Abstract
OBJECTIVE
Observational studies show breaking up prolonged sitting has beneficial associations with cardiometabolic risk markers, but intervention studies are required to investigate causality. We examined the acute effects on postprandial glucose and insulin levels of uninterrupted sitting compared with sitting interrupted by brief bouts of light- or moderate-intensity walking.
RESEARCH DESIGN AND METHODS
Overweight/obese adults (n = 19), aged 45–65 years, were recruited for a randomized three-period, three-treatment acute crossover trial: 1) uninterrupted sitting; 2) seated with 2-min bouts of light-intensity walking every 20 min; and 3) seated with 2-min bouts of moderate-intensity walking every 20 min. A standardized test drink was provided after an initial 2-h period of uninterrupted sitting. The positive incremental area under curves (iAUC) for glucose and insulin (mean [95% CI]) for the 5 h after the test drink (75 g glucose, 50 g fat) were calculated for the respective treatments.
RESULTS
The glucose iAUC (mmol/L) ⋅ h after both activity-break conditions was reduced (light: 5.2 [4.1–6.6]; moderate: 4.9 [3.8–6.1]; both P < 0.01) compared with uninterrupted sitting (6.9 [5.5–8.7]). Insulin iAUC (pmol/L) ⋅ h was also reduced with both activity-break conditions (light: 633.6 [552.4–727.1]; moderate: 637.6 [555.5–731.9], P < 0.0001) compared with uninterrupted sitting (828.6 [722.0–950.9]).
CONCLUSIONS
Interrupting sitting time with short bouts of light- or moderate-intensity walking lowers postprandial glucose and insulin levels in overweight/obese adults. This may improve glucose metabolism and potentially be an important public health and clinical intervention strategy for reducing cardiovascular risk.
Prolonged sitting time is associated with premature cardiovascular and all-cause mortality, independent of leisure-time physical activity and adiposity (1,2). Findings from the 2003–2004 and 2005–2006 U.S. National Health and Nutrition Examination Survey (NHANES) and the 2004–2005 Australian Diabetes, Obesity and Lifestyle (AusDiab) study showed that objectively measured total sedentary time was detrimentally associated with cardiometabolic risk markers (3,4). In contrast, frequent interruptions (breaks) to sedentary time (defined as the transition from sedentary to an active state for ≥1 min) were beneficially associated. In both populations, the mean break duration was approximately 4 min and was characterized by light-intensity activity (approximately 500 accelerometer counts/min). Furthermore, these relationships persisted after accounting for moderate-to-vigorous activity, suggesting that frequent short breaks in sedentary time may impart unique benefit. A next step for the science of sedentary behavior is to identify the metabolic underpinnings of these deleterious and beneficial relationships.
Regular ingestion of high-calorie meals rich in processed carbohydrates and saturated fat can lead to transient exaggerated postprandial spikes in glucose and lipids, which promote oxidative stress that triggers a biochemical inflammatory cascade, endothelial dysfunction, and sympathetic hyperactivity (5–9). These postprandial excursions, when repeated multiple times each day, can create a milieu conducive for the development of atherosclerosis and cardiovascular disease (9,10). Postprandial hyperglycemia or glucose variability has been associated with indices of atherosclerotic progression, including carotid intima-media thickening (11,12) and coronary artery calcium (13) as well as development or progression of retinopathy (14,15), cardiovascular events (16), and death (17) in patients with type 2 diabetes. Guidelines for cardiovascular health include minimizing the magnitude of postprandial hyperglycemia (18).
