Abstract
OBJECTIVE
Diabetes and hypertension often co-occur and share risk factors. Hypertension is known to predict diabetes. However, hyperglycemia also may be independently associated with future development of hypertension. We investigated glycated hemoglobin (HbA1c) as a predictor of incident hypertension.
RESEARCH DESIGN AND METHODS
We conducted a prospective analysis of 9,603 middle-aged participants in the Atherosclerosis Risk in Communities Study without hypertension at baseline. Using Cox proportional hazards models, we estimated the association between HbA1c at baseline and incident hypertension by two definitions 1) self-reported hypertension during a maximum of 18 years of follow-up and 2) measured blood pressure or hypertension medication use at clinic visits for a maximum of 9 years of follow-up.
RESULTS
We observed 4,800 self-reported and 1,670 visit-based hypertension cases among those without diagnosed diabetes at baseline. Among those with diagnosed diabetes at baseline, we observed 377 self-reported and 119 visit-based hypertension cases. Higher baseline HbA1c was associated with an increased risk of hypertension in subjects with and without diabetes. Compared with nondiabetic adults with HbA1c <5.7%, HbA1c in the prediabetic range (5.7–6.4%) was independently associated with incident self-reported hypertension (hazard ratio 1.14 [95% CI 1.06–1.23]) and visit-detected hypertension (1.17 [1.03–1.33]).
CONCLUSIONS
We observed that individuals with elevated HbA1c, even without a prior diabetes diagnosis, are at increased risk of hypertension. HbA1c is a known predictor of incident heart disease and stroke. Our results suggest that the association of HbA1c with cardiovascular risk may be partially mediated by the development of hypertension.
Glycated hemoglobin (HbA1c), a marker of chronic hyperglycemia, is the standard measure for monitoring glucose control in diabetic patients and recently was recommended for use in the diagnosis of diabetes (1,2). Elevated HbA1c strongly predicts the development of diabetes (3–6) and is independently associated with cardiovascular outcomes even in individuals without a diabetes diagnosis (6–9). One pathway by which hyperglycemia may contribute to cardiovascular disease risk is via the development of hypertension. For example, previous research demonstrates associations of hyperglycemia with endothelial dysfunction and vascular stiffness, both of which are linked to increased hypertension and cardiovascular disease risk (10–12). In addition, long-term (observational) follow-up of Diabetes Control and Complications Trial (DCCT) participants with type 1 diabetes demonstrated that intensive glucose control significantly reduced the risk of incident hypertension by nearly 25% (13,14), suggesting that strategies to improve glucose control might lower hypertension risk among people with diabetes.
Approximately 34% of U.S. adults aged ≥20 years are hypertensive, with an additional 25% living with prehypertension (15,16). Hypertension is strongly associated with diabetes and, if poorly controlled, greatly accelerates the progression of diabetes complications (17–20). Diabetes and hypertension frequently coexist. However, the directionality of this association has been inconsistent in the literature (17,19,20). Although diabetes and hypertension often co-occur and share risk factors, evidence that chronic hyperglycemia is associated with the development of hypertension is unclear.
This study aimed to quantify the association between HbA1c and incident hypertension in a community-based study of individuals with and without a previous history of diabetes. We hypothesized that chronic hyperglycemia would be associated with hypertension risk, even among individuals without diagnosed diabetes.
RESEARCH DESIGN AND METHODS
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based cohort study of 15,792 adults from four U.S. communities aged 45–64 years at enrollment during 1987–1989 (21). Participants completed three follow-up examinations, spaced ~3 years apart. Participants also were contacted annually via telephone. HbA1c was measured in samples collected at ARIC visit 2 (1990–1992) and, therefore, this visit served as the baseline examination for the current study. Details regarding the study population and data collection in the ARIC Study have been previously published (21).
