Abstract
OBJECTIVE
Insulin resistance, as measured by surrogate markers, is associated with lower response to hepatitis C virus (HCV) therapy and may improve with HCV eradication. We prospectively evaluated the impact of directly measured insulin resistance and abnormal glucose metabolism on achieving sustained virologic response (SVR) with HCV therapy and assessed whether SVR results in improved insulin sensitivity and fasting glucose.
RESEARCH DESIGN AND METHODS
A total of 50 noncirrhotic, nondiabetic, HCV-infected patients (27 untreated, 23 treated with pegylated interferon/ribavirin, nonrandomized) underwent clinical and histologic evaluation and 75-g oral glucose tolerance test. Insulin sensitivity was assessed directly with insulin suppression test by measuring steady-state plasma glucose (SSPG) concentration during a 240-min infusion of octreotide, glucose, and insulin. Of the subjects, 43 had at least one follow-up evaluation.
RESULTS
Patient characteristics were median age 48, 57% male, and 52% white. SVR was achieved in 61% (14 of 23) of treated subjects. SVR was independently associated with HCV genotypes 2 and 3 (odds ratio 8.8 [95% CI 1.2–61.7]) but was not strongly associated with insulin sensitivity. When controlling for elapsed time between measurements, being on interferon, and BMI, SSPG decreased by 36 mg/dL (−88 to 16) in those with SVR and decreased by 28 mg/dL (−93 to 38) in those without SVR, compared with the untreated group. BMI (coefficient 9.1 per 5 units; 95% CI 5.3–12.9) and interferon use (coefficient 56; 95% CI 6.8–105) were associated with SSPG.
CONCLUSIONS
Insulin resistance does not appear to be strongly associated with SVR. HCV therapy may improve insulin resistance regardless of virologic response; however, BMI and interferon use were clearly associated with insulin resistance.
Individuals infected with chronic hepatitis C virus (HCV) have two- to threefold greater odds of having diabetes than the general population (1,2). HCV infection is an independent risk factor for incident diabetes, particularly in individuals who have other risk factors for the development of diabetes (3). It is thought that the mechanism by which HCV is associated with diabetes is through induction or accentuation of insulin resistance (IR) (4).
Although a definite cause-and-effect relationship between HCV and diabetes has not been established, the successful eradication of HCV may result in an improvement of IR, particularly in individuals who achieve sustained virologic response (SVR) (5,6). However, the impact of viral eradication on downstream consequences of IR, such as impairment of glucose metabolism, has not been clearly established. Although SVR has been associated with reduced rates of new-onset abnormalities in glucose metabolism with short-term follow-up (7), this has not been observed with longer follow-up (8).
IR and diabetes have consequences related to HCV-related liver disease and the response to HCV therapy. Specifically, IR and diabetes are associated with more advanced hepatic fibrosis in HCV-infected patients (9,10), as well as more rapid fibrosis progression (11). In addition, several studies demonstrate that IR is associated with greater odds of not achieving SVR (12–16). These studies, however, use homeostasis model assessment (HOMA)-IR to assess insulin sensitivity, which is a surrogate measure of IR. We have recently shown that surrogate measurements of IR are inferior to direct measurements, such as the insulin suppression test (17). In addition, IR may change over time in the setting of HCV infection (18). Therefore, our aims were to use direct measurements of insulin sensitivity to evaluate 1) the impact of IR on achieving an SVR to HCV therapy and 2) the impact of HCV eradication on IR in comparison with individuals who did not receive HCV therapy.
RESEARCH DESIGN AND METHODS
Nondiabetic HCV-infected subjects between age 18 and 60 with detectable hepatitis C viral load (HCV RNA) were recruited from San Francisco General Hospital (SFGH) at the University of California San Francisco (UCSF) and other UCSF-affiliated clinics. The American Diabetes Association criterion of fasting glucose of ≥126 mg/dL was used to define diabetes. Subjects coinfected with hepatitis B virus or HIV, with other causes of liver disease, or with clinical or histologic evidence of cirrhosis or decompensated liver disease were excluded. Also excluded were patients treated previously for HCV, taking steroids or anabolic drugs, with alcohol use ≥50 g/day, or with medical conditions that impaired their ability to participate in the study. Each subject provided written informed consent prior to enrollment. The study was approved by the UCSF Committee on Human Research and the SFGH Data Governance Committee.
