Abstract
OBJECTIVE
Metformin and statins have shown promise for cancer prevention. This study assessed whether the effect of metformin on prostate cancer (PCa) incidence varied by statin use among type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
The study cohort consisted of 5,042 type 2 diabetic male patients seen in the Veteran Administration Health Care System who were without prior cancer and were prescribed with metformin or sulfonylurea as the exclusive hypoglycemic medication between fiscal years 1999 and 2005. Cox proportional hazards analyses were conducted to assess the differential hazard ratio (HR) of PCa due to metformin by statin use versus sulfonylurea use, where propensity scores of metformin and statin use were adjusted to account for imbalances in baseline covariates across medication groups.
RESULTS
Mean follow-up was ∼5 years, and 7.5% had a PCa diagnosis. Statin use modified the effect of metformin on PCa incidence (P < 0.0001). Metformin was associated with a significantly reduced PCa incidence among patients on statins (HR 0.69 [95% CI 0.50–0.92]; 17 cases/533 metformin users vs. 135 cases/2,404 sulfonylureas users) and an increased PCa incidence among patients not on statins (HR 2.15 [1.83–2.52]; 22 cases/175 metformin users vs. 186 cases/1,930 sulfonylureas users). The HR of PCa incidence for those taking metformin and statins versus those taking neither medication was 0.32 (0.25–0.42).
CONCLUSIONS
Among men with type 2 diabetes, PCa incidence among metformin users varied by their statin use. The potential beneficial influence on PCa by combination use of metformin and statin may be due to synergistic effects.
Prostate cancer (PCa) is the most common cancer detected in men in the U.S., accounting for ∼28% of the new cancer burden. Risk for PCa increases significantly with age, and the lifetime risk for a U.S. man is 1 in 6 (1). Although a large proportion of cases will not progress to a life-threatening state, a diagnosis of PCa can have significant daunting effects on the patient and his family, with concomitant lifestyle changes, particularly due to the high risk of voiding and sexual dysfunction resulting from currently available curative treatment options. Therefore, preventive strategies would have substantial benefit. Although risk for PCa has been shown to be lower for men with diabetes (2), preventing PCa is particularly important in men with type 2 diabetes because this population appears to be at higher risk for high-grade PCa compared with men without diabetes (3,4). Metformin and statins, two drug classes with sound safety profiles that are well tolerated, have shown promise for cancer prevention trials, although their efficacy in the prevention or treatment of PCa still remains to be seen (5–11).
Metformin is a biguanide drug widely prescribed as a first-line oral antihyperglycemic agent for individuals with type 2 diabetes (12). Its glucose-lowering effects may require activation of AMP-activated protein kinase (AMPK) to inhibit hepatic gluconeogenesis (13), increase peripheral uptake of glucose, and delay gastrointestinal glucose absorption (14). In addition, preclinical and clinical data have suggested antineoplastic effects of metformin, and several potential mechanisms include a reduction of hyperinsulinemia, growth inhibition through activation of the AMPK pathway and downstream inhibition of the mammalian target of rapamycin (mTOR) pathway, blockade of cell cycle progression, and alteration of anti-inflammatory properties (5,15–17).
Although the beneficial metabolic effects of metformin make it a good treatment candidate for preventing ensuing metabolic syndrome after androgen-deprivation therapy for PCa (18), there is currently mixed enthusiasm for use of metformin therapy in the prevention of PCa. Observational human studies have examined the effect of metformin on risk or recurrence of PCa. Studies conducted in the general population reported decreased risk for PCa among metformin users (19,20). Given that diabetes has been inversely correlated with PCa risk (2), these early reports may have been subject to this bias. In fact, two studies conducted exclusively among diabetic subjects did not observe a significant reduction in PCa risk by metformin use (21,22); rather, a possible dose-dependent increase in risk was reported (21).
Because these results are unexpected and contrast not only with the preclinical data but also with clinical data for other cancers, such as breast cancer, it is important to examine this question in other cohorts using more rigorous approaches to adjust for heterogeneity and potential confounding. For example, because of the high prevalence of dyslipidemia among individuals with type 2 diabetes, a large number will also be treated with a lipid-lowering medication such as a statin drug (23). The combination treatment with metformin and statin has not been explored. Prior studies, such as in Azoulay et al. (21), have adjusted for statin use by including an indicator variable to capture any previous use or no use in their model because statin use has itself been associated with PCa risk. No study has formally examined the interactive and potentially synergistic effects of the combination treatment with both drugs.