Postprandial glucose levels and insulin sensitivity are beneficially influenced by regular moderate-intensity exercise training (19–21). Recent epidemiologic findings have also shown 2-h plasma glucose to be beneficially associated with objectively measured light-intensity activity (22). Light-intensity activity may be effective for reducing postprandial glucose, and corroborative experimental evidence exists. In middle-aged women, 15- and 40-min bouts of light-intensity activity both led to a reduction in the acute blood glucose response to a carbohydrate-rich meal relative to 2-h seated rest (23). Compared with sitting, light nonexercise activities of intensities between 1.1 and 2.7 metabolic equivalents can improve insulin action in young men and women (24). In addition, 20 min of light exercise (40% of maximal power output) performed 45 min after the ingestion of a standardized pre-exercise carbohydrate load in young men resulted in similar glucose and insulin responses to that of moderate and vigorous exercise (65 and 80%) (25). This evidence, together with observational study findings on breaking up sedentary time (22), points to the need for further experimental studies. Specifically, there is the need to examine the effects of frequent brief interruptions to prolonged sitting involving light-intensity or moderate-intensity activity (as distinct from continuous ambulatory activity) on postprandial glucose and insulin. This may have important implications for settings where continuous ambulatory activity may not be feasible or practical, such as workplaces.
We examined the acute effects of uninterrupted sitting on postprandial plasma glucose and serum insulin, compared with sitting interrupted by short 2-min bouts of light- or moderate-intensity walking in overweight middle-aged adults. We hypothesized that postprandial blood glucose control during sitting would be improved by brief intermittent bouts of activity, irrespective of whether these were of light or moderate intensity.
RESEARCH DESIGN AND METHODS
Study overview
This randomized, three-period, three-treatment crossover trial was approved by the Alfred Hospital Human Ethics Committee and was in accordance with the Declaration of Helsinki. Participants provided signed, written informed consent. The study is registered as a clinical trial with the Australian New Zealand Clinical Trials Registry (ACTRN12609000656235).
Participants attended three separate visits to the laboratory to complete each of the trial conditions in a randomized order: 1) uninterrupted sitting; 2) sitting interrupted by light-intensity activity breaks; and 3) sitting interrupted by moderate-intensity activity breaks. Because an acute bout of physical activity may enhance insulin sensitivity for up to 72 h (26), we used a minimum wash-out of 6 days between each condition to eliminate potential carryover effects of the activity conditions.
Participants and enrollment process
Participants were recruited between April 2009 and August 2010 from the general community (Fig. 1). Eligibility criteria included age between 45 and 65 years and BMI >25 kg/m2 (overweight or obese), representing a population with a heightened diabetes risk (27). Exclusion criteria were pregnancy, clinically diagnosed diabetes, BMI >45 kg/m2, non-English speaking, taking glucose-lowering and/or lipid-lowering medication, employment in a nonsedentary occupation, currently watching <2 h of television per day, regularly engaged in moderate-intensity exercise 150 min/week for at least 3 months, and known physical activity contraindications, major illness/injury (acute or chronic), or other health issues that may have limited the ability to perform the necessary activity bouts.
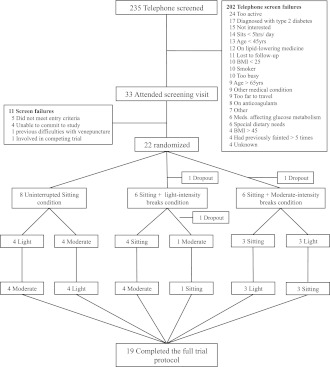
Figure 1.
Trial CONSORT diagram.
Participants were invited to an orientation and were provided with a detailed study overview, gave consent, and were screened for additional exclusion criteria. This included a medical examination performed by a physician (D.A.B.) that involved a medical history, physical examination, resting blood pressure, and a resting 12-lead electrocardiogram. Eligible participants received a brief familiarization and were given the opportunity to become accustomed to the light- and moderate-intensity walking speeds and were also familiarized with use of the Borg Rate of Perceived Exertion (RPE) scale (28). During the moderate-intensity walking familiarization, the treadmill speed that yielded an RPE rating between 12 and 14 for each participant was recorded and used during that experimental condition
Study protocol
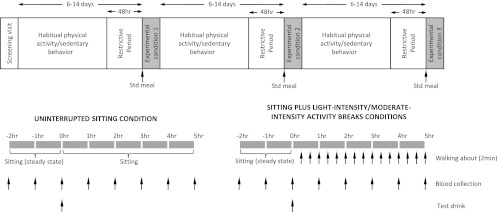
Figure 2 shows the study protocol. Participants were provided with verbal and written instructions to refrain from any exercise, alcohol, and caffeine in the 48 h before each of the three trial conditions. During this time, physical activity was objectively measured with an Actigraph GT1M accelerometer (Actigraph, Pensacola, FL) worn around the hip during waking hours. Data were recorded in 1-min epochs, with accelerometer counts ≥100/min classified as active time. This was further differentiated as moderate-to-vigorous intensity activity (≥1,952 counts/min) and light-intensity activity (100–1,951 counts/min) (29). Wear time and activity duration, type, and intensity undertaken during any nonwear periods were recorded in activity diaries. This information, in conjunction with an automated accelerometer wear-time estimation, was used to derive daily wear time.