Among the 14,348 ARIC Study participants who attended visit 2, we excluded individuals with prevalent diagnosed hypertension (n = 3,893), self-reported race/ethnicity other than black or white (n = 37), and those who were missing variables used in the analyses (n = 815); the final analytic sample for the primary analysis included 9,603 ARIC Study participants with a maximum follow-up time of 18 years. For secondary analyses, we additionally excluded individuals with prevalent undiagnosed hypertension at baseline (based on measured blood pressure). These analyses included 7,551 ARIC Study participants for up to 9 years of follow-up time (the time period was limited based on measured blood pressure data availability).
Measurement of HbA1c
We measured HbA1c in whole blood samples collected at ARIC visit 2 (1990–1992) using a high-performance liquid chromatography method (Tosoh 2.2 Plus in 2003–2004 and Tosoh G7 in 2007–2008; Tosoh, Tokyo, Japan), standardized to the DCCT assay (22). The measurement of HbA1c is standardized by the International Federation of Clinical Chemistry.
Incident hypertension
At each clinic visit, antihypertensive medication use was determined based on a medication inventory. In addition, systolic and diastolic blood pressures were obtained in the sitting position by certified technicians following a 5-min rest period using a random-zero sphygmomanometer (23). During visits 2 and 3, three readings were obtained; the second and third readings were averaged to obtain mean blood pressure values. During visit 4, two readings were obtained and were averaged to obtain mean blood pressure values. After the last follow-up visit (1997–1999), hypertension was assessed during annual telephone calls using the following two questions: “Since we last contacted you, has a doctor said you had high blood pressure?” and “Did you take any medications during the past two weeks for high blood pressure?”
We considered two definitions of incident hypertension in the current study: 1) self-reported hypertension based on medication use at the two follow-up visits or medication use/hypertension diagnosis during annual telephone calls for a maximum of 18 years of follow-up and 2) visit-based hypertension defined by an average systolic blood pressure of ≥140 mmHg, average diastolic blood pressure of ≥90 mmHg, or medication use for lowering blood pressure assessed at either of the two follow-up visits for a maximum of 9 years of follow-up.
Other variables of interest
Age, sex, race/ethnicity, smoking status, alcohol use, physical activity (measured with a modified version of the Baecke physical activity questionnaire) (24), and educational attainment were self-reported by participants at the clinic examination during a structured interview. Previous diabetes history was determined via self-report of a physician diagnosis or use of diabetes medications; for sensitivity analyses, incident cases of diabetes that met the definition of diagnosed diabetes after the baseline examination were considered. Diet was assessed using a 66-item semiquantitative food frequency questionnaire modified from the 61-item food frequency questionnaire by Willett et al. (25). The examination included measurement of height, weight, waist circumference, and hip circumference. BMI was calculated as weight in kilograms divided by the square of height in meters. Participants also were instructed to fast overnight and provided blood specimens for the measurement of glucose, insulin (at visit 1 only), lipids, and creatinine (21).
Statistical analysis
Baseline characteristics for the study population were calculated after stratifying by history of diagnosed diabetes and HbA1c clinical category at baseline; the study population was stratified by diabetes history status to address concerns regarding differential diagnosis of hypertension by diabetes status as well as the possibility of different risk relationships in patients with a clinical diagnosis of diabetes. Thus, those without diagnosed diabetes were separated into three groups (< 5.7%, which represents normal glycemia; 5.7–6.4%, which represented elevated risk for diabetes or prediabetes; and ≥6.5%, which represents at or above the diagnostic threshold for HbA1c); those with previously diagnosed diabetes were dichotomized at the recommended treatment goal (7%) (1). We used Cox proportional hazards regression models to obtain hazard ratios (HRs) and their 95% CIs for the association between baseline HbA1c (modeled as a continuous variable and in clinical categories) and incident self-reported and visit-based hypertension after adjustment for covariates. In analyses with HbA1c modeled according to clinical categories, participants without diabetes and with HbA1c <5.7% served as the reference group for the subgroup without diabetes; for those with diabetes, those with HbA1c <7% served as the reference group. The proportional hazards assumption was confirmed by examining interactions between follow-up time and HbA1c modeled both as a continuous and categorical variable (P > 0.05 for all interactions).
