Abstract
OBJECTIVE
To investigate the effect of flexible intensive insulin therapy (FIIT) and an automated bolus calculator (ABC) in a Danish type 1 diabetes population treated with multiple daily injections. Furthermore, to test the feasibility of teaching FIIT in a 3-h structured course.
RESEARCH DESIGN AND METHODS
The BolusCal Study was a 16-week randomized, controlled, open-label, three-arm parallel, clinical study of 51 adults with type 1 diabetes. Patients aged 18–65 years in poor metabolic control (HbA1c 8.0–10.5%) were randomized to the Control (n = 8), CarbCount (n = 21), or CarbCountABC (n = 22) arm. During a 3-h group teaching, the Control arm received FIIT education excluding carbohydrate counting. CarbCount patients were taught FIIT and how to count carbohydrates. CarbCountABC group teaching included FIIT and carbohydrate counting and patients were provided with an ABC.
RESULTS
At 16 weeks, the within-group change in HbA1c was −0.1% (95% CI −1.0 to 0.7%; P = 0.730) in the Control arm, −0.8% (−1.3 to −0.3%; P = 0.002) in the CarbCount arm, and −0.7% (−1.0 to −0.4%; P < 0.0001) in the CarbCountABC arm. The difference in change in HbA1c between CarbCount and CarbCountABC was insignificant. Adjusting for baseline HbA1c in a regression model, the relative change in HbA1c was −0.6% (−1.2 to 0.1%; P = 0.082) in CarbCount and −0.8% (−1.4 to −0.1%; P = 0.017) in CarbCountABC. Treatment satisfaction measured by the Diabetes Treatment Satisfaction Questionnaire (status version) improved in all study arms, but the improvement was significantly greater in CarbCountABC.
CONCLUSIONS
FIIT and carbohydrate counting were successfully taught in 3 h and improved metabolic control and treatment satisfaction. Concurrent use of an ABC improved treatment satisfaction further.
The importance of near normalization of blood glucose (BG) in preventing microvascular long-term complications of diabetes was demonstrated in the Diabetes Control and Complications Trial (1). In the trial, strict glycemic control was achieved by flexible intensive insulin therapy (FIIT) with multiple daily insulin injections adjusted according to BG, carbohydrate intake, and exercise to mimic insulin secretion in healthy individuals. The therapy was closely supervised by the health care team and, although effective, the therapy was caregiver-dependent and resource demanding. For >3 decades, a patient-empowering approach to FIIT has been practiced in Germany (2). The concept includes a 5-day structured program in which patients are taught to practice FIIT and to handle minor metabolic derangements themselves. Over the years, the approach has been adapted in several other countries (3–7). The DAFNE (Dose Adjustment for Normal Eating) Study Group in the U.K. performed the first randomized study testing the efficacy of the approach and documented significant improvements in glycemic control and diabetes-related quality of life (3). The group recently has reported sustained benefit of the teaching program after 44 months (8).
In Denmark, national guidelines published in 2010 recommend FIIT, including the use of carbohydrate counting to all patients with type 1 diabetes (9). This is in line with American Diabetes Association guidelines (10). In many clinics, however, it is yet to be implemented. It is our experience that insulin pump patients adapt well to FIIT, whereas few multiple daily injection (MDI)-treated patients do. This could rely on the different techniques used for bolus estimation by the two groups. The pump users have the advantage of bolus calculator functions integrated in their insulin pump, whereas MDI-treated patients are left with mental calculations or more simple algorithms (11–14). The calculations can be somewhat complicated, especially when the premeal BG is out of the desired range.
A BG meter with an integrated bolus calculator function (Accu-Chek Aviva Expert; Roche Diagnostics, Mannheim, Germany) recently was launched, offering MDI-treated patients the same advantages as pump patients. We hypothesized that a Danish type 1 diabetic population in poor metabolic control would achieve better metabolic control, treatment satisfaction, and quality of life from FIIT and that the benefits could be further improved with the concurrent use of the Accu-Chek Aviva Expert automated bolus calculator (ABC). The BolusCal Study was a pilot study testing these hypotheses and the feasibility of teaching the principles of FIIT during a 3-h structured course.
RESEARCH DESIGN AND METHODS
The BolusCal Study was a prospective, randomized, controlled, open-label, three-arm parallel, bicentric clinical study of 51 adults with type 1 diabetes. Eligibility criteria were age 18–65 years, type 1 diabetes duration ≥12 months, and use of MDI therapy with long- and rapid-acting insulin analog. Pregnancy, nursing, gastroparesis, and present or former practice of carbohydrate counting were the criteria for exclusion.