Supportive evidence for use of statins as a PCa prevention therapy is growing, and two clinical trials are currently being initiated in the U.S. to examine effects of statin use in PCa patients (24). As 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, statins may affect PCa tumorigenesis by blocking the mevalonate pathway and thus reducing cholesterol and/or through multiple pleiotropic effects, as reviewed in Papadopoulos et al. (25). Clinical data have shown cholesterol levels are strongly correlated with PCa risk, so reduction of cholesterol levels is likely a key factor in the anticancer effects of statins (26,27)
We conducted a 7-year cohort study to compare the PCa incidence rate associated with metformin monotherapy versus sulfonylurea monotherapy among male patients with type 2 diabetes in the Veteran Health Administration Health Care System (VAHCS). Further, we assessed whether the metformin effect on the PCa incidence rate could be modified by statin use to better examine the heterogeneity of metformin effect.
RESEARCH DESIGN AND METHODS
Study cohort
We drew our study sample from the 887,775 VAHCS enrollees who responded to the nationally representative Veteran’s Large Health Survey (VLHS) in 1999 (28). VLHS is the only VAHCS electronic database containing diabetes duration. Patients who were aged younger than 18 years or older than 90 years in 1999 were excluded. To identify patients with type 2 diabetes, we restricted the cohort to 158,062 individuals who had at least one primary care visit (defined as any visit to the general medicine, geriatric, or diabetes clinics) as well as a diagnosis of type 2 diabetes (diagnosis using ICD-9 CM codes of 250.00 or 250.02) each year during fiscal year (FY) 1999 to FY2000. Our criteria of identifying patients with type 2 diabetes could miss at most 3% of those who only had diagnoses for diabetes complications (29). We further narrowed the study cohort from the identified 158,062 with type 2 diabetes to 5,042 men who also met the following criteria: 1) having had prescription(s) of sulfonylureas or metformin as the sole class of glucose-lowering medication for 180 days or more; however, subjects with any PCa event that occurred before 180 days of metformin or sulfonylurea exposure were excluded; 2) no prescription for insulin or a thiazolidinedione (TZD) during the study period; 3) no liver or renal diseases during the study period; 4) no cancer diagnosis before the baseline (starting date of metformin or sulfonylureas); and 5) no missing data on any baseline covariates (age, ethnicity, HbA1c, BMI, diabetes duration, age-adjusted Charlson comorbidity score (30), and smoking status). All study procedures were approved by the institutional review board of the University of Texas Health Science Center San Antonio.
We chose to compare metformin monotherapy versus sulfonylurea monotherapy in men with type 2 diabetes because metformin and sulfonylurea were the two predominating first-line glucose-lowering drugs in the VAHCS during the study period. In addition, we excluded patients who were on TZD and/or insulin to better assess the association between metformin and PCa risk by eliminating potential confounding effects of other glucose-lowering medications that could themselves have an effect on PCa incidence or progression, as reported in the literature (31–33).
Data sources
We used five VAHCS databases for this study. VAHCS Inpatient and Outpatient Medical SAS Datasets were used to identify the cohort of men with type 2 diabetes and their associated characteristics, including demographic variables and comorbidities (based on diagnosis codes). Additional clinical variables were extracted from the VHA Decision Support System (HbA1c results, lipid laboratory results, and dates of measurements) and the VAHCS Corporate Data Warehouse (height and weight values for deriving BMI). Medication prescription records were extracted from the VAHCS Pharmacy Benefits Management Services Database. Duration of diabetes was extracted from VLHS in 1999. Mortality data were extracted from the VAHCS Vital Status files (28).
Outcomes of interest
The outcome of interest in this study was the incidence (rate) of the first PCa diagnosis during the study period. Thus, the dependent variable used in our analyses was the interval between initiations of the medication (sulfonylurea or metformin) to the first PCa diagnosis observed during the study period. A PCa event was defined as having an ICD-9 diagnosis of 185.xx from a primary or secondary diagnosis code. Those who died before the first PCa event observed during the study period, or those who remained alive but never had a PCa event during the study period, were treated as censored data. The study termination date for each patient corresponded to the date of the first PCa event, 30 September 2006 (study end), or the date of death, whichever came first.
