin, angiotensin-converting enzyme inhibitors, β-blockers, calcium-channel antagonists, nitrates, and diuretics.
Body weight, HbA1c, and glucose over time
Median HbA1c was 6.9% (5–95%; range, 5.3–9.6%) at baseline and showed a slight but steady rise over the study, with an increase of 0.22% (P < 0.001) at 5 years—an average increase of 0.045% per year. Median fasting glucose was 8.5 mmol/L at baseline (5–95%; range, 5.6–13.7 mmol/L) and declined by an average of 0.46 mmol/L (P < 0.001) over 5 years. Median body weight was 86.3 kg and decreased by an average of 0.4 kg over 5 years (P = 0.002).
Medication use over time
Over 5 years, the proportion of patients using lifestyle measures alone had decreased from 27 to 13%, rates of use of oral hypoglycemic treatment (without insulin) remained similar at 56%, and insulin treatment increased to 31% (21% combined with oral hypoglycemic medication).
Of the patients who entered the study on lifestyle measures alone, only 44% remained on lifestyle measures alone after 5 years, 54% were receiving oral hypoglycemic medication without insulin, and 3% were on insulin therapy (Fig. 1). For those who were on oral hypoglycemic medication without insulin at baseline, 25% progressed to insulin use within 5 years, but 92% remained on at least one oral hypoglycemic medication (20% in combination with insulin; Fig. 1). For those patients on insulin at baseline, 97% were still on insulin treatment after 5 years.
At study entry, 118 patients (2.4%) were taking an oral hypoglycemic medication other than metformin or sulfonylurea (mainly acarbose or guar gum), and none was taking a thiazolidinedione. Of 375 patients who commenced thiazolidinedione therapy (pioglitazone or rosiglitazone) during the trial, 212 (4.3%) remained on this form of therapy at study close, 196 in combination with metformin or sulfonylureas, or both, and 97 patients (2.0%) were taking other oral drugs at study end (mostly acarbose and guar gum).
Baseline predictors of treatment progression over time
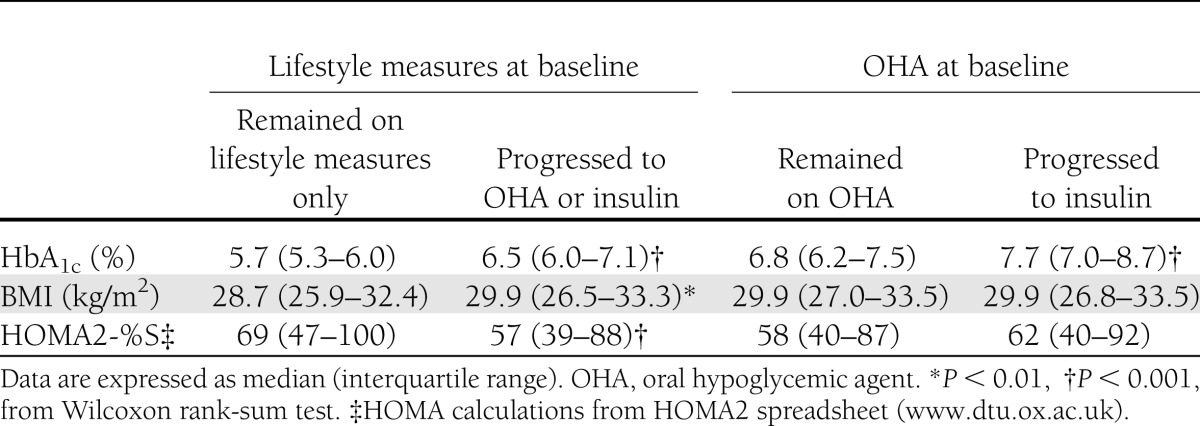
As expected, starting oral hypoglycemic medication or insulin in those on lifestyle measures alone and starting insulin therapy in those on oral hypoglycemic medication were associated with higher HbA1c at baseline (Table 2). The group that stayed on lifestyle measures alone had lower BMI and better insulin sensitivity than those who progressed to more intensive therapy. In multivariate models, however, only baseline HbA1c and HOMA-S remained independently significant predictors of treatment progression (both P < 0.05). Baseline BMI did not predict treatment progression after adjustment for these variables in patients on lifestyle measures alone or in those on oral hypoglycemic medications at baseline.
Table 2.
Treatment progression in patients on lifestyle measures only or oral hypoglycemic agents at baseline related to baseline HbA1c, BMI, and HOMA2-%S (10)
Threshold for changes in hypoglycemic therapy
The median threshold of HbA1c for starting any first oral hypoglycemic medication was 7.1%, and for a subsequent oral agent, 7.3%. Insulin therapy was initiated at higher levels of HbA1c, at a median of 8.2%.