We identified all patients fulfilling the eligibility criteria from medical records from the diabetes outpatient clinics at two hospitals in the capital area of Denmark, Hvidovre University Hospital and Bispebjerg University Hospital. All study activities took place at Hvidovre University Hospital and were led by members of a group of diabetes health care professionals based at this site.
Patients provided written informed consent. The study was approved by the Danish Data Protection Agency, performed according to the principles of the Declaration of Helsinki, and registered at clinicaltrial.gov (NCT01223547). We presented the study for the Regional Committee on Biomedical Research Ethics (H-3-2010-140), but the committee categorized the study as “product development,” which is not subject to ethical approval.
Randomization
Allocation was done by randomization with a 1:3:3 ratio in blocks of 14 with sealed, opaque envelopes containing the group assignments. The envelopes had been prepared by a person not otherwise involved in the study, and the patients themselves drew the envelopes. After randomization but before the intervention was given, we optimized patients’ long-acting insulin dose based on 3-day diabetes worksheets.
Intervention
All patients included in the study participated in a structured 3-h group teaching delivered by a registered diabetes nurse and a dietitian. The curriculum covered different aspects of diabetes: food recommendations, self-monitoring of BG techniques, insulin profiles, and appropriate management of hypo- and hyperglycemia in general and in relation to stress, infections, menstrual periods, alcohol intake, and exercise. Emphasis was put on empowering patients to make rational day-to-day insulin dosage adjustment. Patients in the control arm (Control) were taught the principles of a healthful diabetes diet, but they were not trained in estimating the carbohydrate content of foods. In addition to the curriculum described above, patients in the first intervention arm (CarbCount) were also taught carbohydrate counting, and individual insulin-to-carbohydrate ratios (ICRs) and insulin sensitivity factors (ISFs) were estimated using the 500 and 1800 rule, respectively (15). Carbohydrate counting education included both theory and practical exercises. The patients in the second intervention arm (CarbCountABC) attended the 3-h group teaching, were taught carbohydrate counting, estimated individual ICRs and ISFs, and, furthermore, were provided with and instructed in the use of the ABC. In all three study arms, the group teaching lasted for 3 h. To compensate for the substantially differing information load between the control and the intervention arms, we used longer breaks and stimulated patients to share personal experiences during the former. One Control arm group teaching, three CarbCount arm group teachings, and three CarbCountABC arm group teachings were held from November 2010 to March 2011.
After the group teaching, patients in the Control arm continued estimating their premeal insulin doses and correction boluses in an empirical manner. In practice, this meant that patients continued following the meal dose recommendations provided by their physician but adjusted the dose, typically by adding or subtracting 2 units, if the premeal BG was out of target range or if the meal differed in size from their average meal. Patients in the CarbCount and CarbCountABC arms implemented the new, systematic approach to insulin dose estimation (i.e., FIIT). Some used kitchen scales for carbohydrate counting, but the majority quickly learned to estimate the carbohydrate content by eye. Methods for insulin dose estimation applied by the patients in the CarbCount arms included mental calculations, use of pen and paper, and the simple calculator function of their mobile phones. All patients were encouraged to perform a minimum of four BG tests per day. Test strip costs were covered by the national health care system.
The intervention also included a 1-h follow-up session and two 15-min telephone consultations. At 2 weeks after the group teaching, patients in all three study arms had an individual 1-h follow-up consultation with the nurse and the dietitian. Patients brought diabetes worksheets from at least 3 days, and on the basis of these, patients were given suggestions for optimization of long- and rapid-acting insulin dosage. The two intervention arms were also given advice regarding ICRs, ISFs, and carbohydrate counting. Another two consultations were performed via telephone by a diabetes specialist (K.N. and B.G.-R.) at 4 and 10 weeks, and the patients attended an end-of-study consultation at 16 weeks (Supplementary Fig. 1).
The ABC
The Accu-Chek Aviva Expert is a palm-sized integrated BG meter and bolus calculator device similar to the meter of the Accu-Chek Combo insulin pump from the same manufacturer. On the basis of the patient’s current BG, target BG, ICR, ISF, insulin on board, time of day, amount of carbohydrates to be consumed, and exercise level, it gives insulin bolus advice. The device has memory function and different graphic display possibilities for the stored data. The device has recently been launched in a series of European countries, but at the time of the study, it had not yet been launched in Denmark. Consequently, the device language had to be set to English because Danish was not an option.