Figure 2.
Study protocol. Std, standard.
Participants reported to the laboratory between 0700 and 0800 h, having fasted overnight. A catheter was inserted into an antecubital vein for hourly blood sampling. After the initial blood collection (time point: −2 h), participants remained seated for 2 h to achieve a steady state before the consumption of a standardized test drink (time point: 0 h). The 200-mL test drink consisted of 75 g carbohydrate (100% corn maltodextrin powder; Natural Health, Australia) and 50 g fat (Calogen; Nutricia, Australia). The specific nutritional components were energy, 3,195 kJ; fat, 50.0 g; saturated fat, 5 g; monounsaturated fat, 30.4 g; polyunsaturated fat, 14.3 g; carbohydrate, 75 g; total sugars, 12.8 g; protein, nil; fiber, <1 g; sodium, 46.9 mg; and water, 90 g. The grounds for inclusion of the fat content were based on 1) better simulation of a mixed meal and 2) its influence of slowing the ingested glucose production (gastrointestinal emptying) to spread the plasma glucose and insulin responses over more of the selected 5-h treatment period. Participants were then guided through the respective trial condition protocols for the remaining 5 h. The hourly blood collection was undertaken before activity bouts during the activity days (trial conditions 2 and 3 outlined below). The research staff directly supervised participants throughout each trial to ensure that full compliance with the trial protocols was achieved.
The trial conditions are listed as follows:
Uninterrupted sitting: Participants remained seated throughout the experimental period and were instructed to minimize excessive movement, only rising from the chair to void.
Sitting + light-intensity activity breaks: Participants rose from the seated position every 20 min throughout the experimental period (to achieve three breaks per hour), and completed a 2-min bout of light-intensity walking on a motorized treadmill with a level surface at 3.2 km/h. They then returned to the seated position. This procedure was undertaken on 14 occasions, providing a total of 28 min of light-intensity activity.
Sitting + moderate-intensity activity breaks: Identical procedure to the sitting + light-intensity activity breaks condition, but participants completed 2-min bouts of moderate-intensity walking on the treadmill at between 5.8 and 6.4 km/h every 20 min, providing a total of 28 min of moderate-intensity activity.
Participants watched television or DVDs; read books, magazines, or newspapers; performed light paperwork; or worked on a laptop computer throughout the three conditions. As expected, during the 5-h postprandial period in the moderate-intensity activity breaks condition, the mean ± SE accelerometer-measured time spent in moderate-intensity activity was 24.0 ± 1.8 min compared with 0.6 ± 0.4 min in the light-intensity condition. Activity levels were further monitored at the completion of each activity bout using the Borg RPE scale. The mean ± SE (range: min–max) RPEs during the light-intensity and moderate-intensity activity break conditions were 8 ± 0.4 (6–11) and 12 ± 0.4 (8–14), respectively.
Randomization and masking
Participants were randomized to one of six possible trial-condition orders using balanced blocks prepared for male and female participants. An independent research institution prepared the computer-generated randomization lists and sealed envelopes for randomization (BSR for Windows, Johns Hopkins Oncology Center, Baltimore, MD). Once informed consent was obtained, a third party opened the sealed randomization envelope revealing the trial-condition order. The pathology technicians and team statistician were also blinded to trial condition.