Glycemic control and body weight after change in therapy
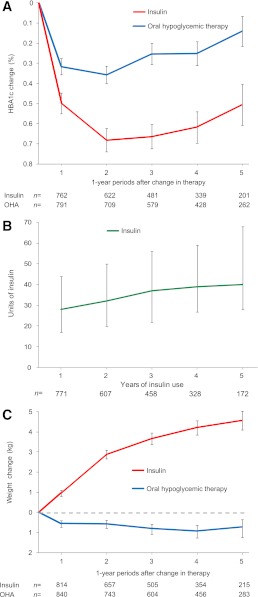
Median HbA1c dropped from 7.1% before to 6.8% a year after initiation of oral hypoglycemic therapy (mean difference, –0.32%; P < 0.001) and was still lower after 5 years (mean difference, –0.14%; P = 0.06; Fig. 2A). Median HbA1c dropped from 8.2% before to 7.5% a year after initiation of insulin therapy (mean difference, –0.5%; P < 0.001), and remained stable over 5 years (mean difference, –0.51%; P < 0.001; Fig. 2A). Maintaining this HbA1c level was achieved by a gradual increase of the median insulin dose from 28 units in the first year of therapy to 40 units after 5 years (Fig. 2B). The median weight before insulin therapy was 86 kg, which had increased by a mean of 4.6 kg at 5 years (P < 0.001; Fig. 2C). Mean weight was slightly lower after the change to oral hypoglycemic therapy, decreasing 0.7 kg to 5 years (P = 0.04; Fig. 2C).
Figure 2.
A: Mean (with SE) changes in HbA1c after commencement of an oral hypoglycemic agent (OHA) or insulin. B: Median (interquartile range) insulin dose (units) over years of insulin usage in patients not on insulin at baseline. C: Mean (with SE) changes in body weight after commencement of an OHA or insulin.
Weight gain after thiazolidinedione therapy was similar to that with insulin therapy. The average weight gain after 2 years of either treatment was 2.9 kg. Reductions in HbA1c due to insulin or thiazolidinedione therapy were also similar. The average reduction in HbA1c after 2 years was 0.7% for those on insulin and 0.6% for those on thiazolidinediones.
As expected, hypoglycemia, here defined as an episode that required presentation to the hospital, was more frequent in patients on insulin. There were no episodes in patients on lifestyle measures only, 12 in patients on oral hypoglycemic agents without insulin (1 per 1,232 patient-years of treatment), and 34 in patients on insulin (1 per 168 patient-years of treatment).
Effects of country and clinic
Insulin usage at baseline varied significantly across the three countries (Australia, 8%; New Zealand, 19%; Finland, 29%; P < 0.001). However, patient characteristics had similar patterns across categories of diabetes treatment and progression in all three countries and across tertiles of clinic recruitment numbers (<130, 130–189, 190+ patients).
CONCLUSIONS
Through intensification of therapy, predominantly with metformin, sulfonylureas, and insulin, the HbA1c increase over 5 years in this large cohort of 4,900 patients with type 2 diabetes was limited to 0.22%, less than would have been predicted from other studies (5,8). At the time of the FIELD study, HbA1c below 7.0% was the accepted level for glycemic control (12), and good practice toward this goal is reflected by the median HbA1c of 7.1% at which oral hypoglycemic therapy was commenced. Oral hypoglycemic therapy was not associated with weight gain, and the cohort overall lost weight during the study.
The median HbA1c for initiation of insulin therapy was 8.2%. There are many barriers to the initiation of insulin, including the well-recognized associated weight gain, which averaged 4.6 kg over 5 years, similar to the reported 4.0-kg gain in the UKPDS in those using older, long-acting insulin (8).
The pattern of diabetes therapy reported here aligns with the recent consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes on the medical management of hyperglycemia in type 2 diabetes (13). Few patients took oral hypoglycemic agents other than metformin or sulfonylureas. The data reflect the effectiveness of usual diabetes care in this large patient cohort distributed across 63 districts of Australia, New Zealand, and Finland. These countries have comparable health care systems: there is universal health insurance and they are primary-care led, with family medicine practitioners as the first point of contact. However, patients were recruited mainly through voluntary national diabetes registers and hospital-based diabetes clinics and volunteered to participate in a long-term study, possibly a more motivated patient group. Also, participation in any clinical trial may in itself lead to a greater emphasis on diabetes management by both patient and doctor. Nevertheless, the HbA1c thresholds at which oral therapy and insulin were introduced and their subsequent effectiveness were similar to those in an Australian community-based longitudinal observation study (14), and the participants are representative of patient cohorts that take part in studies of new diabetes therapies.
During the past 10 years, the UKPDS has been the most widely quoted example of a study showing that type 2 diabetes progresses despite intensive treatment with standard therapies (6). Even in the UKPDS intensive-treatment arm, HbA1c rose progressively by about 0.75% over 5 years (8), and this deterioration is seen to show a need for newer treatments for type 2 diabetes. Progression of type 2 diabetes was also evidenced by a more recent study of three forms of oral hypoglycemic therapy (15), and the difficulty of combating it has been reflected in textbook statements such as, “No cohort of patients of substantial size has ever been reported in which an average HbA1c level less than 7% has been achieved over a time frame that exceeds more than a few months” (16).
