Abstract
OBJECTIVE
The benefits of real-time continuous glucose monitoring (CGM) have been demonstrated in patients with type 1 diabetes. Our aim was to compare the effect of two modes of use of CGM, patient led or physician driven, for 1 year in subjects with poorly controlled type 1 diabetes.
RESEARCH DESIGN AND METHODS
Patients with type 1 diabetes aged 8–60 years with HbA1c ≥8% were randomly assigned to three groups (1:1:1). Outcomes for glucose control were assessed at 1 year for two modes of CGM (group 1: patient led; group 2: physician driven) versus conventional self-monitoring of blood glucose (group 3: control).
RESULTS
A total of 257 subjects with type 1 diabetes underwent screening. Of these, 197 were randomized, with 178 patients completing the study (age: 36 ± 14 years; HbA1c: 8.9 ± 0.9%). HbA1c improved similarly in both CGM groups and was reduced compared with the control group (group 1 vs. group 3: −0.52%, P = 0.0006; group 2 vs. group 3: −0.47%, P = 0.0008; groups 1 + 2 vs. group 3: −0.50%, P < 0.0001). The incidence of hypoglycemia was similar in the three groups. Patient SF-36 questionnaire physical health score improved in both experimental CGM groups (P = 0.004). Sensor consumption was 34% lower in group 2 than in group 1 (median [Q1–Q3] consumption: group 1: 3.42/month [2.20–3.91] vs. group 2: 2.25/month [1.27–2.99], P = 0.001).
CONCLUSIONS
Both patient-led and physician-driven CGM provide similar long-term improvement in glucose control in patients with poorly controlled type 1 diabetes, but the physician-driven CGM mode used fewer sensors.
Since the report of the results of the Diabetes Control and Complications Trial (1), so-called intensified insulin therapy has been considered the gold standard for patients with type 1 diabetes, together with frequent self-monitoring of blood glucose (SMBG), patient education about diabetes management, and close follow-up and support by the attending health care team. This intensified insulin therapy corresponds to insulin regimens involving multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). The availability of insulin analogs has enabled further improvements in the safety and efficacy of basal-bolus insulin therapy (2). However, a significant subset of patients with type 1 diabetes still fails to reach optimal HbA1c levels and/or experiences debilitating BG variability and recurrent hypoglycemia (3,4). The availability of devices for real-time continuous glucose monitoring (CGM) recently has aroused considerable interest among patients and physicians, who anticipated that such devices might provide potential benefits in terms of BG control. Indeed, several randomized controlled studies demonstrate that use of CGM could improve HbA1c levels in patients with type 1 diabetes with no increased risk of hypoglycemia (5–7). However, most of the reported studies were short-term (3 to 6 months) and included close monitoring by the investigators, thus precluding any conclusions about expected outcomes in common clinical practice and in the long-term. While compliance with CGM therapy was identified as a strong predictor of HbA1c reduction, the role of specific patient education in the use of CGM data to adjust insulin therapy and the contribution of patient empowerment have not been widely examined. Hence, it is still not known whether patient-led or physician-driven CGM use and management have different outcomes. Answering such questions would be of great value, considering the additional cost of CGM use. We therefore designed this randomized multicenter controlled study to assess in poorly controlled patients with type 1 diabetes the outcomes on glucose control and quality of life (QoL) of two CGM approaches following a similar initial specific education procedure: patient self-management with CGM or physician-prescribed use of CGM.
RESEARCH DESIGN AND METHODS
Patients were recruited at 19 diabetes care centers. Inclusion criteria included age between 8 and 60 years, type 1 diabetes diagnosed >12 months earlier, treatment by insulin analogs using either CSII or MDI, HbA1c level ≥8.0%, and SMBG performed at least twice daily. Before inclusion, the patients were instructed in the technical use of the study CGM device and were required to wear it during a 10-day test period to confirm their ability and willingness to use CGM. The trial was approved by the ethics committee of the Paris VI Medical Faculty. All patients (or the parents of minors) had read the patient information sheet and signed informed consent forms before enrolment (Clinical trial reg. no. NCT00726440).
Study protocol
Randomization.