Predictors and measures
Medication exposure.
For this study, we regarded sufficient medication exposure as a minimum of 180 days, because most clinical trials of these medications were 24 weeks or longer and other studies have used a similar exposure cut point (22). The metformin group consisted of patients who had a metformin prescription of any dose for 180 days or more but never had any TZD or insulin during the study period or a prescription for sulfonylurea for 180 days or more. Similarly, the sulfonylurea group consisted of patients who had a sulfonylurea prescription for 180 days or more but never had any TZD or insulin during the study period or a prescription for metformin for 180 days or more. The statin group consisted of patients who had any type of statin prescription at any dose for 180 days or more. The predominating statin prescription was simvastatin for 85% of statin users.
We also assessed whether longer exposure to a higher dose of metformin or statin across the study period was associated with PCa risk. We defined the proportion of time on a higher metformin daily dose as the proportion of days with metformin daily dose ≥1,000 mg among the total days with prescription(s) for metformin during the study. A high dose of statin was defined as the equivalent dose that reduces LDL by 30% or more on average.
Covariates
Covariates adjusted for in the analyses included demographic and clinical characteristics of the patient. Demographic characteristics included age and race/ethnicity (Caucasian, African American, Hispanic, others). Clinical characteristics included diabetes duration category in FY1999 (≤10, >10 years), age-adjusted Charlson comorbidity score, smoking status, and the mean LDL levels and HbA1c during the study period.
Statistical analyses
The Cox proportional hazards model adjusting for covariates and the propensity score of medication group membership (metformin and statin use) was conducted to compare hazard rates associated with PCa between the metformin and sulfonylurea groups. The propensity score of medication group membership was incorporated as the inverse probability weight in the Cox regression analyses. The propensity score can be defined as a measure of the likelihood of being treated conditionally on the basis of a subject’s pretreatment characteristics. This score is used to weight individuals differently to achieve balance in covariates at baseline between the four medication groups (metformin/no statin, metformin + statin, no metformin/no statin, no metformin + statin); therefore, potential confounding due to pretreatment characteristics is minimized