We considered three models for the association between HbA1c and incident hypertension. Model 1 was adjusted for age (years), sex (male or female), race/ethnicity (black or white), and clinic site (Forsyth County, NC; Jackson, MS; Minneapolis, MN; or Washington County, MD). Model 2 was additionally adjusted for smoking status (current, former, or never), physical activity level (sport index score), educational attainment (≤11, 12–16, ≥17 years), and log-transformed triglycerides. Finally, model 3 additionally included BMI and waist-to-hip ratio (WHR). Other variables that were tested as potential confounders but were removed from the final model because of null or nonsignificant effects on the main association of interest included alcohol intake, estimated glomerular filtration rate (eGFR), dietary factors (total caloric intake, sodium, potassium, saturated fat, and fiber), and fasting insulin. We also fitted restricted cubic splines to characterize the continuous association between HbA1c and incident hypertension; plots were centered at the median HbA1c value, the data were truncated to exclude extreme values, and knot locations were determined as described by Harrell (26).
We considered models both with and without adjustment for baseline blood pressure. In addition, we conducted sensitivity analyses excluding individuals with prevalent coronary heart disease, stroke, or congestive heart failure (n = 9,166, excluding 437 participants from the primary analytic sample). Among participants without a diagnosis of diabetes at baseline, we also conducted sensitivity analyses censoring cases of incident diabetes that developed during the follow-up period to determine whether the association between HbA1c and incident hypertension was mediated by the intervening development of diabetes. Finally, effect modification by race/ethnicity, sex, and age was evaluated. All analyses were performed using Stata Statistical Software (release 11.2; StataCorp LP, College Station, TX).
RESULTS
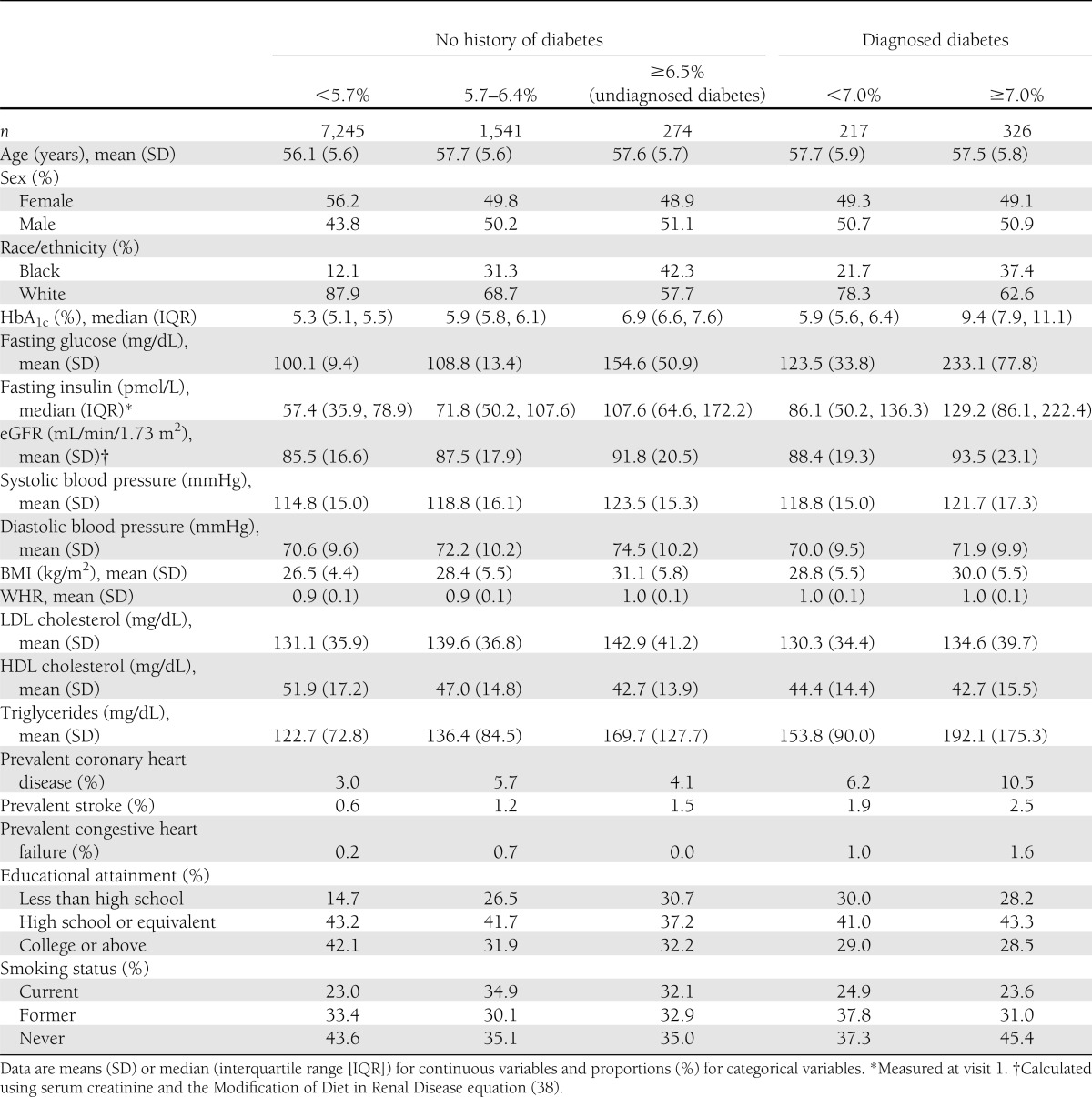
Baseline characteristics of the 9,603 participants (543 with a history of diabetes) are shown by diabetes status and HbA1c clinical category in Table 1. Subjects with elevated HbA1c had higher mean fasting glucose, fasting insulin, eGFR, blood pressure, BMI, LDL cholesterol levels, and triglycerides. The mean HbA1c for the study population was 5.6% (range 3.5–18.2).
Table 1.
Baseline (visit 2) participant characteristics, stratified by diabetes history status and HbA1c clinical category
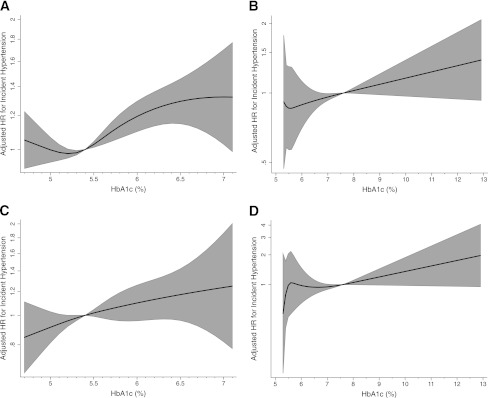
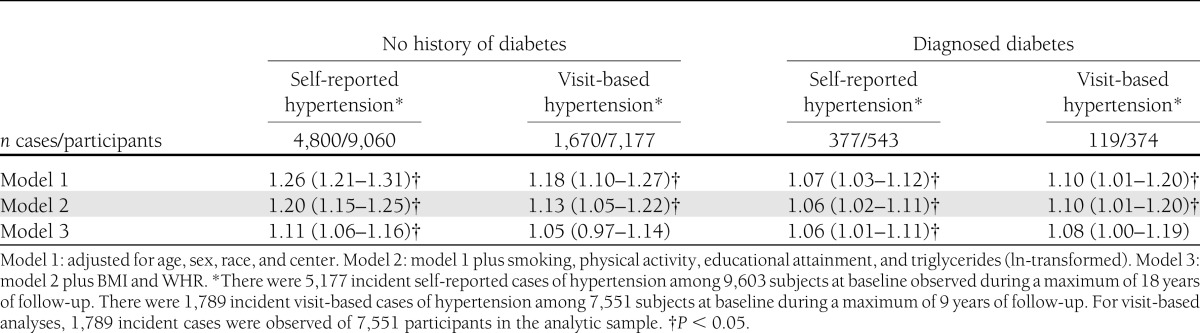
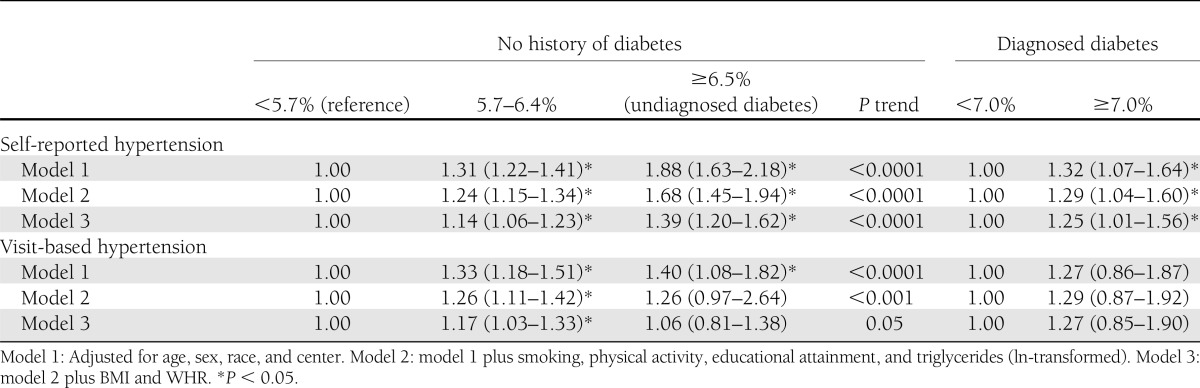
We observed 4,800 incident self-reported hypertension cases over a median follow-up time of 12 years in those without a history of diabetes (45 cases per 1,000 person-years) and 377 cases over a median follow-up time of 9 years in those with diagnosed diabetes (76 cases per 1,000 person-years). The continuous associations between HbA1c and incidence of self-reported hypertension are shown in Fig. 1. The HR per 1% point higher HbA1c in the fully adjusted