Patients meeting the inclusion criteria were randomly assigned 1:1:1 to one of the three groups: patient-led use of CGM (group 1), physician-prescribed CGM (group 2), or conventional SMBG (group 3 [control group]) for 12 months.
In group 1, use of the CGM device was managed entirely by the patients themselves. Patients were advised to use CGM continuously throughout the study, as they would with a glucose meter, especially if glucose targets were not achieved. In group 2, use of the CGM device was prescribed by the patient’s physician, who asked the patient to use the sensors intermittently according to guidelines based on glucose outcomes: 2 weeks’ sensor use per month during the first 3 months, thereafter continuing either in the same manner or with more intensive use during the following 3 months if at any visit the patient presented one of the following criteria: HbA1c ≥7.5%, greater than four mild hypoglycemic episodes per week, or at least one severe hypoglycemic episode. Thus, use of the sensors could be gradually increased every 3 months to 20, 25, or even 30 days/month. In the control group (group 3), patients were asked to carry out standard home SMBG. Abbott Diabetes Care provided the Navigator CGM systems and their consumables, as well as the home glucose meters and test strips used for the study.
Training.
At the time of inclusion, all patients received intensive education about target glucose values, insulin dose management, and insulin-to-carbohydrate ratios and correction factors, and they were asked to perform SMBG at least three times daily. Patients randomized to groups 1 and 2 were provided with the FreeStyle Navigator glucose needle-type sensor system for 1 year (Abbott Diabetes Care, Alameda, CA) (8). These patients received specific training in how to analyze and make use of the CGM data and to confirm glucose values using the meter included in the Navigator device before making therapeutic decisions (see Supplementary Appendices 1–3). The patients’ skills were assessed quarterly during the study by means of a short questionnaire comprising six questions: Is the patient able to 1) identify fasting and premeal glucose levels, 2) interpret these glucose results and adapt the insulin doses, 3) identify postmeal glucose levels, 4) interpret these glucose results and adapt the insulin doses, 5) identify low glucose levels during the night, and 6) interpret the nocturnal glucose levels and adapt the basal insulin doses?
Visits.
Visits were scheduled 20 days after randomization and at 3, 6, 9, and 12 months. Patients were asked to perform an 8-point BG profile in the week preceding each visit. At each of these visits, HbA1c was measured and adverse events were reported. Adverse events collected included mild hypoglycemia (defined as an SMBG value <70 mg/dL or symptoms of low BG) during the preceding week, severe hypoglycemia (defined as an event requiring assistance from another person), ketoacidosis, unexpected study- or device-related events, and any serious adverse event, regardless of cause, since the previous visit. In both CGM groups, the glucose data were downloaded from the device memory and the amount of actually used sensors was recorded. Insulin dose adjustment was discussed with the patient on the basis of CGM graphs and according to individual glucose targets. Further attention was paid to specific circumstances, such as physical activity or unusual meals with high carbohydrate content. Physicians evaluated the patients’ skill in managing insulin doses using the six-item questionnaire. Training was considered optimal if the six items were answered positively at each visit throughout the entire study and nonoptimal if at least one item was not answered positively at any visit. Alarm settings, sensor insertion, and calibration techniques were checked. QoL was assessed at baseline and at the end of the study using the Diabetes Quality of Life (DQoL) (9) and SF-36 questionnaires (9–11).
Evaluation criteria
The main aim of this study was to assess the impact on BG control of 1 year of CGM using two approaches to sensor use (patient led or physician driven) comparatively with current standard SMBG in patients with poorly controlled type 1 diabetes. The primary end point was the reduction in HbA1c at 12 months versus baseline. A 0.5% change in HbA1c value was considered clinically meaningful. On the basis of a single-sided two-sample t test with a 95% CI, a sample size of 50 patients per group was required to ensure statistical power of 80% to demonstrate a 0.5% difference between the groups regarding absolute change in HbA1c between baseline and 12 months. A total of 180 patients had to be randomized to achieve an evaluable dataset of 150 patients while allowing for a 15% drop-out rate per group and equal distribution of patients between centers. Because of the nature of the treatment, the study was not blinded.