Outcome measures
The primary outcome of the study was change in HbA1c. Secondary outcomes included the following: change in treatment satisfaction and perceived frequency of hyper- and hypoglycemia measured by the Diabetes Treatment Satisfaction Questionnaires (DTSQs [status version] and DTSQc [change version]) (16,17), change in average weighted impact of diabetes on quality of life and present quality of life measured by the Audit of Diabetes-Dependent Quality of Life questionnaire (ADDQoL) (18), change in the perception of problem areas in diabetes using the Problem Areas in Diabetes questionnaire (PAID) (19), change in fear of hypoglycemia using the Hypoglycemia Fear Survey 13-item worry subscale (HFS) (20), change in distribution of BG values estimated by blinded continuous glucose monitoring (CGM) (iPro2; Medtronic, Northridge, CA), change in total daily insulin dose (TDD), and change in weight. These outcome measures were made at baseline and again at 16 weeks. The DTSQs was also administered at the follow-up consultation 2 weeks after the group teaching. In addition, at the end of the study, patients reported the total number of severe hypoglycemic events during the 16 weeks, and the two intervention arms reported self-estimated use of the principles of FIIT.
Sample size determination and statistical analysis
We expected to find a clinically important difference in HbA1c of 0.8% between the Control and the CarbCount arms and a difference of 1.2% between the Control and the CarbCountABC arms. We wanted to compare the three arms using the ANOVA test as well as investigate the within-group change in HbA1c using the paired Student t test and the difference in change in HbA1c between the CarbCount and the CarbCountABC arms using the Student t test. On the basis of these assumptions, a 0.67% SD, and a two-sided 5% significance level, the study power would be 80% with a total of 57 patients evaluated—7 in the control arm and 25 in each of the intervention arms. We decided not to limit the number of participants but to invite all patients fulfilling the eligibility criteria to attend the study.
In the evaluation of secondary outcomes, we compared all three groups using parametric and nonparametric ANOVA, repeated-measures mixed model in the analysis of DTSQs, and χ2 test in the analysis of number of hypoglycemic events. Again, for the within-group comparison, we used the paired Student t test. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
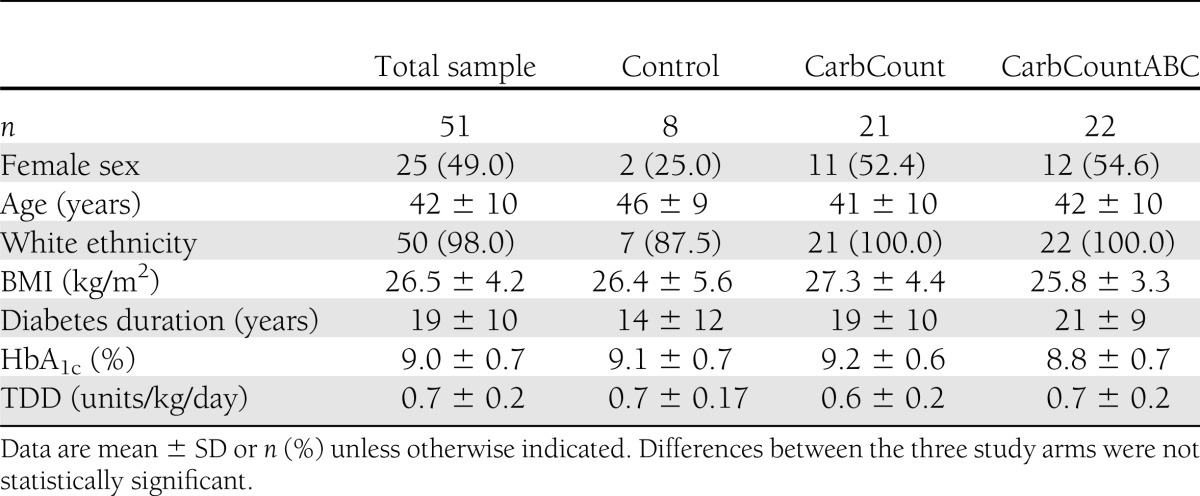
The flow diagram of the study is presented in Supplementary Fig. 1. An electronic medical record database search identified 164 eligible patients, of which 63 were willing to participate and fulfilled the inclusion criteria. These 63 were randomized; 12 patients (19%) dropped out or were excluded and 51 completed the study. Baseline characteristics of the randomized patient sample did not differ significantly between the three study arms. The baseline characteristics of the patient sample that completed the study are presented in Table 1. Differences between groups in this sample also were not significant. Here we present the results of analyses of the 51 patients who completed the study.