Subjects underwent a medical interview, physical examination including anthropometric measurements, fasting laboratory evaluation, and liver biopsy at the screening visit. Subjects were admitted to the UCSF Clinical and Translational Science Institute–Clinical Research Center at SFGH for metabolic testing. A nonrandom subset of subjects underwent standard anti-HCV therapy with pegylated interferon and ribavirin for 6 months (genotype 2 or 3) or 12 months (genotype 1). All subjects, irrespective of HCV therapy, had a set of metabolic tests at baseline. Subjects then underwent metabolic testing at 6 months after study entry. At the time the study was performed, the standard of care stopping rule for HCV therapy was based on virologic response at 6 months. Therefore, all patients who received therapy were treated for a minimum of 6 months regardless of genotype. Patients infected with HCV genotype 1 who had detectable HCV RNA 6 months after initiation of therapy were considered nonresponders and had antiviral therapy discontinued. SVR was defined as undetectable HCV RNA 6 months after the completion of antiviral therapy. Subjects who did not achieve SVR (non-SVR) included nonresponders and relapsers. Subjects who received HCV therapy with or without SVR underwent another set of metabolic testing at 6 months after discontinuation of HCV therapy.
Metabolic testing
An oral glucose tolerance test (OGTT) was performed after a 12-h fast, which consisted of plasma glucose and insulin measurements at 0, 30, 60, 120, and 180 min after ingestion of 75-g glucose. After another 12-h fast, each subject underwent a modified insulin suppression test. During this study, octreotide was infused at a rate of 0.27 μg ⋅ m−2 ⋅ min−1 to suppress endogenous insulin secretion. Insulin and glucose were infused at rates of 6 mU ⋅ m−2 ⋅ min−1 and 50 mg ⋅ m−2 ⋅ min−1, respectively, during the low-dose period to simulate basal conditions and then at rates of 32 mU ⋅ m−2 ⋅ min−1 and 267 mg ⋅ m−2 ⋅ min−1, respectively, during the high-dose period to achieve physiologic hyperinsulinemia. Blood was drawn for plasma glucose and insulin measurement at 0, 90, 100, 110, 120, 210, 220, 230, and 240 min. The four values obtained from 210 to 240 min were averaged to represent the steady-state plasma glucose (SSPG) and steady-state plasma insulin concentrations. Because steady-state plasma insulin concentrations are similar in all patients given identical infusion rates of insulin, the SSPG concentration is a direct measure of the ability of insulin to mediate the disposal of the infused glucose load. Higher SSPG concentrations, reported in milligrams per deciliter, represent higher degrees of peripheral IR.
Laboratory evaluation
Plasma glucose concentrations were measured by the glucose oxidase method (YSI 2300 STAT-Plus Analyzer, Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured by using a single-antibody radioimmunoassay without cross-reactivity with human proinsulin (Millipore, Billerica, MA). The intra- and interassay coefficients of variation for plasma insulin measurements were averaged at 3.2 and 3.9%, respectively. HCV RNA was measured using a branched-DNA signal amplification nucleic-acid probe assay (Versant HCV RNA Assay, version 3.0; Siemens). The lower limit of detection of this assay is 615 IU/mL.