and data from an observational study can be causally interpreted. The propensity score was generated by logistic regression analysis. The treatment variable was the dependent variable, and the pretreatment characteristics were the independent variables. We included all significant predictors (i.e., the pretreatment/baseline covariates) in the propensity score models to ensure credible estimation of the main outcome (PCa incidence) model (34). The propensity score model of metformin use membership included HbA1c, age, diabetes duration, and race/ethnicity. The significant baseline predictors for the propensity score model of statin use membership included age and the Charlson comorbidity score.
The interaction between statins and metformin was assessed by the coefficient associated with the product of indicator of metformin use and the indicator of statin use. A Wald test with P value <0.05 was considered significant.
RESULTS
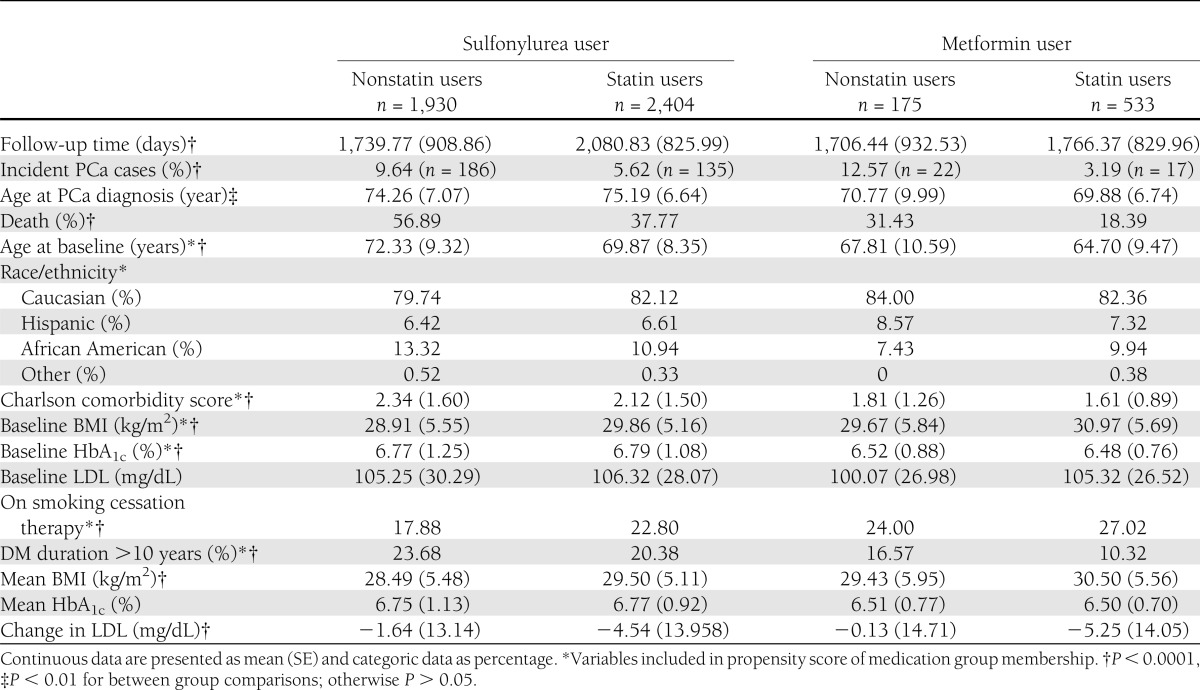
In our final cohort of 5,042 men, 4,334 (86%) had prescription(s) of sulfonylureas as the sole class of glucose-lowering medication, and 708 (14%) had prescription(s) of metformin as the sole class of glucose-lowering medication. Among 2,937 men (58% of the cohort) who had prescription(s) of statins, 2,404 were sulfonylurea users and 533 were metformin users. Among 2,105 men (42%) who did not have prescription(s) of statins, 1,930 were sulfonylurea users and 175 were metformin users. The characteristics of the subjects by medication group are reported in Table 1. The mean age at cohort entry was 70.2 ± 9.2 years, and the mean study period was 5.2 ± 2.4 years. The mean HbA1c at baseline was 6.7 ± 1.1%, and the type 2 diabetes duration exceeded 10 years in 1,031 subjects (23.8%). During the study period, 376 patients (7.5%) were diagnosed with PCa.
Table 1.
Characteristics of subjects by medication group
As can be seen in Table 1, we observed heterogeneity (imbalance) in subjects’ characteristics at baseline among the four medication groups, and some of these variables are associated with PCa outcome. The χ2 tests showed that age, BMI, HbA1c, Charlson comorbidity score, smoking, and diabetes duration were significantly different among the four medication groups. The results in Table 1 suggested that to assess the interaction between medications and PCa incidence as if they were balanced in terms of baseline covariates (pretreatment characteristics), subjects must be weighted or stratified by their propensity scores for medication group membership.
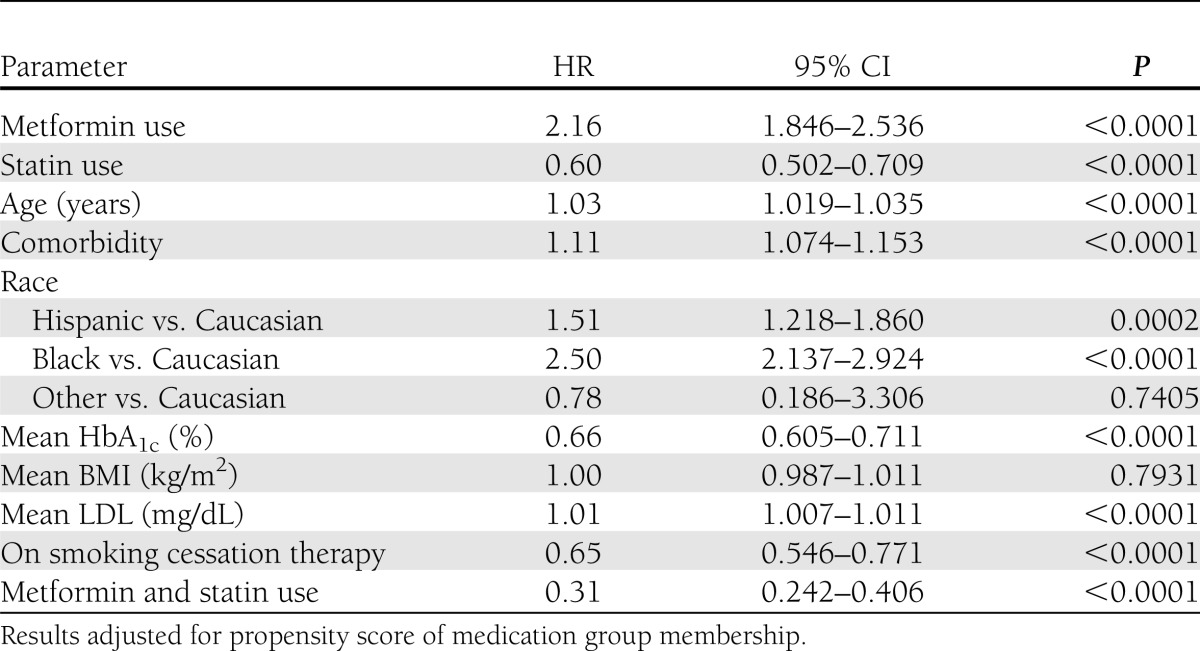