Table 1.
Baseline characteristics of patients who completed the study
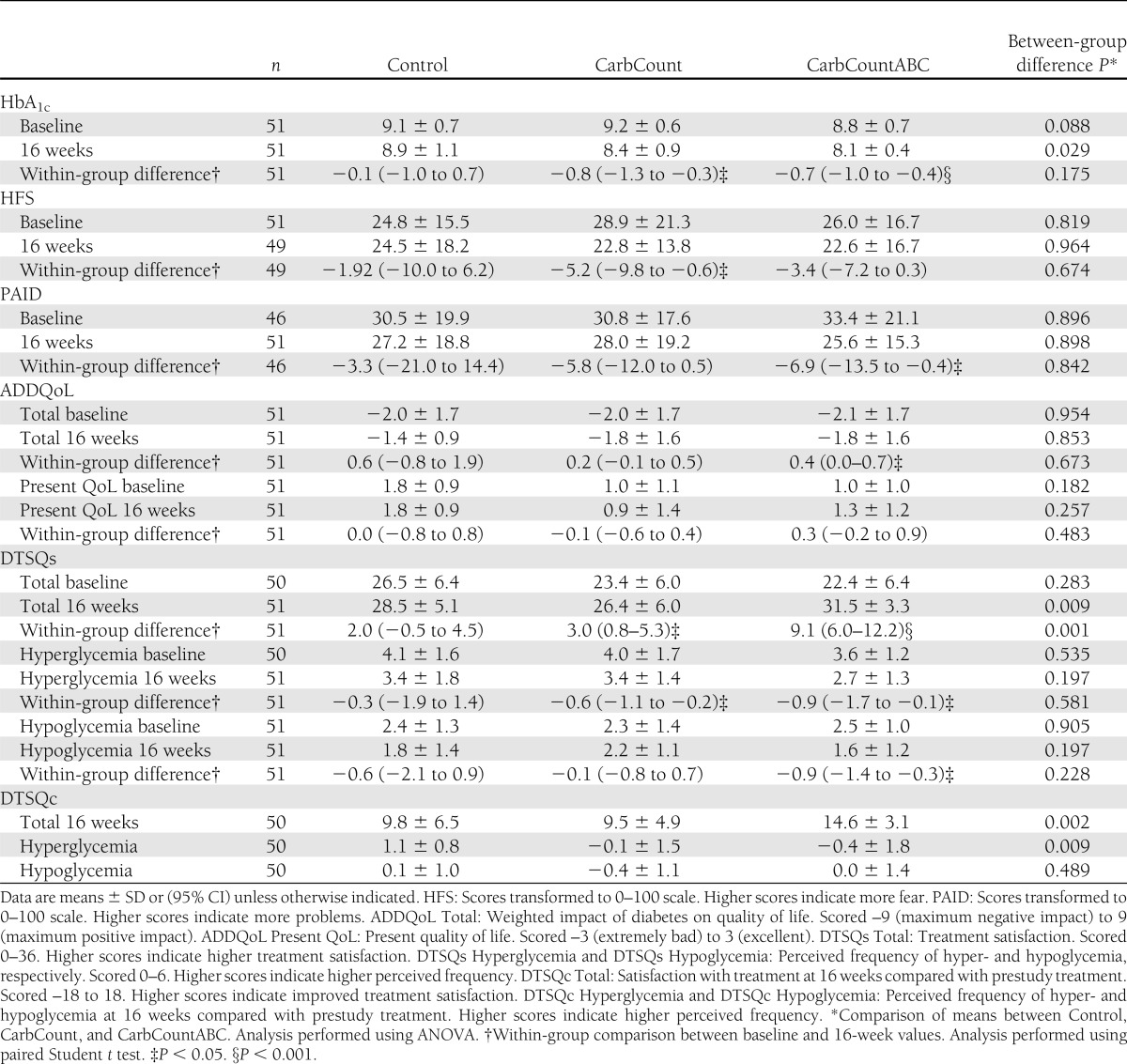
The primary outcome, change in HbA1c, was statistically significant within both intervention arms (paired Student t test) (Table 2). When we compared the change in HbA1c between the two intervention arms (Student t test), there was no significant difference (0.1% [95% CI −0.6 to 0.4]; P = 0.756), and there was no significant difference when we compared the change in HbA1c between all three study arms (ANOVA) (Table 2). The most substantial decrease in HbA1c occurred in the patients with the highest baseline HbA1c values (r = −0.6; P < 0.0001). When we included the baseline HbA1c values in a regression model, the difference in change in HbA1c between the three groups was borderline significant (P = 0.056), but the relative differences between groups altered to a −0.6% (−1.2 to 0.1; P = 0.082) change in HbA1c in the CarbCount arm and a −0.8% (−1.4 to −0.1; P = 0.017) change in HbA1c in the CarbCountABC arm compared with the Control arm (reference group).