model was 1.11 (95% CI 1.06–1.16) among those with no history of diabetes and 1.06 (1.01–1.11) among those with diagnosed diabetes; both HRs were statistically significant even after adjustment for BMI and WHR (Table 2). Hypertension risk was higher with increasing HbA1c category. The multivariable-adjusted HRs for those without diabetes were 1.14 (1.06–1.23) for individuals with HbA1c 5.7–6.4% and 1.39 (1.20–1.62) for individuals with HbA1c ≥6.5% compared with those with HbA1c in the normal range. Among those with prevalent diabetes at baseline, individuals with poorer glycemic control (HbA1c ≥7% compared with those with HbA1c <7%) were at 1.25 times higher risk (95% CI 1.01–1.56) for incident hypertension (Table 3).
Figure 1.
Fully adjusted HRs for incident self-reported hypertension among participants without (A) and with (B) prevalent diagnosed diabetes at baseline, as well as HRs for incident visit-based hypertension among participants without (C) and with (D) prevalent diagnosed diabetes at baseline using the restricted cubic spline model. *Adjusted for age, sex, race, center, smoking, physical activity, educational attainment, triglycerides (ln-transformed), BMI, and WHR. The top and bottom 1% values for HbA1c are truncated to exclude extreme values, and the model is centered at the median of the distribution (5.4% for participants without prevalent diabetes at baseline and 7.6% for participants with prevalent diabetes at baseline). HRs are plotted on the natural logarithm scale, and the grayed area represents the 95% CI.