The secondary objectives were evaluation of changes in glucose variability, frequency of mild and/or severe hypoglycemic events, changes in QoL, efficacy of CGM in patients treated by CSII and MDI, and efficacy of CGM in optimally educated patients versus nonoptimally educated patients. Secondary end points included SD of BG calculated from the 8-point BG profiles, number of mild and severe hypoglycemic events, number of ketoacidosis episodes, change in QoL scores from baseline to 12 months, and daily insulin requirements. Finally, the relationship between compliance (i.e., mean number of sensors used per month) and ΔHbA1c was evaluated in each study group.
Statistical analysis
The full analysis set (FAS) was defined as all randomized patients having at least one postbaseline HbA1c available. Missing HbA1c values during follow-up were replaced by the mean of the previous and following measurements where available (2.5% of HbA1c measurements). Missing HbA1c values at the end of follow-up were replaced using the last observation carried forward method (9.7% of HbA1c measurements). The level of significance was α = 0.05. For comparison of CGM-use groups versus the control group (groups 1 + 2 vs. 3, group 1 vs. 3, and group 2 vs. 3), a superiority hypothesis was tested (one-sided situation). For comparison of group 1 versus group 2, a difference hypothesis was tested (two-sided situation). Repeated assessments over time were analyzed by means of a mixed model with repeated measures (ANCOVA), using an autoregressive correlation structure taking treatment groups, visits, and their interaction as fixed factors and baseline values and age as covariates. Changes from baseline were analyzed using ANCOVA, taking baseline values and age as covariates. Binary criteria assessing HbA1c at 12 months (HbA1c level <7.5%), changes in HbA1c at the end of follow-up (a relative decrease >10% between baseline and 12 months), and severe hypoglycemia episodes during follow-up were compared between groups using logistic regression with adjustment of two covariates (baseline values and age). Sensor consumption was well compared using a Wilcoxon rank sum test between groups 1 and 2.
RESULTS
Patients
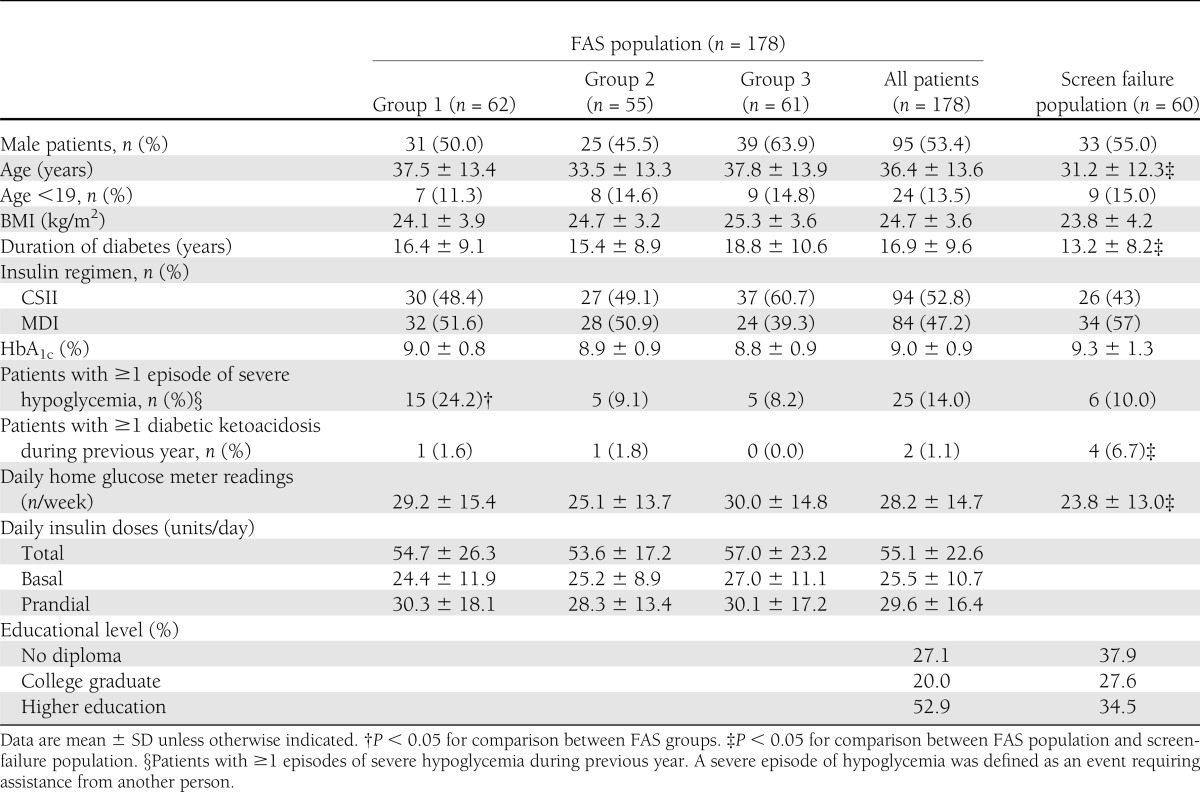