Plasma preparation and analytic methods
Code-labeled samples were sent to an independent laboratory for the determination of glucose and insulin levels. Glucose was measured in plasma (fluoride/oxalate) on the day of testing using a hexokinase method on an Architect ci16200 analyzer (Abbott Diagnostics, North Ryde, NSW, Australia). Serum, collected hourly, was stored at −80°C for insulin analysis after the study. Serum insulin was measured by chemiluminescent microparticle immunoassay (Architect ci16200 analyzer, Abbott Diagnostics). Samples collected for each participant during the respective trial conditions were included in the same insulin assay to avoid interassay variability. The intra-assay coefficient of variation for insulin was between 2 and 4%.
Statistical analysis
Sample size calculations were based on pilot data of mean changes in the incremental area under the curve (iAUC) for glucose and insulin (10% for glucose, 60% for insulin), estimates of population variability (SD 1% for glucose, 30% for insulin), and assuming a correlation coefficient of 0.5 between repeated measures of the outcomes. We estimated that 19 paired observations were needed to achieve a power of 0.90 to detect the smallest expected effect size in the primary outcome variables between the three interventions, while adopting a two-tailed testing and a 0.05 probability level.
Generalized estimating equations with exchangeable working correlation to account for dependency in the data (repeated measures) were used to evaluate the differential effects of the trial conditions on the outcomes (30,31). All models were adjusted for potentially important covariates explaining residual outcome variance (age, sex, and weight), baseline predrink outcome values, and period effects. Carryover effects were not formally tested, given the >6-day washout between trials and that there were no differences in baseline biochemical, anthropometric, dietary, or physical activity measures before each intervention (Table 1) (26,31). Postestimation contrasts were used to examine the differential effects of pairs of conditions. Generalized estimating equation models were used to examine between-trial condition differences in pretrial waist circumference, weight, plasma glucose, serum insulin, physical activity, and dietary measures. Level of P < 0.05 was considered significant. All statistical analyses were performed using Stata 10.1 software (StataCorp LP). Data are reported as mean ± SD and mean (95% CI) in the text and tables unless otherwise indicated, and in Fig. 3 marginal means ± SE are presented.
Table 1.
Biochemical, anthropometric, physical activity, and dietary values before each trial
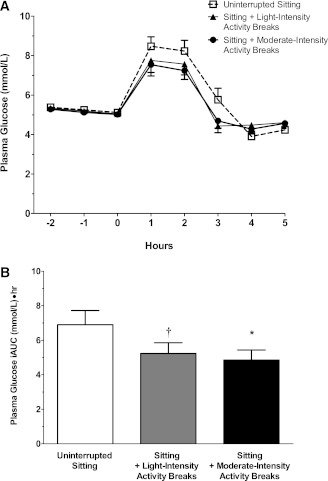
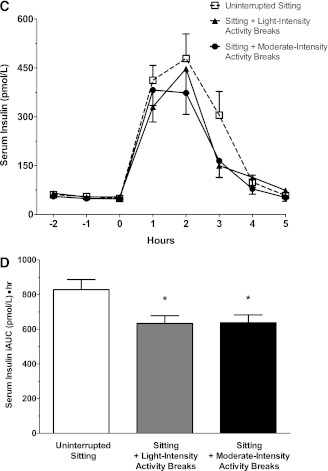
Figure 3.
The effect of the three trial conditions on postprandial plasma glucose levels (A); positive (5-h postprandial) glucose iAUC (B); postprandial serum insulin levels (C); and positive (5-h postprandial) iAUC (D). Data for postprandial plasma glucose and serum insulin levels represent mean ± SE. Data for glucose and insulin positive iAUC represent marginal means ± SE (adjusted for age, sex, body weight, period effects, and predrink levels). *Significantly different from uninterrupted sitting condition, P < 0.001. †Significantly different from uninterrupted sitting condition, P < 0.01.