Table 2.
Results for HbA1c and questionnaires
One person in the CarbCount arm presented a change in HbA1c of −4.3% (from 10.3 to 6.0%). This outlier data point had a large impact on the results because of the relatively small sample size. In a secondary analysis of the primary outcome, we excluded this patient. The resulting mean change in HbA1c in the CarbCount arm was −0.6 ± 0.6%. The difference in change in HbA1c between the three study arms and the difference in change in HbA1c between CarbCount and CarbCountABC were still statistically insignificant after exclusion of the outlier (P = 0.134 and P = 0.649, respectively). However, when we included the baseline HbA1c in a regression model, the relative difference between the three study arms was significant (P = 0.027), with a change in HbA1c of −0.4% (95% CI −1.0 to 0.1) in the CarbCount arm and −0.7% (−1.2 to −0.2) in the CarbCountABC arm compared with the Control arm (reference group). The difference in change in HbA1c between the two intervention arms (outlier excluded and adjusted for baseline HbA1c) was 0.3% in favor of CarbCountABC (−0.0 to 0.7; P = 0.075).
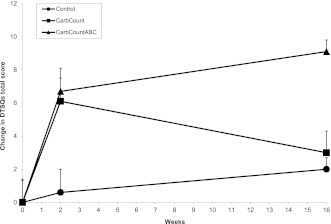
The results of the HFS, PAID, ADDQoL, and DTSQs and the changes in scores from baseline to 16 weeks are given in Table 2. Figure 1 depicts the changes in DTSQs total scores from baseline to 2 weeks and from 2 to 16 weeks. At baseline and 2 weeks, there were no significant differences between DTSQs total scores in the three study arms (P = 0.283 and P = 0.789) or between the two intervention arms (P = 0.602 and P = 0.964), but at 16 weeks, the differences were highly significant (P = 0.009 and P = 0.002). The DTSQc was markedly more responsive than the DTSQs. The difference in DTSQc scores was also highly significant (P = 0.001) when we compared the three study arms.
Figure 1.
Changes in DTSQs scores. The change in DTSQs score over time was significantly different between the three study arms (P = 0.008) and between the two intervention arms, CarbCount and CarbCountABC (P = 0.004).
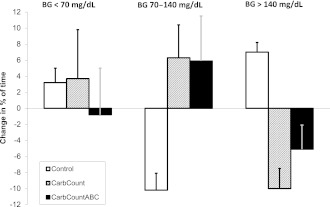
The change in distribution of BG values measured by CGM is illustrated in Fig. 2. All changes observed in the CarbCountABC arm favored better glycemic control. The opposite was the case in the Control arm. In the CarbCount arm, the percentage of time spent >140 mg/dL was reduced, but time spent <70 mg/dL was increased. The change in average BG was 16, −9, and −13 mg/dL in the Control, CarbCount, and CarbCountABC arm, respectively. None of the changes in the distribution of BG values measured by CGM reached the significance level in any of the study arms.
Figure 2.
Change in distribution of glucose values from baseline to 16 weeks measured by blinded CGM.
One person in the control arm and two persons in each of the intervention arms experienced one episode of severe hypoglycemia where help was needed from another person during the 16-week study period. The difference between study arms in number of self-reported episodes of hypoglycemia was not significant. The average weight change in the study arms was <1 kilo, and the average change in the TDD was also small, 0.01 ± 0.07, −0.03 ± 0.11, and −0.03 ± 0.15 units/kg/day in the Control, CarbCount, and CarbCountABC arm, respectively, but with large individual differences. The distribution between long- and rapid-acting insulin was unchanged from baseline to 16 weeks.