From May 2008 to June 2009, 257 patients with type 1 diabetes were screened for inclusion in the study. After the 10-day test period using CGM, 197 patients fulfilled the inclusion criteria and were randomly assigned. An additional 19 patients were excluded from the study analysis owing to lack of any HbA1c results between baseline and the end of the study. The FAS population thus comprised 178 patients. Compared with the FAS population, patients failing the screening test (n = 60) were younger, had diabetes of shorter duration, had presented more ketoacidosis events, and had lower levels of education (Table 1). Using the study randomization procedure, the FAS population included 62 patients in group 1, 55 in group 2, and 61 in group 3. The patients’ baseline characteristics were comparable between the three groups except for a higher number of patients with one or more episodes of severe hypoglycemia during the previous year in group 1 (Table 1). The per-protocol population excluded 3 FAS patients on account of major protocol deviations: switch from MDI to CSII in 2 participants and pregnancy in 1 participant.
Table 1.
Characteristics of patients with type 1 diabetes screened for inclusion in the study
HbA1c levels and insulin doses
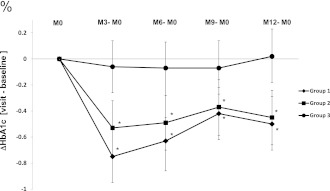
The reduction in HbA1c from baseline to 12 months was significantly greater in group 1 (−0.50% [95% CI −0.70 to −0.29]) and group 2 (−0.45% [−0.66 to −0.24]) than in group 3 (0.02% [−0.18 to 0.23]; group 1 vs. 3: P = 0.0006; group 2 vs. 3: P = 0.0018) (Fig. 1). This result was also observed when the results of both CGM groups were combined: ΔHbA1c groups 1 + 2 (−0.48% [−0.63 to −0.33]) versus group 3: P < 0.0001. The decrease in HbA1c was similar in groups 1 and 2 (group 1 vs. group 2: –0.05% [−0.34 to 0.25]; P = 0.7644). These results were reported from 3 months and continued throughout the study. More patients in groups 1 and 2 had a relative HbA1c reduction >10% versus baseline (29.0, 25.5, and 11.5% in groups 1, 2, and 3, respectively; group 1 vs. group 3: P = 0.044; group 2 vs. group 3: P = 0.036), and more patients achieved the HbA1c target of <7.5% (9.7, 14.6, and 1.6% in groups 1, 2, and 3, respectively; group 1 vs. group 3: P = 0.025; group 2 vs. group 3: P = 0.026). In both CGM groups, patients on CSII had a more pronounced reduction in HbA1c by the end of the study than patients on MDI compared with the control group. In patients on CSII, the intergroup HbA1c was −0.67% (−1.01 to −0.33; P = 0.0001) for groups 1 + 2 (n = 60) versus group 3 (n = 24); in patients on MDI, the intergroup HbA1c was only −0.28% (−0.67 to 0.10; NS) for groups 1 + 2 (n = 56) versus group 3 (n = 37). Changes in basal or prandial insulin doses ranged from −2 to 2 units/day during the study period, not reaching significance.
Figure 1.