RESULTS
The trial Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Fig. 1. Of the 22 participants who commenced the study, 19 (11 men, 8 women; mean age, 53.8 ± 4.9 years; mean BMI, 31.2 ± 4.1 kg/m2) completed all three trials and were included in the analyses. A completed case analysis (rather than intention-to-treat) was undertaken because the reasons for withdrawal (difficulties with intravenous cannulation for two participants, a syncopal episode for the other) were unrelated to initial values or their response during the first condition.
The biochemical, anthropometric, dietary, and accelerometer-derived physical activity data before each of the respective trials are reported in Table 1. There were no significant differences between trials for any of these measures.
After adjustment for age, sex, weight, period effects, and predrink levels, the mean net glucose response to the test drink (5-h positive iAUC) was 24.1% lower after sitting with light-intensity activity (5.2 mmol/L ⋅ h [4.1–6.6], P < 0.01) and 29.6% lower after sitting with moderate-intensity activity (4.9 mmol/L ⋅ h [3.8–6.1]; P < 0.0001) compared with uninterrupted sitting (6.9 mmol/L ⋅ h [5.5–8.7]) (Fig. 3A and B). The mean plasma glucose concentrations at 2-h postdrink were 8.0 mmol/L (7.2–8.8) for uninterrupted sitting, 7.5 mmol/L (6.8–8.3) for sitting with light-intensity physical activity, and 7.2 mmol/L (6.5–8.0) for sitting with moderate-intensity physical activity (P > 0.05 for light- and moderate-intensity conditions relative to uninterrupted sitting).
After adjustment for age, sex, weight, period effects, and predrink levels, the mean insulin-positive iAUC was reduced by 23% after the sitting with light-intensity (633.6 pmol/L ⋅ h [552.4–727.1], P < 0.0001) and moderate-intensity conditions (637.6 pmol/L ⋅ h [555.5–731.9], P < 0.0001) relative to the uninterrupted (828.6 pmol/L ⋅ h [722.0–950.9]) sitting (Fig. 3C and D). The mean serum insulin concentration at 2-h postdrink was significantly lower for sitting with moderate-intensity physical activity (412.9 pmol/L [340.4–500.7], P < 0.05), but not for sitting with light-intensity physical activity (324.3 pmol/L [267.4–393.3], P = 0.4), relative to uninterrupted sitting (375.7 pmol/L [308.2–458.0]).
No significant differences were observed in the positive iAUC observed between the two activity conditions, nor any age or sex interactions for any of the outcomes.
CONCLUSIONS
These findings provide initial experimental confirmation of hypotheses generated by epidemiologic observational studies on the deleterious health consequences of prolonged sedentary time (too much sitting as distinct from too little exercise). Of particular importance is the potential for reducing cardiovascular disease risk by briefly breaking up prolonged periods of sitting with activity of at least light intensity. Brief interruptions to sitting led to significant reductions in postprandial glucose and insulin, irrespective of the activity intensity. The 24–30% lowering in the plasma glucose iAUC and the 23% lowering in the insulin iAUC seen after the activity break conditions, at the very least, is comparable in magnitude to an acute bout of moderate-intensity aerobic or resistance exercise in overweight/obese individuals (32).
Our findings are the first to document, among overweight/obese adults, an elevated postprandial glucose and insulin response during an acute bout of prolonged sitting, relative to sitting with brief activity interruptions. Among young nonobese adults, significant reductions in whole-body insulin sensitivity have been observed after 1 day of prolonged sitting (24). The magnitudes of changes observed in postprandial glucose and insulin during the uninterrupted sitting condition in our study are consistent (∼23–30%) with the changes in metabolic outcomes reported after 1 day of prolonged sitting (24). The lowering of postprandial glucose and insulin suggests both increased insulin sensitivity and reduced insulin secretion, the latter effect consistent with preservation of pancreatic β-cell function. Intervention study findings demonstrating that blunting of postprandial spikes in glucose improves inflammation and endothelial function (33) and reduces carotid intima-media thickness (11,33) suggest that the blunted glucose response seen in our study through the inclusion of three brief (2-min) activity breaks per hour during prolonged sitting may ameliorate such consequences of postprandial hyperglycemia (9,18).