Using a Cox proportional hazards model adjusted for the medication propensity scores and covariates associated with PCa outcome, we observed significantly different hazard ratios (HRs) by medication use (Table 2 and Fig. 1). Statin use modified the effect of metformin use on PCa outcome, and this interaction effect was significant (P < 0.0001). Among nonstatin users, the HR for metformin use versus sulfonylurea use was 2.15 (95% CI 1.83–2.52; P < 0.0001). Among statin users, the HR for metformin use versus sulfonylurea use was 0.69 (0.50–0.92; P = 0.01). The HR for PCa incidence of those on combination treatment with metformin and statins versus sulfonylurea only was 0.32 (0.25–0.42). Although this study was designed primarily to examine PCa incidence with respect to metformin use, the effect of statin is worthy of attention. Among sulfonylurea users, the HR for statin use versus no statin use was 0.60 (0.49–0.70; P < 0.0001), whereas among metformin users, the HR for statin use versus no statin use was 0.19 (0.14–0.25; P < 0.0001).
Table 2.
Multivariate Cox proportional hazards analysis of metformin and statin effect on PCa incidence rate in men with type 2 diabetes
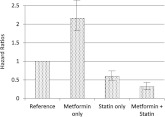
Figure 1.
HRs for prostate cancer by medication status. Results are shown for Cox proportional hazards model adjusting for all variables listed in Table 2 that have a P value <0.05. The 95% CIs are shown as vertical lines on bars. The number of incident PCa cases and total cohort members for each group is provided in Table 1. The HR of the left-most reference group refers to the group on sulfonylurea only and is provided for orientation purposes.
Higher mean HbA1c was associated with decreased risk of PCa among metformin users regardless of whether they were also prescribed a statin (HR 0.66 [95% CI 0.61–0.72] P < 0.001). BMI across the study period was greater among those subjects taking metformin and more so among those taking both metformin and statin(s). For those subjects with baseline BMI ≥25 kg/m2, combination treatment with metformin and statin was associated with a significantly decreased PCa risk (HR 0.25 [0.13–0.45], P < 0.0001) compared with sulfonylurea users. For those with a baseline BMI <25 kg/m2, dual drug use was not associated with PCa risk (1.02 [0.57–1.83], P = 0.93). Mean LDL was positively associated with an increased PCa incidence (HR 1.01 [1.007–1.012] for every 1-mg/dL increase in LDL; P < 0.0001). We noted a significant drop in LDL among those subjects taking statins (P < 0.0001). The group on combination treatment of metformin and statins had the greatest average reduction in LDL (−5.25 ± 14.05 mg/dL).