HbA1c change from baseline (M0) at 3, 6, 9, and 12 months in groups 1, 2, and 3. Values are means (95% CIs). The data correspond well to the estimated mean and CI of the model. *P < 0.05 for comparisons between the two experimental groups and the control group at each time point. M, month.
Glucose stability, hypoglycemia, and ketoacidosis
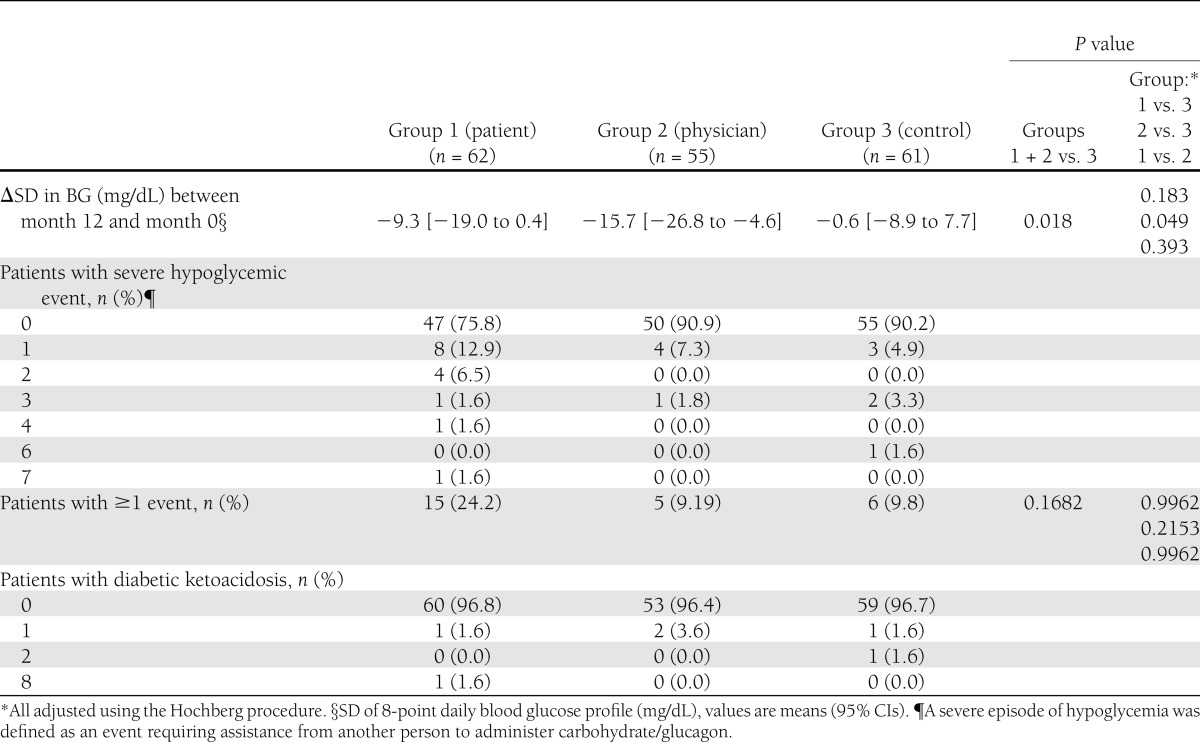
At 1 year, glycemic stability significantly improved in group 2 (Table 2). The frequency of severe hypoglycemia episodes did not differ among groups after adjustment for both age and the presence of at least one severe hypoglycemia episode during the year before inclusion. However, a nonsignificant increase was observed in group 1, mainly with regard to one patient who experienced seven episodes of severe hypoglycemia. Ketoacidosis events were infrequent in all groups. In patients on CSII, the frequency of severe and mild hypoglycemia was similar in the CGM groups compared with the control group.
Table 2.