Statistical analysis
Baseline characteristics of subjects were summarized using mean ± SD, median (range), and frequencies. Univariable analysis was performed using Mann-Whitney U test for continuous variables and χ2 tests (or Fisher exact test when appropriate) for dichotomous and categorical variables. Multiple logistic regression was used to evaluate variables associated with achievement of SVR. Mixed effects multiple linear regression, with a random intercept term to account for within-patient correlation, was used to evaluate variables that may influence SSPG and its changes over time, including achievement of SVR. Similar models were also used to evaluate variables that may influence fasting glucose. Variables that were deemed clinically relevant, as well as others with univariate P values <0.1, were considered for inclusion in multivariable models. P < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Cohort characteristics
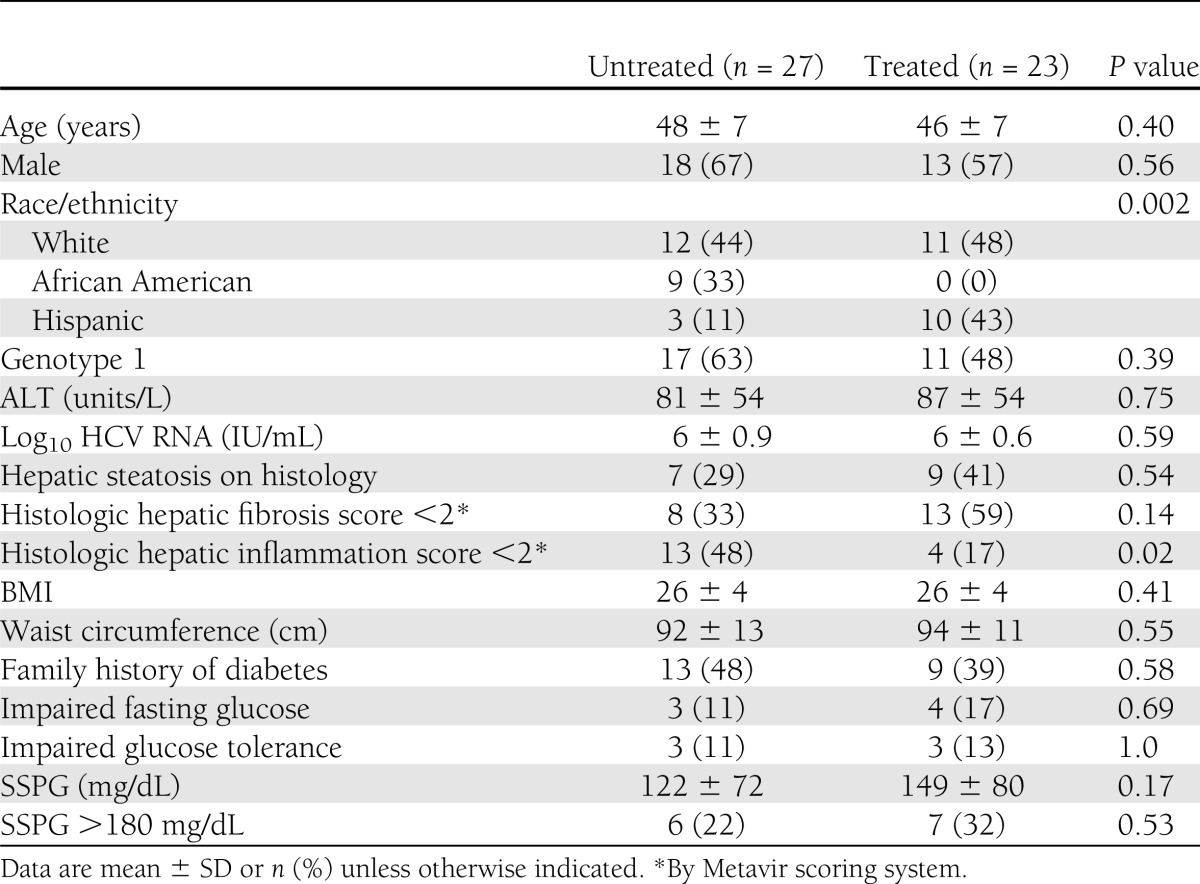
A total of 50 subjects were enrolled and underwent baseline insulin suppression tests and OGTT. In all subjects, the 2-h postglucose load was <200 mg/dL during OGTT, confirming their nondiabetic status by OGTT. Next, 23 subjects received anti-HCV therapy, and 22 of these patients and 24 of the untreated patients underwent liver biopsies at baseline. Of the patients who underwent HCV therapy, 14 achieved SVR. The overall cohort had a median age of 48 (interquartile range 34–57), 13 (57%) were male, and 12 (52%) were white. The host and viral characteristics of the cohort were mostly similar between those who underwent treatment and those who did not (Table 1). The SSPG levels were 149 ± 80 and 122 ± 72 mg/dL in the treated and untreated groups, respectively (P = 0.17). In addition, the proportion of subjects with IR (SSPG >180 mg/dL) was 32 and 22%, respectively, in those who did and did not receive anti-HCV therapy (P = 0.53). Although the proportion of white subjects was similar in the treated and untreated groups, the proportion of black subjects was higher (33 vs. 0%) in the untreated group, and the proportion of Latinos was higher (43 vs. 11%) in the treated group. In addition, a higher proportion of untreated subjects had mild degrees of liver inflammation (P = 0.02), but the degrees of fibrosis and proportion with steatosis were similar among the treated and untreated groups.