Table 2.
HRs (95% CI) per 1% point of HbA1c for incident hypertension among ARIC Study participants, stratified by diabetes status at baseline
Table 3.
HRs (95% CI) for incident hypertension in participants with and without prevalent diabetes at baseline using HbA1c clinical categories
In the visit-based hypertension analysis, we observed 1,670 incident cases of hypertension among those without diabetes (43 cases/1,000 person-years), and 119 incident cases occurred among those with diagnosed diabetes (62 cases/1,000 person-years), roughly one-third of the total hypertension cases. Associations between HbA1c and incidence of visit-based hypertension are shown in the Fig. 1. HRs for incident visit-based hypertension by baseline HbA1c are presented in Tables 2 and 3. Both in individuals with and without history of diabetes, higher baseline HbA1c was significantly associated with the risk of hypertension, after adjustment for age, sex, race, center, smoking, physical activity, educational attainment, and triglycerides (model 2). HRs from model 2 were 1.13 (95% CI 1.05–1.22) per 1% higher HbA1c for those without diabetes history and 1.10 (1.01–1.20) for those with diagnosed diabetes (Table 2). However, this association was no longer significant after further adjustment for BMI and WHR (1.05 [0.97–1.14] in those without diabetes and 1.08 [1.00–1.19] in subjects with diagnosed diabetes). In the fully adjusted model with HbA1c in clinical categories (Table 3), only those individuals without diabetes in the prediabetic range for HbA1c (5.7–6.4%) were at significantly higher risk for incident hypertension (1.17 [1.03–1.33]) compared with those with HbA1c <5.7% (P for trend = 0.05). Individuals with diagnosed diabetes and with HbA1c ≥7% were not at significantly increased risk for incident hypertension compared with those with diagnosed diabetes and with HbA1c <7%.
Results were similar after additional adjustment for baseline blood pressure and kidney function (using eGFR) and remained significant for the self-reported hypertension outcome. In addition, results were similar but slightly weaker for the association between fasting glucose and incident hypertension (Supplementary Tables 1 and 2). Sensitivity analyses excluding participants with prevalent coronary heart disease, congestive heart failure, and stroke at baseline did not materially alter the association between HbA1c and incident hypertension. Censoring incident cases of diabetes that occurred prior to incident hypertension also did not materially alter the results. There were no significant interactions with race/ethnicity or sex in the association of HbA1c with incident hypertension, although the association was attenuated among older participants (results not shown).
CONCLUSIONS
In this large community-based prospective study, higher HbA1c values at baseline were associated with increased risk of hypertension during long-term follow-up. This association was independent of known risk factors and was observed for HbA1c values even below the diagnostic threshold for diabetes. Poor glycemic control also was associated with increased risk of hypertension among those with diagnosed diabetes. The associations were similar for both of the hypertension definitions used, although our findings were more robust for the self-reported hypertension outcome as compared with the visit-based definition, perhaps because of increased statistical power (5,177 self-reported vs. 1,789 visit-based hypertension cases). Adjustment for measures of adiposity strongly attenuated the observed association, suggesting that obesity may partially explain the higher risk of hypertension among individuals with hyperglycemia. Nonetheless, a significant effect of elevated HbA1c on hypertension risk remained even after adjustment for multiple risk factors and two measures of adiposity.
Few prospective studies have examined the association between HbA1c and subsequent hypertension risk. However, previous studies have reported positive associations between hyperglycemia and blood pressure levels (27–30), although some of these associations were weak or not statistically significant (31). In addition, previous studies have shown a more favorable blood pressure profile among individuals with diabetes receiving glucose-lowering treatment (19,20). Our findings are of similar magnitude to results from the Women’s Health Study reported by Britton et al. (27), which reported an association between HbA1c and risk of self-reported hypertension that was similarly attenuated after adjustment for BMI. However, the Women’s Health Study relied on self-report only and did not have information on measured blood pressure to identify undiagnosed cases of hypertension.