Glycemic stability, hypoglycemia, and diabetic ketoacidosis
Sensor prescription and real use
In group 1, actual sensor use was 3.42 per month (median [Q1–Q3] [2.20–3.91]), that is, 57 ± 20% of the prescribed time. No correlation between sensor use and HbA1c level was noted in this group (P = 0.117, R2 = 0.0449). In group 2, the number of sensors prescribed per month was as follows: at baseline, three sensors for all patients; at 3 months, three sensors for 13% and four sensors for 87% of patients; at 6 months, three sensors for 7%, four sensors for 56%, and five sensors for 37% of patients; and at 9 months, three sensors for 2%, four sensors for 45%, five sensors for 21%, and six sensors for 32% of patients. In this group, sensor use was 2.25 per month (1.27–2.99), that is, 65 ± 29% of the prescribed time. Compliance in terms of sensor use was negatively correlated with HbA1c level (P = 0.026, R2 = 0.1050). Total sensor consumption during 1 year was significantly lower (34%) in group 2 than in group 1 (P = 0.001).
From baseline to the end of the study, SMBG per week significantly decreased in both CGM groups versus the control group (−9 ± 12 vs. 1 ± 12, P < 0.0001).
Training
In groups 1 and 2, 47.6% of patients had received optimal training, as assessed by the six-item questionnaire. These optimally trained patients exhibited greater improvement in HbA1c than the others. This difference remained significant after adjustment for compliance with CGM use (ΔHbA1c: −0.71 ± 0.81 vs. −0.30 ± 0.81, P = 0.033).
QoL
At 1 year, a significant improvement in the physical component summary of the SF-36 questionnaire was reported in groups 1 and 2 in comparison with group 3 (groups 1 + 2: 1.47 ± 6.52; group 3: −2.48 ± 6.52; P = 0.0042). The mental component summary did not differ significantly between the groups (groups 1 + 2: 0.65 ± 10.55; group 3: −1.03 ± 10.62; NS), and neither did the global DQoL score. However, patient satisfaction, assessed by one scale of the DQoL, improved significantly in groups 1 and 2 (groups 1 + 2: 2.83 ± 12.61 vs. group 3: −2.12 ± 12.61; P = 0.0447).
CONCLUSIONS
The aim of this randomized multicenter study was to compare the efficacy of 1 year of use of CGM with that of standard SMBG. Moreover, it assessed the performance of two approaches in CGM use, patient led and physician driven. After 1 year, there was a significant difference in HbA1c of 0.5% between the control group and the other two groups using CGM. Furthermore, after 1 year, the decrease in HbA1c was similar in both the patient-led and physician-driven groups. The 0.5% decrease in HbA1c observed with CGM compared with traditional SMBG was similar to that reported in previous studies assessing the efficacy of CGM in patients with suboptimally controlled type 1 diabetes (5–7,12). Maximum reduction in HbA1c was achieved at 3 months (−0.75 and −0.53% in groups 1 and 2, respectively), probably as a result of a study effect consisting of first access to an innovative and appealing technology followed by only slight impairment in glucose control thereafter. However, significant improvement in glucose control was maintained for 1 year through CGM, a result already observed in another study (13). The conditions of the only study with the same duration reported thus far (the Sensor-Augmented Pump Therapy for A1C Reduction Study 3 [STAR-3]) were different since it compared the effectiveness of an insulin pump combined with CGM versus that of insulin injections combined with SMBG (12). Of note, the authors of that study found the same degree of efficacy. It is interesting that metabolic efficacy with CGM was identical in both the patient- and physician-driven groups, although sensor use by patients in the physician-driven group was 34% lower. In addition, compliance with CGM use was greater in the physician-driven group, with patients adhering more closely to the prescribed time of CGM use (57 and 65% of the prescribed times in the patient- and physician-driven groups, respectively). The correlation between CGM use and HbA1c level was significant only in the physician-driven group. Glucose stability was also better in these patients than in the patients from the control group, a finding that tallies with the results observed in several other studies (7,13–15). The step-by-step approach to prescription of sensor use seems to be more economical than patient-led prescription. In the physician-driven group, the quantity of sensors prescribed depended on individual metabolic outcomes at each visit. Finally, at the end of the study, half of the patients in this group required CGM for 15 to 20 days/month, with the remainder needing to use CGM for 25 to 30 days/month. CGM requirements thus vary widely from one patient to another. A number of previous studies suggest that the devices must be worn as much as possible to produce any significant benefit (6,12). However, sensors are relatively cumbersome, invasive, and expensive. Our observations suggest that the initial prescription of CGM 15 days/month, followed by an incremental use if needed, may be more agreeable and, finally, cost effective.