Table 1.
Patient characteristics at study entry (n = 50)
Host, metabolic, and viral factors associated with SVR
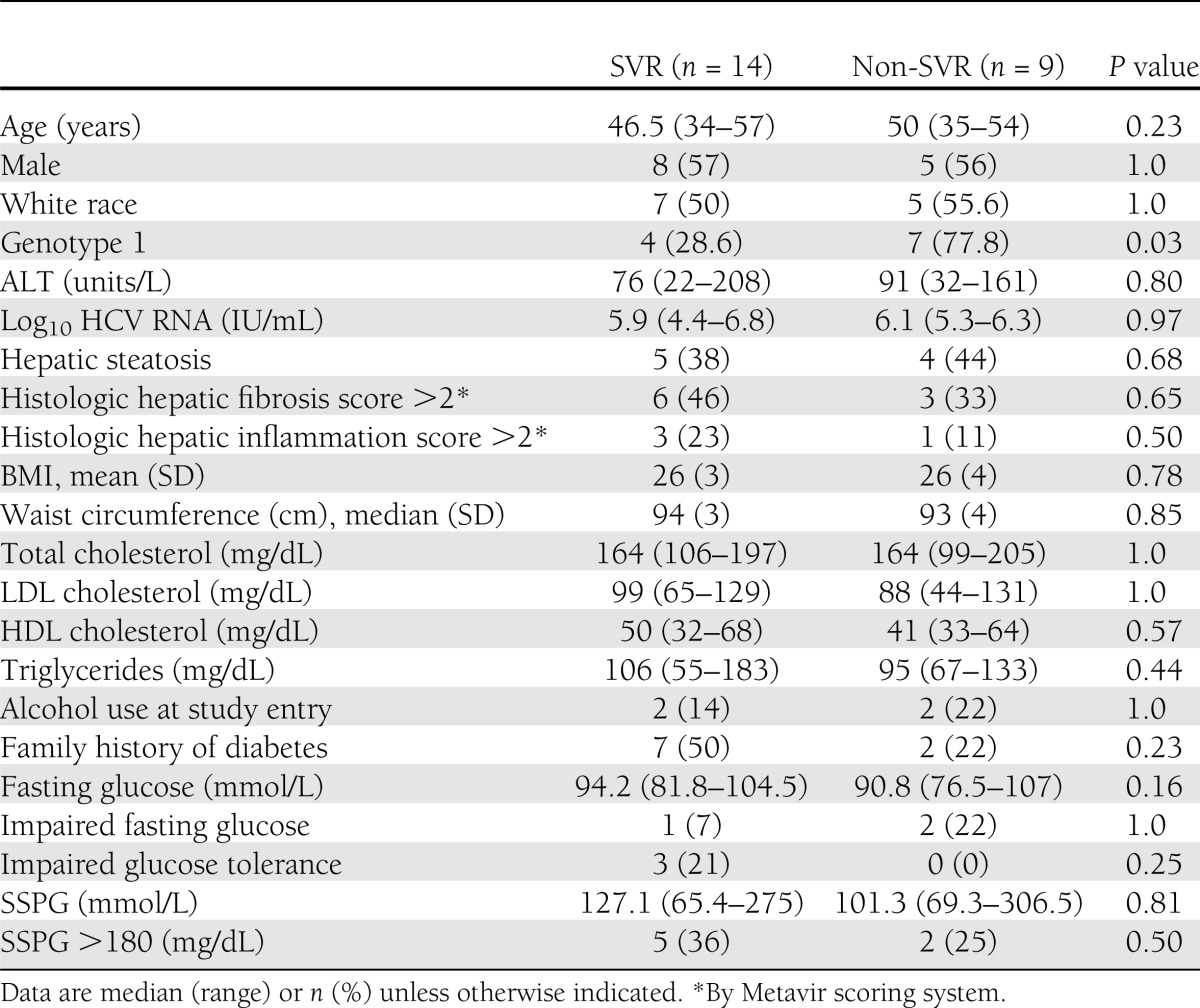
SVR was achieved in 36% of individuals infected with genotype 1 and in 83% of those infected with genotypes 2 and 3 (P = 0.03). The odds of achieving SVR were 8.8 times higher (95% CI 1.2–61.7; P = 0.03) in subjects infected with HCV genotypes other than 1. Other host and viral factors, including age, sex, race, BMI, alanine aminotransferase (ALT), HCV RNA, lipid profile, severity of liver disease, and presence of impaired fasting glucose (≥100 mg/dL) or glucose intolerance (≥140 and <200 mg/dL at 120 min after OGTT), were similar between subjects who did or did not achieve SVR (Table 2). When pretreatment IR (SSPG) was evaluated as a predictor of SVR, the odds of achieving SVR were only 1.02 times higher for each 10 mg/dL decrease in SSPG, and this was not statistically significant (0.91–1.15; P = 0.68). Controlling for genotype did not alter the odds ratio for the association between IR and SVR (1.02 [0.89–1.16]; P = 0.81).
Table 2.
Comparison of individuals who received anti-HCV therapy by response type
Impact of HCV therapy on glucose metabolism
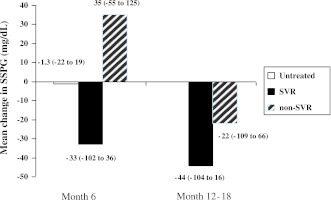
Of the patients, 43 (27 untreated, 11 SVR, and 5 non-SVR) underwent at least one follow-up evaluation with repeat metabolic testing. Average SSPG did not change substantially at the 6-month follow-up visit (−1.3 mg/dL [95% CI −22 to 19]) in those who did not receive antiviral