Although larger reductions in postprandial glycemia have yielded clinical benefits to surrogate cardiovascular end points, including carotid intima-media thickness (8), smaller differentials in the postprandial glucose seen in response to pharmaceutical agents, generally comparable in magnitude to those seen in the activity break conditions, have been linked to reductions in oxidative stress, circulating adhesion molecules, and endothelial function in patients with and without type 2 diabetes (6,8,33). Additional research using measures of inflammation and endothelial function will be necessary to further elucidate the influence of frequent breaks from sitting and whether the exaggerated postprandial glucose response observed after 5 h of uninterrupted sitting is more pronounced when imposed over multiple days. Potential long-term consequences, both beneficial and deleterious, of different sitting-time patterns are needed to strengthen the case for relevant public health and clinical initiatives.
These findings support the hypothesis that brief interruptions to sedentary time with a minimum of light-intensity physical activity can attenuate acute postprandial plasma glucose and serum insulin response during prolonged sitting. Importantly, the brevity of the interruptions to sitting (2 min) indicates that such breaks would not count toward the minimum amount of aerobic activity necessary for substantial health benefits within current physical activity guidelines because at least 10-min episodes of activity are stipulated (34). However, consistent with studies of the effects of continuous exercise on glucose metabolism (35), the sum of total activity time over a 5-h period was 28 min. A logical next step would be to build on these findings to design a study that would provide a head-to-head comparison of a single continuous exercise bout to the breaking up of prolonged sitting protocol used in this study.
The moderate-intensity activity break condition was relatively strenuous for some of our study participants. Our original protocol called for a consistent 6.4 km/h for all participants, but we found it necessary to slightly reduce the speed for some so that they could complete the prescribed 2-min moderate-intensity bouts without discomfort. Thus, the higher-intensity breaks condition was close to the practical maximal feasible intensity for these overweight/obese individuals and likely close to the tolerable upper limit of activity for expected physiologic benefit.
Our trial does have some potential limitations. First, it examined acute effects of a 1-day exposure to prolonged uninterrupted versus interrupted sitting; thus, implications cannot be extrapolated to long-term exposures. Second, it examined activity bouts of fixed frequency and length. Systematic variations in length and frequency of activity bouts and possible moderating effects of factors such as sex and adiposity status should be examined.
Our experimental protocol involved brief and regular interruptions (2 min of activity for every 20 min sitting) to prolonged sitting time. Interrupting sedentary time in this way could be feasible in domestic and workplace settings where adults sit for prolonged periods (36). However, further experimental evidence that can define dose-response relationships is required. For example, evidence from animal studies (37) suggests that it may be important primarily to interrupt the contractile inactivity in postural muscles that takes place during prolonged sitting. From body weight–dependent equations (38), it is estimated that treadmill walking at 3.2 km/h in people weighing 90.5 kg demands an energy expenditure of only 18.8 kJ/min, yet most forms of nonexercise activity that naturally interrupt sitting would be expected to yield even less energy expenditure. Although the body weight–dependent equations do not cover the 5.6–6.4 km/h walking speed used in this trial, it would be realistic to expect that the energy expenditure for the moderate walking bouts would be expected to exceed the 25.6 kJ/min estimated for walking at 4.8 km/h. Simply regularly standing up for a short period may have beneficial metabolic effects. Estimating the caloric expenditure of such changes would be informative; the increased substrate utilization that is required to meet differences in energy demands when transitioning from sitting to standing may be an important mechanism (37,38). However, whether simple brief standing, as opposed to longer activity bouts, will be protective for metabolic health, remains to be determined.
Given the high prevalence of overweight and obesity among those of low socioeconomic status living in affluent populations (39) and the average “greying” of demographic profiles, our study participants are representative of large numbers at risk for developing type 2 diabetes and subsequent cardiovascular complications. Pragmatic trials with large numbers of participants would be highly informative (40), such as demonstrating metabolic health effects of reducing and breaking up sitting time in the workplace over sustained periods of time (i.e., months or years) among representative groups of working adults.