In the patient-driven group, a nonsignificant trend toward higher incidence of severe hypoglycemia events was noted in comparison with the control group. Other randomized studies assessing the efficacy of CGM found no increase in the frequency of severe hypoglycemic events with CGM, except for the STAR 1 study (16). Some studies even note an improvement in glycemic stability (7,13,14). An explanation of this difference is that one subject in the patient-driven group presented seven events during the study. Furthermore, at baseline, patients in this group had already experienced more frequent episodes of severe hypoglycemia. These two observations could account for the trend noted. Comparison of the frequency of severe hypoglycemia between groups was made after adjustment for baseline values, including severe hypoglycemic events, and, consequently, there is no significant difference in severe hypoglycemia rates between the groups.
Our study underscores the value of a test period during which patients are invited to use the sensors before starting long-term CGM to evaluate their technical skills and their motivation to use CGM, as has been previously suggested (17). Patients unwilling to use the CGM device on a long-term basis after a 10-day test period were younger, less compliant with SMBG, and probably less likely to use CGM than those finally included in the study. Thus, such a test period could be useful to ensure selection of patients motivated to use CGM.
Our study also shows that CGM is more effective in patients treated with CSII. This result is consistent with the fact that when using an insulin pump, a patient can more easily adjust insulin delivery in real time according to both CGM values and trends. However, since “extra boluses” were not recorded during the study, it is difficult to determine clearly whether patients were acting in response to CGM data or to alarms.
CGM is also of greater benefit to patients trained to adjust their insulin doses according to CGM data, whence the importance of structured training to help patients translate CGM technology into an efficient self-management tool, as suggested by others (18,19).
The pediatric population was composed of 24 subjects aged <19 years. Because of the small size of this subgroup, it was not possible to draw any specific conclusions about the pediatric population; however, major differences did not show up.
Although long-term CGM might possibly increase the overall burden of diabetes management, patient QoL in this study was not impaired after 1 year, and an improvement was in fact seen in both the physical component summary of the SF-36 questionnaire and the satisfaction component of the DQoL questionnaire. As suggested by others, use of CGM may thus potentially produce positive psychological and behavioral effects (18,20).
One limitation of our study concerns the inability to compare glucose profiles between the control group and the study groups, since masked CGM was not used in the control group.
In summary, this study demonstrates that use of CGM significantly improves both HbA1c level and glucose stability in the long-term compared with conventional SMBG in patients with poorly controlled type 1 diabetes. This benefit is particularly evident in patients on insulin pump therapy. In addition, patient satisfaction was improved in a study that lasted 1 year. The uniqueness of this study is in assessing two approaches in the use of CGM (patient led or physician driven) with step-by-step physician-driven sensor use. We report similar metabolic efficacy with both approaches. Since the physician-driven group used 34% fewer sensors, this strategy appears cost effective.
Acknowledgments
This study was supported by the Association Française des Diabétiques and the Leon Fredericq Foundation of the University of Liège (for the Belgian part of this trial).
FreeStyle Navigators, home glucose meters, and test strips used for the study were provided by Abbott Diabetes Care. J.-P.R. participates in advisory boards and as a consultant for Abbott Diabetes Care, LifeScan, sanofi-aventis, and Eli Lilly and has received honoraria and payment for presentations, travel, and accommodation expenses from Abbott Diabetes Care and Novo Nordisk. E.R. is a consultant for Abbott Diabetes Care and has received honoraria and payment for the development of educational presentations, travel, and accommodation expenses from Abbott Diabetes Care. A.P. is a member of the boards of Boehringer Ingelheim, Merck-Serono, Novartis, Novo Nordisk, and sanofi-aventis; has received payment for the development of educational presentations including speakers' office services from Abbott, Merck Sharp & Dohme, Novartis, Novo Nordisk, and sanofi-aventis; and has had travel and accommodation expenses covered or reimbursed by Abbott, Boehringer Ingelheim, Merck Sharp & Dohme, and sanofi-aventis. N.T.-R. is a member of a scientific advisory panel. P.-Y.B. participates in advisory boards and as a consultant for LifeScan, sanofi-aventis, and Eli Lilly and has received fees for speaking in symposia organized by Abbott Diabetes Care, Medtronic, Eli Lilly, Roche Diagnostics, and Novo Nordisk. H.H. participates in advisory boards and as a consultant for Abbott Diabetes Care, Medtronic, sanofi-aventis, Eli Lilly, and Novo Nordisk and has received fees for speaking in symposia organized by Abbott Diabetes Care, Medtronic, Eli Lilly, and LifeScan. No other potential conflicts of interest relevant to this article were reported.