The association between hyperglycemia and hypertension may reflect the presence of shared risk factors, particularly adiposity. In addition, inflammatory processes have been implicated in both the development of hyperglycemia and hypertension (32). However, several potential mechanisms may explain why higher HbA1c values predict the development of hypertension. Because hyperglycemia leads to high glucose flux across endothelial cell membranes (33–35), the resulting increased oxidative stress reduces the bioavailability of nitric oxide and contributes to endothelial dysfunction that may subsequently alter blood pressure (11). Furthermore, previous evidence suggests that hypertension risk may be elevated in those with higher levels of circulating glucose via increased systemic vascular resistance and stiffness (10,12). Excess circulating glucose also may bind proteins, lipids, and nucleic acids, resulting in the formation and accumulation of advanced glycation end products (36,37); accumulation of advanced glycation end products in the vessel wall may contribute to inflammation, oxidative stress, and endothelial dysfunction.
There are certain limitations of this study that should be considered in the interpretation of the results. Only a single measurement of HbA1c at baseline was available, which may have resulted in misclassification. Future analyses with additional measurements of HbA1c may reveal stronger associations with incident hypertension. Furthermore, despite rigorous measurement of cardiovascular risk factors, we cannot rule out the possibility of residual confounding in this observational study. In particular, we observed that the association was attenuated after adjustment for BMI and waist circumference; it is possible that adjusting for these measures did not fully account for potential confounding by adiposity. In addition, although we excluded individuals with prevalent hypertension at baseline to establish the directionality of the association between hyperglycemia and hypertension, the causal relationship remains unclear. Finally, some analyses may have had limited power to detect small to moderate effects (e.g., we had only 119 incident visit-based hypertension cases among those with diagnosed diabetes at baseline). Nonetheless, our study benefited from the large sample size and both measured blood pressure and self-reported information to identify cases of hypertension during a long duration of follow-up.
In summary, we found a positive association between elevated HbA1c at baseline and incident hypertension, suggesting that hyperglycemia may play a role in the development of hypertension even in individuals without a prior history of diabetes. In particular, HbA1c in the prediabetic range and HbA1c ≥7.0% among those with diagnosed diabetes (i.e., those with poorer glucose control) were independently associated with incident hypertension. Combined with evidence demonstrating that HbA1c is a marker of long-term cardiovascular risk, our results suggest that individuals with elevated HbA1c, even in the absence of diabetes, are at increased risk for hypertension and should be targeted for cardiovascular risk factor management and hypertension prevention strategies.
Acknowledgments
This research was supported by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R21-DK-080294. J.K.B. was also supported by NIH/National Heart, Lung, and Blood Institute (NHLBI) grant T32 HL007024, and E.S. was supported by NIH/NIDDK grant K01-DK-076595. The ARIC Study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
No potential conflicts of interest relevant to this article were reported.
L.J.A., K.M., J.H.Y., A.A., and E.S. contributed to the analysis plan and interpretation of the results and reviewed and edited the manuscript. F.L.B. contributed to the interpretation of the results and reviewed and edited the manuscript. J.K.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 2012 Scientific Sessions of the American Heart Association, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, San Diego, California, 13–16 March 2012.