The average self-reported usage of the principles of FIIT in bolus estimation was 70 ± 27% of the time in the CarbCount arm and 89 ± 14% in the CarbCountABC arm (P = 0.010).
CONCLUSIONS
The BolusCal Study is the first randomized, controlled study investigating the effect of a new ABC in poorly controlled patients with type 1 diabetes. Furthermore, this is the first report on successful communication of the principles of FIIT during a structured group teaching only 3 h in length. The main findings of this pilot study were a clinically relevant and statistically significant change in HbA1c in the two intervention arms and a statistically significant improvement in treatment satisfaction in the intervention arms, most pronounced in the CarbCountABC arm. All study outcomes improved from baseline to 16 weeks in the CarbCountABC arm, though not all changes were significant. In the CarbCount arm, significant and insignificant improvements were also observed except in the distribution of BG values and “present quality of life.” In the Control arm, minor improvements and deteriorations were observed, none of which were significant.
The original German 5-day teaching program was organized as an inpatient course (2), the DAFNE group converted to a 5-day outpatient program (3), and a Swiss group has implemented a concept of seven weekly evening sessions (21). The duration of the group teaching in our study contrasts with these resource-demanding programs. We chose to test the feasibility of teaching FIIT in only 3 h for two reasons: First, if the course proved effective, it could be directly implemented in most diabetes clinics in Denmark. Second, we assumed that patients who are in poor metabolic control despite MDI therapy are less motivated about their diabetes treatment and would be reluctant to attend a time-consuming course conflicting with their work and other social commitments.
The mean decrease in HbA1c in the Control arm was 0.1%, whereas a clinically relevant decrease of 0.8 and 0.7% was observed in the CarbCount arm and the CarbCountABC arm, respectively. This demonstrates that FIIT, as shown in both intervention arms, is an effective regimen to improve glycemic control. All patients, irrespective of study arm, followed the same study course and, thus, we conclude that the improvements in HbA1c observed in the intervention arms were not simply a result of increased health care provider attention.
The baseline HbA1c values were not significantly different between study arms, but the average baseline value in the CarbCountABC arm was 0.4% lower than in the CarbCount arm. To estimate the impact of this difference, we adjusted for HbA1c baseline values in a regression model. The result favored the CarbCountABC arm that showed the most pronounced relative change in HbA1c (−0.8% [95% CI −1.4 to −0.1]) followed by the CarbCount arm (−0.6% [−1.2 to 0.1]). The model assumes a linear relation between baseline HbA1c and change in HbA1c; however, it is well known that it is easier to obtain decreases in HbA1c with a higher baseline value. Had this curvilinear relation been included in the model, the relative change in the CarbCountABC arm with the lowest baseline value would have been even further pronounced.
We had expected to see a significant difference in HbA1c between the two intervention arms, but this was not the case. However, with regard to diabetes treatment satisfaction, the intervention arms differed. It is interesting that a similar increase in satisfaction was observed at 2 weeks, but at 16 weeks, the satisfaction score in the CarbCount arm had decreased again, whereas a further increase in satisfaction was observed in the CarbCountABC arm. One explanation for this phenomenon, though speculative, could be an initial excitement in both intervention arms after the introduction of a new treatment regimen, which later proved to be too complicated for patients using mental calculations but functional for patients using the ABC. The difference in self-reported usage of FIIT supports this explanation.
Although there were significant changes in HFS, PAID, and ADDQoL scores from baseline to 16 weeks within study arms, there were no differences when comparing the changes in the three study arms. The improvements may be attributed to the group teaching and follow-up program common to all patients in the study.
The combined use of FIIT and the ABC in the CarbCountABC arm led to an improvement in the distribution of BG values as documented by CGM. More time was spent in the glycemic target range (70–140 mg/dL) and less time in hypo- and hyperglycemia. In the CarbCount arm, time in target was also increased, but while time above target decreased, time in hypoglycemia increased. Although a blinded CGM was used, some could argue that recordings were biased because the patients were aware of the monitoring and therefore behaved differently during the CGM periods. However, if this was the case, the bias was likely to be the same at baseline and 16 weeks, and looking at changes in variability, we eliminated the potential bias.