therapy (Fig. 1). SSPG decreased from baseline by 33 mg/dL (95% CI −102 to 36) at 6 months and 44 mg/dL (95% CI −104 to 16) at the time of SVR (6 months after discontinuation of therapy) in those treated individuals who achieved an SVR. Subjects who underwent antiviral treatment but did not achieve SVR initially had increases in mean SSPG (35 mg/dL [95% CI −55 to 125]) at 6-month follow-up but subsequently decreased at 6 months after discontinuation of therapy, as compared with baseline values (−22 mg/dL [95% CI −109 to 66]). When controlling for elapsed time between SSPG measurements, SSPG decreased by 17.2 mg/dL at 6 months after discontinuation of therapy (95% CI −64 to 30; P = 0.46) in those with SVR and increased by 14.7 mg/dL (95% CI −36.3 to 66; P = 0.56) in those without SVR compared with those who did not receive therapy. When additionally controlling for receiving interferon and for BMI at the time of the SSPG measurement, SSPG decreased by 36 mg/dL (95% CI −87.6 to 15.5; P = 0.17) in those with SVR and decreased by 27.6 mg/dL (95% CI −92.9 to 38; P = 0.40) in those without SVR compared with the untreated group, but these changes did not reach statistical significance. There was little difference in SSPG between treated patients with or without SVR (coefficient 8.5 mg/dL [95% CI −45.9 to 63.0]; P = 0.75). BMI (coefficient 9.1 mg/dL per 5 units [95% CI 5.3–12.9]; P < 0.0001) and taking interferon at the time of SSPG measurement (coefficient 56 [95% CI 6.8–105]; P = 0.03) were positively associated with SSPG.