There were some differences in diabetes treatment between the UKPDS and the nonintervention arm of the FIELD study. The UKPDS used older sulfonylureas, which have a greater propensity to lead to hypoglycemia. Not many patients were on combination therapy because a separate arm of the study used metformin in overweight patients. In addition, very long-acting beef insulin was generally used, and self-monitoring of blood glucose was not available for a significant part of the study. In the nonintervention arm of the FIELD study, combination therapy with metformin and sulfonylureas was widely used, and oral agents were usually continued with insulin.
Several more recent studies have also demonstrated maintenance of glycemic control over 3 to 5 years (17–19). In two of these, there was widespread background use of thiazolidinediones (17–19), which have also been associated with problematic weight gain, overt heart failure, and osteoporosis (20–22). Hypoglycemia was also a significant clinical problem in these trials. Therefore, insulin-sparing agents that achieve glycemic control without weight gain or hypoglycemia represent ideal therapy for type 2 diabetes. Incretin-based therapies offer this prospect (23,24), and the current outcome studies will be important to determine their effectiveness in diabetes care.
In conclusion, standard pharmacologic therapy for type 2 diabetes with metformin, sulfonylureas, and insulin in the setting of nationally coordinated, primary health care–led health systems, can achieve glycemic control consistent in most patients with current guidelines. Because this goal was achieved at the expense of significant weight gain and hypoglycemia, newer insulin-sparing treatments for type 2 diabetes might offer important advantages for maintenance of long-term glycemic control.
Acknowledgments
The FIELD study was supported by grants from Laboratoires Fournier SA, Dijon, France (subsequently part of Solvay Pharmaceuticals, which is now part of Abbott Laboratories) and the NHMRC of Australia, and was coordinated independently by the NHMRC Clinical Trials Centre, University of Sydney, Sydney, New South Wales, Australia. The sponsors of the study had no role in data collection or data analysis. This secondary study did not have specific funding. No other potential conflicts of interest relevant to this article were reported.
J.D.B. designed the study, wrote the manuscript, and interpreted the results. P.L.D., T.M.E.D., M.-R.T., Y.A.K., and R.S. revised the draft and interpreted the results. C.P. and M.V. analyzed the data, interpreted the results, and revised the manuscript. A.C.K. wrote the manuscript and interpreted the results. All authors approved the manuscript for submission. J.D.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008, and at the 45th European Association for the Study of Diabetes Meeting, Vienna, Austria, 29 September–2 October 2009.
The authors would like to thank Rhana Pike, from the NHMRC Clinical Trials Center, for editing the article.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1307/-/DC1.
References
- 1.Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Prim Care 1999;26:771–789 [DOI] [PubMed] [Google Scholar]
- 2.Eriksson J, Franssila-Kallunki A, Ekstrand A, et al. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med 1989;321:337–343 [DOI] [PubMed] [Google Scholar]
- 3.U.K. Prospective Diabetes Study Group U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 4.Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med 1998;15:290–296 [DOI] [PubMed] [Google Scholar]
- 5.Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004;27:17–20 [DOI] [PubMed] [Google Scholar]
- 6.Turner RC, Cull CA, Frighi V, Holman RR, UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Thorne KI, Farmer AJ, et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 9.Keech A, Simes RJ, Barter P, et al. FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849–1861 [DOI] [PubMed] [Google Scholar]
- 10.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365:1591–1595 [DOI] [PubMed] [Google Scholar]
- 11.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Supplement 1. American Diabetes Association: clinical practice recommendations 2000. Diabetes Care 2000;23Suppl. 1:S1–S116 [PubMed] [Google Scholar]
- 13.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis TME, Davis Cyllene Uwa Edu Au WA, Bruce DG. Glycaemic levels triggering intensification of therapy in type 2 diabetes in the community: the Fremantle Diabetes Study. Med J Aust 2006;184:325–328 [DOI] [PubMed] [Google Scholar]
- 15.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 16.Buse JB. Management of type 2 diabetes mellitus. In Endocrinology 5th ed. Vol 3. DeGroot LJ, Jameson JL, Eds. Philadelphia, Saunders, 2005, p. 1231–1248 [Google Scholar]
- 17.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 20.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129–1136 [DOI] [PubMed] [Google Scholar]
- 21.Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 22.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 2009;180:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011;124(Suppl.):S3–S18 [DOI] [PubMed] [Google Scholar]
- 24.Cernea S, Raz I. Therapy in the early stage: incretins. Diabetes Care 2011;34(Suppl. 2):S264–S271 [DOI] [PMC free article] [PubMed] [Google Scholar]