We registered only minor, insignificant decreases in the average TDD, and the distribution between long-acting and rapid-acting insulin did not change. The reduction in the TDD in the study arms that experienced a significant reduction in HbA1c is in line with a previous report from the DAFNE group (22). The need for less insulin could rely on more appropriate insulin dosing and less correction of hyperglycemic deteriorations.
When we conducted the study, the ABC had not yet been launched in Denmark. Accordingly, the device menus and the user manual were not available in Danish, and the health care team did not have long-term experience with the device. We introduced the patients to the bolus estimation functions, but the ABC also has a variety of options for displaying stored data. Some of the patients quickly discovered these options themselves and used them for evaluation and motivation, while others used only the basic calculation functions. All these factors could have affected the outcome in the CarbCountABC arm.
Nonpharmacological interventions in general and diabetes self-management programs in particular are often complex, consisting of various interconnected components (motivation and competence of the health care team and organizational and financial conditions) on which the outcome is dependent (23). To compensate for these components, we included a control arm in our study design, which is a strength of our study compared with, for example, the DAFNE Study (3). Because of the nature of the intervention, it was impossible to blind the study. As in all open-label randomized studies, the research team could, unintentionally, have been more enthusiastic in the treatment of the intervention arms, but being aware of this risk, we did our utmost not to differentiate our enthusiasm. We have described the structured teaching program in detail, and another clinic should easily be able to conduct the course in a similar manner. Nevertheless, the outcome of the study is dependent on the expertise and motivation of the BolusCal Study Group, and this might affect transferability of the results.
We included in the study patients in poor metabolic control (HbA1c 8.0–10.5%). Many of them had a history of frequent missed appointments at the diabetes clinic and none of them had previously been engaged in adjusting their own insulin therapy. This patient group is notoriously difficult to treat, and despite significant HbA1c decreases in both intervention arms, only one patient had an HbA1c <7.0% at 16 weeks. Nevertheless, the sustained high treatment satisfaction observed in the CarbCountABC arm provides hope for continued treatment adherence. The study observation period was relatively short, and further improvements could be obtained from long-term practice of FIIT and use of the ABC. Patients who are already in good metabolic control might not obtain improvements in HbA1c from FIIT and use of the ABC, but treatment satisfaction could still possibly be enhanced. It should be noted, however, that the patients included in the study were willing to change their treatment regimen and to invest a little extra time in their diabetes care and they may differ from the patients who declined to participate.
BolusCal was a pilot study and the sample size was small. The number of eligible patients proved smaller than anticipated and the drop-out rate larger, and as a result, fewer patients than intended completed the study. Nevertheless, the study provides support for the benefits of FIIT and carbohydrate counting in a Danish type 1 diabetic population in poor metabolic control. The principles of FIIT were successfully communicated to patients in a structured 3-h course. In addition, the study gives indications of increased treatment satisfaction and adherence with the concurrent use of an ABC. A larger, long-term study will be needed to further explore the benefits of use of ABCs in combination with MDI therapy, and in such a study, the structured BolusCal teaching program could be used.
Acknowledgments
The BolusCal Study was an investigator-initiated, -designed, and -performed study. The study was a DiaCon Study Group project funded by the Danish Strategic Research Council (DiaCon, NABIIT 2106-07-0034).
The BolusCal Study received further funding from Novo Nordisk, Scandinavia. Home glucose meters and bolus calculators were kindly provided to the study by Roche, Denmark. Medtronic, Denmark, kindly provided glucose sensors and lent out iPro2s. K.N., M.M., and C.S. have received speaker honorariums from Roche. K.N. has received payment for development of educational presentations from Roche. No other potential conflicts of interest relevant to this article were reported.
S.S. identified eligible patients, conducted the study, contributed to discussion, researched data, and wrote and approved the manuscript. M.M., N.S., C.S., and B.G.-R. conducted the study, contributed to discussion, and approved the manuscript. T.M.C. identified eligible patients, conducted the study, contributed to discussion, and approved the manuscript. K.N. acquired funding, conducted the study, contributed to discussion, and reviewed and approved the manuscript. S.S. and K.N. are the guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 5th International Conference on Advanced Technologies & Treatments for Diabetes, Barcelona, Spain, 8–11 February 2012.
The authors recognize the efforts of the patients and thank them for their participation. The authors also thank Clare Bradley, Royal Holloway University of London, for granting us permission to use the DTSQs, DTSQc, and ADDQoL questionnaires; Linda Gonder-Frederick, University of Virginia, for granting us permission to use the HFS questionnaire; and Katie Weinger, Joslin Diabetes Center, for granting us permission to use the PAID questionnaire.