Figure 1.
Mean (95% CI) change in SSPG measurements from baseline by treatment category (untreated vs. SVR vs. non-SVR).
When controlling for the elapsed time between measurements, the fasting glucose decreased slightly by 1.5 mg/dL (95% CI −8.6 to 5.6; P = 0.68) in those with SVR and increased by 3.2 mg/dL (95% CI −4.6 to 10.9; P = 0.42) in those without SVR compared with the untreated group. When also controlling for the presence of interferon use and BMI at the time of SSPG measurement, fasting glucose decreased by 5.2 mg/dL (95% CI −13.5 to 3.1; P = 0.22) in those with SVR compared with untreated patients, but those without SVR also had a decrease in fasting glucose (−3.0 mg/dL [95% CI −13.5 to 7.6]; P = 0.57), and neither of these reached statistical significance versus untreated patients. The fasting glucose was also not substantially different between those with SVR compared with those without SVR (2.2 mg/dL [95% CI −6.6 to 11]; P = 0.62). Only BMI at the time of glucose measurement (3.6 mg/dL per 5 units [95% CI 0.34–6.8]; P = 0.03) was statistically significantly associated with fasting glucose levels.
CONCLUSIONS
This is the largest study to date that has used direct measurements to evaluate IR in an ethnically diverse cohort of HCV patients and monitored the relationship between IR and HCV treatment response. We found that infection with HCV genotypes 2 and 3 predicted SVR, but baseline IR was not substantially associated with the achievement of SVR. As expected in this study, higher BMI was positively associated with IR. Active interferon use was also associated with higher degrees of IR. IR appeared to improve with HCV therapy, irrespective of viral eradication.
Several prior studies using surrogate measures of IR (mainly HOMA-IR) report IR as a predictor of nonresponse to anti-HCV therapy (9,12–16). In a study of 159 Spanish patients, individuals with SVR had lower baseline HOMA-IR scores compared with those with non-SVR (2.4 vs. 3.8) (16). In addition, after adjusting for genotype and liver fibrosis scores, the odds of nonsustained response to anti-HCV therapy were ∼1.8 times higher with increasing HOMA-IR scores (16). In the Virahep-C Study, which compared African Americans and Caucasian Americans infected with HCV genotype 1, race, viral load, liver fibrosis score, and, once again, presence of IR and interferon dosage were negatively associated with SVR (19).
The reported association between SVR and IR, however, has not been consistently demonstrated (13,20). In a recent study of 412 Italian patients, SVR was associated with genotypes 2 and 3 and younger age, but baseline HOMA-IR scores between individuals who did and did not achieve SVR did not reach statistical significance (20). In another study, there were no improvements in SVR despite pharmacologically induced improvement of IR with metformin (21). In a similar manner, in our study, genotypes 2 and 3 were associated with SVR, but SSPG appeared to have little association. The discrepancies observed in reported associations between IR and SVR may reflect homogeneous patient populations with limited ethnic diversity; variation in host factors that are well known to affect IR, such as BMI; and limitations in the surrogate measurements of IR compared with direct measurements. For example, the patients in the Virahep-C Study had an especially high prevalence of obesity and high degrees of IR compared with other reports; the mean HOMA-IR in that study was 3.5 in individuals without hepatic steatosis and 6.1 in those with moderate degrees of steatosis (19). Other U.S. HCV cohorts with more ethnically diverse populations have reported lower mean HOMA-IR scores of 3.7 among the overweight and 4.9 among the obese patients. In addition, it has been shown that the correlation between surrogate estimates and direct measurement of IR is affected by degrees of obesity and ethnicity (17). In addition, commonly used HOMA-IR cutoffs of >2 or >3 to define IR have high misclassification rates (17). Moreover, there is a significant within-person variation in repeated HOMA-IR measurements in the obese individuals compared with normal weight patients.