Acknowledgments
This work was supported by National Health and Medical Research Council (NHMRC) Project Grant (NHMRC #540107), an NHMRC Program Grant (NHMRC # 569940), a Victorian Public Health Research Fellowship and an Australian Research Council Future Research Fellowship to D.W.D., an NHMRC Principal Research Fellowship to B.A.K., an NHMRC (NHMRC #569861)/National Heart Foundation of Australia (PH 08B 3905) Post-Doctoral Fellowship to G.N.H., a National Heart Foundation Grant-In-Aid (G 07 M3168), an NHMRC (NHMRC #586623) Senior Research Fellowship to J.E.S., an NHMRC Senior Principal Research Fellowship to N.O. (NHMRC #1003960), and an Inactivity Physiology Program grant by Coca-Cola and the Edward G. Schlieder Foundation to M.T.H. and was supported in part by the Victorian Government’s Operational Infrastructure Support Program. No other potential conflicts of interest relevant to this article were reported.
D.W.D., B.A.K., N.O., R.L., J.E.S., G.N.H., E.C., P.Z.Z., J.S., and M.T.H. were responsible for the concept and design of the study, obtained funding, analyzed and interpreted data, participated in critical revision of the report for intellectual content, and provided final approval of the submitted version. R.L. supervised the study. E.C. did the statistical analysis. G.N.H. provided clinical and technical support. D.A.B. provided clinical and technical support, analyzed and interpreted data, participated in critical revision of the report for intellectual content, and provided final approval of the submitted version. D.W.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was presented by D.W.D. at the 58th Annual Scientific Meeting of the American College of Sports Medicine, Denver, Colorado, 1–4 June 2011, and at the International Society for Behavioural Nutrition and Physical Activity Annual Conference, Melbourne, Victoria, Australia, 15–18 June 2011.
The authors thank Miriam Clayfield, Baker IDI Heart and Diabetes Institute, for assisting in the clinical management of the study participants and thank the research participants whose cooperation and dedication made this study possible.
Footnotes
Clinical trial reg. no. ACTRN12609000656235, www.anzctr.org.au.
References
- 1.Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 2010;121:384–391 [DOI] [PubMed] [Google Scholar]
- 2.Patel AV, Bernstein L, Deka A, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol 2010;172:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008;31:661–666 [DOI] [PubMed] [Google Scholar]
- 4.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 2011;32:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A, Quagliaro L, Piconi L, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 2004;53:701–710 [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–2072 [DOI] [PubMed] [Google Scholar]
- 8.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 9.O’Keefe JH, Bell DSH. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 2007;100:899–904 [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Davidson J, Hanefeld M, et al. International Prandial Glucose Regulation Study Group Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis 2006;16:453–456 [DOI] [PubMed] [Google Scholar]
- 11.Esposito K, Giugliano D, Nappo F, Marfella R, Campanian Postprandial Hyperglycemia Study Group Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004;110:214–219 [DOI] [PubMed] [Google Scholar]
- 12.Hanefeld M, Fischer S, Schmechel H, et al. Diabetes Intervention Study. Multi-intervention trial in newly diagnosed NIDDM. Diabetes Care 1991;14:308–317 [DOI] [PubMed] [Google Scholar]
- 13.Snell-Bergeon JK, Roman R, Rodbard D, et al. Glycaemic variability is associated with coronary artery calcium in men with type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med 2010;27:1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimeno-Orna JA, Castro-Alonso FJ, Boned-Juliani B, Lou-Arnal LM. Fasting plasma glucose variability as a risk factor of retinopathy in Type 2 diabetic patients. J Diabetes Complications 2003;17:78–81 [DOI] [PubMed] [Google Scholar]