The authors thank the staff and participants of the ARIC Study for their important contributions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2248/-/DC1.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva, World Health Org., 2011. (Report no. WHO/NMH/CHP/CPM/11.1) [PubMed] [Google Scholar]
- 3.Heianza Y, Hara S, Arase Y, et al. HbA1c 5·7–6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011;378:147–155 [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Matsumoto M, Akimoto K. Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabet Med 2008;25:1157–1163 [DOI] [PubMed] [Google Scholar]
- 5.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med 2007;120:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams RJ, Appleton SL, Hill CL, et al. Independent association of HbA(1c) and incident cardiovascular disease in people without diabetes. Obesity (Silver Spring) 2009;17:559–563 [DOI] [PubMed] [Google Scholar]
- 8.Sarwar N, Aspelund T, Eiriksdottir G, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med 2010;7:e1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–431 [DOI] [PubMed] [Google Scholar]
- 10.Beevers G, Lip GYH, O’Brien E. ABC of hypertension: the pathophysiology of hypertension. BMJ 2001;322:912–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deedwania PC, Fonseca VA. Diabetes, prediabetes, and cardiovascular risk: shifting the paradigm. Am J Med 2005;118:939–947 [see comment] [DOI] [PubMed] [Google Scholar]
- 12.McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab 2001;86:713–718 [DOI] [PubMed] [Google Scholar]
- 13.de Boer IH, Kestenbaum B, Rue TC, et al. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 2008;168:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–2050 [DOI] [PubMed] [Google Scholar]
- 16.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004;44:398–404 [DOI] [PubMed] [Google Scholar]
- 17.Barret-Connor E, Criqui MH, Klauber MR, Holdbrook M. Diabetes and hypertension in a community of older adults. Am J Epidemiol 1981;113:276–284 [DOI] [PubMed] [Google Scholar]
- 18.Bell DSH. Treatment of diabetic hypertension. Diabetes Obes Metab 2009;11:433–444 [DOI] [PubMed] [Google Scholar]
- 19.Imperatore G, Cadwell BL, Geiss L, et al. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971–2000. Am J Epidemiol 2004;160:531–539 [DOI] [PubMed] [Google Scholar]
- 20.Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation 2009;120:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 22.Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care 2011;34:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atherosclerosis Risk in Communities Coordinating Center Operations Manual no. 11: Sitting Blood Pressures. Chapel Hill, NC, University of North Carolina School of Public Health, 1990 [Google Scholar]
- 24.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Berlin, Germany, Springer Verlag, 2001 [Google Scholar]
- 27.Britton KA, Pradhan AD, Gaziano JM, et al. Hemoglobin A1c, body mass index, and the risk of hypertension in women. Am J Hypertens 2011;24:328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagot-Campagna A, Balkau B, Simon D, Ducimetière P, Eschwège E. Is insulin an independent risk factor for hypertension? The Paris Prospective Study. Int J Epidemiol 1997;26:542–550 [DOI] [PubMed] [Google Scholar]
- 29.He J, Klag MJ, Caballero B, Appel LJ, Charleston J, Whelton PK. Plasma insulin levels and incidence of hypertension in African Americans and whites. Arch Intern Med 1999;159:498–503 [DOI] [PubMed] [Google Scholar]
- 30.Liese AD, Mayer-Davis EJ, Chambless LE, et al. Atherosclerosis Risk in Communities Study Investigators Elevated fasting insulin predicts incident hypertension: the ARIC study. J Hypertens 1999;17:1169–1177 [DOI] [PubMed] [Google Scholar]
- 31.Vaccaro O, Imperatore G, Iovino V, Iovine C, Rivellese AA, Riccardi G. Does impaired glucose tolerance predict hypertension? A prospective analysis. Diabetologia 1996;39:70–76 [DOI] [PubMed] [Google Scholar]
- 32.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–384 [DOI] [PubMed] [Google Scholar]
- 33.Cosentino F, Lüscher TF. Effects of blood pressure and glucose on endothelial function. Curr Hypertens Rep 2001;3:79–88 [DOI] [PubMed] [Google Scholar]
- 34.Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. BMJ 2006;333:475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790 [DOI] [PubMed] [Google Scholar]
- 36.Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 2008;93:1143–1152 [DOI] [PubMed] [Google Scholar]
- 37.Monnier VM, Sell DR, Genuth S. Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci 2005;1043:567–581 [DOI] [PubMed] [Google Scholar]
- 38.Levey A, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 2000;11:155A [Google Scholar]