Although studies report an association between HCV infection and diabetes, as well as IR, a cause-and-effect relationship has not been established. The proposed relationship between HCV infection and IR is complex. HCV appears to affect both IR and interferon signaling in the liver (22). The HCV core protein activates suppressor of cytokine signaling 3, which in turn inhibits the insulin receptor substrates (22). However, while some have shown a statistically significant decrease in HOMA-IR scores with viral eradication and achievement of SVR (6,16), others have not shown a significant impact of viral clearance on HOMA-IR (23). In our study, SSPG levels decreased during antiviral therapy in all treatment viral response categories compared with untreated patients, but the difference in mean change in SSPG was not statistically significant between SVR and non-SVR during and after treatment. SSPG >180 mg/dL has been associated with an increased risk of development of clinical syndromes associated with IR (24,25). The changes in SSPG resulting from HCV therapy may be sufficient to reduce SSPG to <180 mg/L, thereby reducing the risk of these clinical sequelae. It has been shown that interferon use can directly increase IR (26,27). On the other hand, a potential adverse effect of interferon therapy may be weight loss, which can also influence metabolic parameters (28). When considering the independent effect of both BMI and interferon at the time of metabolic testing, similar to other studies, higher BMI and taking interferon at the time of metabolic testing were associated with higher degrees of IR. The inclusion of the control group of untreated patients in this study was important considering that it allowed for assessment of the influence of interferon use itself on IR. In addition, because IR is a known precursor to diabetes, prior studies have evaluated the impact of successful HCV therapy on incident diabetes but have failed to prove that there is an independent association between achievement of SVR and risk of incident diabetes or impaired glucose tolerance with long-term follow-up of 8 years (8). In our study, while not reaching statistical significance, we did observe decreases in SSPG and fasting glucose levels with therapy regardless of SVR status. As observed with IR, higher BMI was associated with increases in fasting glucose levels on follow-up.
Similar to other studies using the direct measurements of IR, our study is limited by small sample size (29), which may not have allowed for detection of other known host and viral factors that influence SVR. However, accurate assessment of peripheral IR by direct measurement allows for meaningful comparisons, and performing this test would be logistically challenging and impractical in a larger patient population. In addition, the main purpose of this study was to evaluate the influence of IR on SVR as well as SVR on improvement of IR, so use of direct measurement was important.
In conclusion, when using precise measurements of IR, IR did not appear to be substantially associated with achievement of SVR. Successful viral eradication did not appear to substantially influence IR when compared with HCV therapy that does not result in SVR. However, HCV therapy, regardless of achievement of SVR, appeared to improve IR. This suggests that individuals with higher degrees of IR may benefit from receipt of HCV therapy in an attempt to decrease their risk of clinical sequelae of IR. This study highlights the potential limitations of use of surrogate measures of IR within the context of HCV therapy and also the importance of controlling for change in BMI with antiviral therapy, which significantly influences insulin action. Further studies are necessary to determine if additional changes in IR occur after successful viral eradication beyond 6 months after discontinuation of HCV therapy.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01-DK-074673 (to M.K.), UL1-RR-024131 (NIH/National Center for Research Resources UCSF–Clinical and Translational Science Institute), Hepatology Training Grant DK-060414 (to D.B.), and P30-DK-026743 (UCSF Liver Center) and American Diabetes Association Grant 1-08-CR-30 (to M.K.).
No conflicts of interest relevant to this article were reported.