- 15.Shiraiwa T, Kaneto H, Miyatsuka T, et al. Post-prandial hyperglycemia is an important predictor of the incidence of diabetic microangiopathy in Japanese type 2 diabetic patients. Biochem Biophys Res Commun 2005;336:339–345 [DOI] [PubMed] [Google Scholar]
- 16.Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006;91:813–819 [DOI] [PubMed] [Google Scholar]
- 17.Muggeo M, Verlato G, Bonora E, Zoppini G, Corbellini M, de Marco R. Long-term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non-insulin-dependent diabetes mellitus: the Verona Diabetes Study. Circulation 1997;96:1750–1754 [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, Colagiuri S, Gerich J, Tuomilehto J, Guideline Development Group Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis 2008;18:S17–S33 [DOI] [PubMed] [Google Scholar]
- 19.Aldred HE, Hardman AE, Taylor S. Influence of 12 weeks of training by brisk walking on postprandial lipemia and insulinemia in sedentary middle-aged women. Metabolism 1995;44:390–397 [DOI] [PubMed] [Google Scholar]
- 20.Hughes VA, Fiatarone MA, Fielding RA, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol 1993;264:E855–E862 [DOI] [PubMed] [Google Scholar]
- 21.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996;335:1357–1362 [DOI] [PubMed] [Google Scholar]
- 22.Healy GN, Dunstan DW, Salmon J, et al. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 2007;30:1384–1389 [DOI] [PubMed] [Google Scholar]
- 23.Nygaard H, Tomten SE, Høstmark AT. Slow postmeal walking reduces postprandial glycemia in middle-aged women. Appl Physiol Nutr Metab 2009;34:1087–1092 [DOI] [PubMed] [Google Scholar]
- 24.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 2011;60:941–949 [DOI] [PubMed] [Google Scholar]
- 25.Achten J, Jeukendrup AE. Effects of pre-exercise ingestion of carbohydrate on glycaemic and insulinaemic responses during subsequent exercise at differing intensities. Eur J Appl Physiol 2003;88:466–471 [DOI] [PubMed] [Google Scholar]
- 26.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988;254:E248–E259 [DOI] [PubMed] [Google Scholar]
- 27.Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002;25:829–834 [DOI] [PubMed] [Google Scholar]
- 28.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381 [PubMed] [Google Scholar]
- 29.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–781 [DOI] [PubMed] [Google Scholar]
- 30.Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials 2009;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senn S. Cross-over Trials in Clinical Research. 2nd ed. Chichester, Wiley, 2002 [Google Scholar]
- 32.Ho SS, Dhaliwal SS, Hills A, Pal S. Acute exercise improves postprandial cardiovascular risk factors in overweight and obese individuals. Atherosclerosis 2011;214:178–184 [DOI] [PubMed] [Google Scholar]
- 33.Ceriello A, Assaloni R, Da Ros R, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation 2005;111:2518–2524 [DOI] [PubMed] [Google Scholar]
- 34.Haskell WL, Lee IM, Pate RR, et al. American College of Sports Medicine. American Heart Association Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1081–1093 [DOI] [PubMed] [Google Scholar]
- 35.Jennings G, Nelson L, Nestel P, et al. The effects of changes in physical activity on major cardiovascular risk factors, hemodynamics, sympathetic function, and glucose utilization in man: a controlled study of four levels of activity. Circulation 1986;73:30–40 [DOI] [PubMed] [Google Scholar]
- 36.Jans MP, Proper KI, Hildebrandt VH. Sedentary behavior in Dutch workers: differences between occupations and business sectors. Am J Prev Med 2007;33:450–454 [DOI] [PubMed] [Google Scholar]
- 37.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007;56:2655–2667 [DOI] [PubMed] [Google Scholar]
- 38.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am J Clin Nutr 2000;72:1451–1454 [DOI] [PubMed] [Google Scholar]
- 39.Jones-Smith JC, Gordon-Larsen P, Siddiqi A, Popkin BM. Cross-national comparisons of time trends in overweight inequality by socioeconomic status among women using repeated cross-sectional surveys from 37 developing countries, 1989-2007. Am J Epidemiol 2011;173:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev 2010;38:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]