Footnotes
Clinical trial reg. no. NCT01223547, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2044/-/DC1.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Mühlhauser I, Jörgens V, Berger M, et al. Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia 1983;25:470–476 [DOI] [PubMed] [Google Scholar]
- 3.DAFNE Study Group Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemozy-Cadroy S, Crognier S, Gourdy P, et al. Intensified treatment of type 1 diabetes: prospective evaluation at one year of a therapeutic patient education programme. Diabetes Metab 2002;28:287–294 [PubMed] [Google Scholar]
- 5.McIntyre HD, Knight BA, Harvey DM, Noud MN, Hagger VL, Gilshenan KS. Dose adjustment for normal eating (DAFNE) - an audit of outcomes in Australia. Med J Aust 2010;192:637–640 [DOI] [PubMed] [Google Scholar]
- 6.Mühlhauser I, Bruckner I, Berger M, et al. Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin-dependent) diabetes. The Bucharest-Düsseldorf Study. Diabetologia 1987;30:681–690 [DOI] [PubMed] [Google Scholar]
- 7.Plank J, Köhler G, Rakovac I, et al. Long-term evaluation of a structured outpatient education programme for intensified insulin therapy in patients with type 1 diabetes: a 12-year follow-up. Diabetologia 2004;47:1370–1375 [DOI] [PubMed] [Google Scholar]
- 8.Speight J, Amiel SA, Bradley C, et al. Long-term biomedical and psychosocial outcomes following DAFNE (Dose Adjustment For Normal Eating) structured education to promote intensive insulin therapy in adults with sub-optimally controlled type 1 diabetes. Diabetes Res Clin Pract 2010;89:22–29 [DOI] [PubMed] [Google Scholar]
- 9.Type 1 DM 2010 [guideline online], 2010. Available from http://www.endocrinology.dk/index.php/des2-4/links Accessed 6 October 2011
- 10.American Diabetes Association Executive summary: standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg SK, Bookout TR, McFann KK, et al. Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technol Ther 2008;10:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser NS, Iden SB, Green-Burgeson D, et al. Benefits of an insulin dosage calculation device for adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2004;17:1641–1651 [DOI] [PubMed] [Google Scholar]
- 13.Maurizi AR, Lauria A, Maggi D, et al. A novel insulin unit calculator for the management of type 1 diabetes. Diabetes Technol Ther 2011;13:425–428 [DOI] [PubMed] [Google Scholar]
- 14.Rossi MC, Nicolucci A, Pellegrini F, et al. Interactive diary for diabetes: A useful and easy-to-use new telemedicine system to support the decision-making process in type 1 diabetes. Diabetes Technol Ther 2009;11:19–24 [DOI] [PubMed] [Google Scholar]
- 15.Walsh PA, Roberts R. Pumping Insulin. San Diego, CA, Torrey Pines Press, 2006 [Google Scholar]
- 16.Bradley C. The Diabetes Treatment Satisfaction Questionnarie: DTSQ. In Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Bradley C, Ed. Chur, Switzerland, Harwood Academic Publishers, 1994, p. 111–132 [Google Scholar]
- 17.Bradley C. Diabetes Treatment Satisfaction Questionnaire. Change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care 1999;22:530–532 [DOI] [PubMed] [Google Scholar]
- 18.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res 1999;8:79–91 [DOI] [PubMed] [Google Scholar]
- 19.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 [DOI] [PubMed] [Google Scholar]
- 20.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 21.Bendik CF, Keller U, Moriconi N, et al. Training in flexible intensive insulin therapy improves quality of life, decreases the risk of hypoglycaemia and ameliorates poor metabolic control in patients with type 1 diabetes. Diabetes Res Clin Pract 2009;83:327–333 [DOI] [PubMed] [Google Scholar]
- 22.Leelarathna L, Ward C, Davenport K, et al. Reduced insulin requirements during participation in the DAFNE (dose adjustment for normal eating) structured education programme. Diabetes Res Clin Pract 2011;92:e34–e36 [DOI] [PubMed] [Google Scholar]
- 23.Mühlhauser I, Berger M. Patient education - evaluation of a complex intervention. Diabetologia 2002;45:1723–1733 [DOI] [PubMed] [Google Scholar]