D.B. analyzed and interpreted data and wrote and revised the manuscript. P.B. performed statistical analysis and critically reviewed and revised the manuscript. C.E.A. acquired data, performed study experiments, provided technical support, and critically revised the manuscript. J.J.M. interpreted data and critically revised the manuscript. M.K. designed the study concept, acquired data, interpreted results, provided material support, supervised the study, and revised the manuscript. M.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology 1999;29:328–333 [DOI] [PubMed] [Google Scholar]
- 2.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med 2000;133:592–599 [DOI] [PubMed] [Google Scholar]
- 3.Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003;38:50–56 [DOI] [PubMed] [Google Scholar]
- 4.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology 2008;47:2127–2133 [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Borrego A, Kamegaya Y, Jordan SH, Agrawal S, Valim C, Chung RT. HCV synergizes with body weight in the promotion of insulin resistance. J Viral Hepat 2011;18:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol 2007;102:570–576 [DOI] [PubMed] [Google Scholar]
- 7.Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol 2008;48:721–727 [DOI] [PubMed] [Google Scholar]
- 8.Giordanino C, Bugianesi E, Smedile A, et al. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol 2008;103:2481–2487 [DOI] [PubMed] [Google Scholar]
- 9.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol 2005;100:1509–1515 [DOI] [PubMed] [Google Scholar]
- 10.Petta S, Cammà C, Di Marco V, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol 2008;103:1136–1144 [DOI] [PubMed] [Google Scholar]
- 11.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected in: Gastroenterology 2004;126:634]. Gastroenterology 2003;125:1695–1704 [DOI] [PubMed] [Google Scholar]
- 12.Khattab M, Eslam M, Sharwae MA, Shatat M, Ali A, Hamdy L. Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol 2010;105:1970–1977 [DOI] [PubMed] [Google Scholar]
- 13.Grasso A, Malfatti F, De Leo P, et al. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol 2009;51:984–990 [DOI] [PubMed] [Google Scholar]
- 14.Moucari R, Ripault MP, Martinot-Peignoux M, et al. Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut 2009;58:1662–1669 [DOI] [PubMed] [Google Scholar]
- 15.Poustchi H, Negro F, Hui J, et al. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol 2008;48:28–34 [DOI] [PubMed] [Google Scholar]
- 16.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005;128:636–641 [DOI] [PubMed] [Google Scholar]
- 17.Lam KD, Bacchetti P, Abbasi F, et al. Comparison of surrogate and direct measurement of insulin resistance in chronic hepatitis C virus infection: impact of obesity and ethnicity. Hepatology 2010;52:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SK, Cho YK, Park JH, et al. Change of insulin sensitivity in hepatitis C patients with normal insulin sensitivity: a 5-year prospective follow-up study variation of insulin sensitivity in HCV patients. Intern Med J 2010;40:503–511 [DOI] [PubMed] [Google Scholar]
- 19.Conjeevaram HS, Kleiner DE, Everhart JE, et al. Virahep-C Study Group Race, insulin resistance and hepatic steatosis in chronic hepatitis C. Hepatology 2007;45:80–87 [DOI] [PubMed] [Google Scholar]
- 20.Fattovich G, Covolo L, Pasino M, et al. Italian Hepatitis C Cohort Study Collaborative Group The homeostasis model assessment of the insulin resistance score is not predictive of a sustained virological response in chronic hepatitis C patients. Liver Int 2011;31:66–74 [DOI] [PubMed] [Google Scholar]
- 21.Romero-Gómez M, Diago M, Andrade RJ, et al. Spanish Treatment of Resistance to Insulin in Hepatitis C Genotype 1 Group Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology 2009;50:1702–1708 [DOI] [PubMed] [Google Scholar]
- 22.Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol 2009;15:4356–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawaguchi Y, Mizuta T, Oza N, et al. Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C. Liver Int 2009;29:871–877 [DOI] [PubMed] [Google Scholar]
- 24.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 2001;86:3574–3578 [DOI] [PubMed] [Google Scholar]
- 25.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab 1998;83:2773–2776 [DOI] [PubMed] [Google Scholar]
- 26.Imano E, Kanda T, Ishigami Y, et al. Interferon induces insulin resistance in patients with chronic active hepatitis C. J Hepatol 1998;28:189–193 [DOI] [PubMed] [Google Scholar]
- 27.Koivisto VA, Pelkonen R, Cantell K. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes 1989;38:641–647 [DOI] [PubMed] [Google Scholar]
- 28.Conjeevaram HS, Wahed AS, Afdhal N, Howell CD, Everhart JE, Hoofnagle JH, Virahep-C Study Group Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology 2011;140:469–477 [DOI] [PubMed] [Google Scholar]
- 29.Milner K, van der Poorten D, Trenell M, et al. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology 2010;138:932–941 [DOI] [PubMed] [Google Scholar]