Abbott Diabetes Care was not involved in study design, data collection, or analysis.
J.-P.R., G.C., and H.H. contributed to funding and to the conception and design of the study; acquired, analyzed, and interpreted data; and wrote, reviewed, and edited the manuscript. P.S. contributed patients with diabetes and clinical data and wrote the manuscript. L.C., E.R., A.S.-G., A.P., N.T.-R., V.S., B.C., C.L., R.P.R., C.T., F.M., P.-Y.B., B.G., A.-M.L., L.M., and C.S. contributed patients with diabetes and clinical data and reviewed the manuscript. J.-P.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and at the 47th Annual Meeting of the European Association for the Study of Diabetes, Portugal, Lisbon, 12–16 September 2011.
Study coordination, monitoring visits, data management, and data analysis were performed independently by CERITD, a nonprofit clinical translational research center located in Corbeil Hospital (Corbeil-Essonnes, France). The authors would like to thank Frederic Mistretta, MSC, and Didier Not of Randomized Clinical Trials, Lyon, France, for their assistance with statistical analysis, as well as Lydie Canipel and Caroline Peschard of CERITD for their involvement in the study management. The authors are very grateful to the patients for their participation in the study.
Footnotes
Clinical trial reg. no. NCT00726440, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2021/-/DC1.
A slide set summarizing this article is available online.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care 2005;28:2361–2366 [DOI] [PubMed] [Google Scholar]
- 4.Thompson CJ, Cummings JF, Chalmers J, Gould C, Newton RW. How have patients reacted to the implications of the DCCT? Diabetes Care 1996;19:876–879 [DOI] [PubMed] [Google Scholar]
- 5.Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 6.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 7.Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care 2009;32:2245–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarraugh GV, Clarke WL, Kovatchev BP. Comparison of the clinical information provided by the FreeStyle Navigator continuous interstitial glucose monitor versus traditional blood glucose readings. Diabetes Technol Ther 2010;12:365–371 [DOI] [PubMed] [Google Scholar]
- 9.Renard E, Apostol D, Lauton D, Boulet F, Bringer J. Quality of life in diabetic patients treated by insulin pumps. Quality of Life Newsletter 2002;28:11–13 [Google Scholar]
- 10.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994;17:267–274 [DOI] [PubMed] [Google Scholar]
- 11.Leplège A, Ecosse E, Verdier A, Perneger TV. The French SF-36 Health Survey: translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol 1998;51:1013–1023 [DOI] [PubMed] [Google Scholar]
- 12.Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 13.Bode B, Beck RW, Xing D, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care 2009;32:2047–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW, Hirsch IB, Laffel L, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther 2008;10:377–383 [DOI] [PubMed] [Google Scholar]
- 17.Hermanides J, DeVries JH. Sense and nonsense in sensors. Diabetologia 2010;53:593–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins AJ, Krishnamurthy B, Best JD, et al. An algorithm guiding patient responses to real-time-continuous glucose monitoring improves quality of life. Diabetes Technol Ther 2011;13:105–109 [DOI] [PubMed] [Google Scholar]
- 19.Jenkins AJ, Krishnamurthy B, Best JD, et al. Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial [erratum in: Diabetes Care 2010;33:1911]. Diabetes Care 2010;33:1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diabetes Research in Children Network (DirecNet) Study Group Psychological aspects of continuous glucose monitoring in pediatric type 1 diabetes. Pediatr Diabetes 2006;7:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]