In light of the study by Azoulay et al. (21) that reported an increase in PCa risk with a greater number of metformin prescriptions, we also conducted an exploratory Cox regression analysis to assess the effects of medication dose on PCa incidence. Approximately 85% of subjects taking metformin had been prescribed a daily dose of ≥1,000 mg, and this number did not differ by statin use. We assessed whether longer exposure to this higher dose of medication across the study period was associated with PCa risk. Among metformin users, the average proportion of time on a metformin daily dose ≥1,000 mg was 80%. We found that a longer proportion of time on a metformin daily dose of ≥1,000 mg was associated with an increased PCa incidence (HR 1.44, P < 0.0036). Among statin users, the average proportion of time on a higher dose of statin, defined as the equivalent dose that reduces LDL by ≥30%, on average, was low (0.8%). The percentage of subjects prescribed the higher statin daily dose did not differ by metformin use (62.9% nonmetformin users vs. 65.7% metformin users). We found that a longer proportion of time on a higher statin daily dose was associated with a somewhat decreased PCa incidence (mean HR 0.997, P = 0.0008). Our sample, however, was not powered to provide a stable estimate of the dose-dose interaction effect.
CONCLUSIONS
For those without medication contraindications, metformin is widely prescribed to individuals with type 2 diabetes because it is the recommended first-line oral glucose-lowering agent by the consensus of the American Diabetes Association (ADA) and European Association for the Study of Diabetes (12). Statins are recommended by the American College of Physicians for all individuals with type 2 diabetes because of the high prevalence of dyslipidemia comorbidity in this population. Given that ∼13 million U.S. men (11.2%) have type 2 diabetes, a continuously rising epidemic in the U.S., it is important to use rigorous statistical methods to study the effects of metformin and statins on PCa in this growing population. In this study, we examined the effects of metformin compared with sulfonylurea on PCa incidence among male enrollees of the U.S. VAHCS who had type 2 diabetes. To avoid a biased estimation of medication effects due to dissimilar pretreatment characteristics, we incorporated propensity scores as weights to reduce the potential confounding. In addition, we investigated heterogeneity among metformin users by examining an interaction between metformin and statin use on PCa outcome.
We observed that the effect of metformin use on incidence of PCa varied, dependent on statin use by the subject. Users of metformin had an increased HR for PCa incidence compared with sulfonylurea users, which is consistent with results reported by Azoulay et al. (21); however, this increased risk was only among those not taking statin medications. A combination treatment with metformin and statins showed a significantly decreased risk for PCa that was greater than either medication alone.
The apparent favorable effect of both metformin and statin on PCa may be due to their synergistic effects via lipid-lowering or a combination of pleiotropic effects. Because glucose metabolism is interrelated with lipid synthesis, the synergistic effect of metformin and statins on reduced PCa risk may possibly be partly mediated by their joint lipid-lowering effect. Our data showing that subjects on combination treatment of metformin and statins had the greatest reduction in LDL supports this mechanism; however, this mechanism cannot explain the observed increase in PCa incidence among subjects on metformin without a statin. Subjects in this study on metformin/no statin showed a marginal reduction in LDL on average. In support of the latter hypothesis of pleiotropic effects, metformin and atorvastatin combination treatment has been shown to cooperate in protecting the liver in type 2 diabetes with hyperlipidemia in mice; importantly, the combination treatment did not result in better glycemic or lipid profiles but did improve systemic and liver inflammatory and oxidative stress markers such as C-reactive protein (35). A similar improvement on inflammatory conditions within the prostate may occur. Other evidence of enhanced anticancer effects of combination treatment has been reported in preclinical studies. For example, an enhancement of melanoma cell death in culture was observed when AICAR treatment (an AMPK activator) was combined with statin treatment (36).
In this study comprising a diabetic male U.S. VA population, higher HbA1c was associated with decreased risk of PCa. This observation is consistent with prior epidemiologic studies, such as the study by Stocks et al. (37), that reported a decrease in PCa risk with duration or severity of diabetes. These results may reflect the possibility that subjects with greater insulin resistance benefit more from metformin/statins or that these subjects have lower androgen levels, or both. In vivo studies have shown that antineoplastic activities of metformin are more pronounced in mice receiving a high-energy diet associated with hyperinsulinemia and rapid tumor growth compared with mice receiving a control diet (38). If comparable in humans, these data suggest that metformin may have a more detectable effect on cancers among hyperinsulinemic individuals. Unfortunately, neither insulin measures nor androgen levels were available for these subjects with which to further test either hypothesis. Our data indicating that the synergistic effect on PCa by metformin and statin varies by BMI, with higher BMI being associated with decreased PCa risk among those taking both drugs, suggests that these individuals may indeed have higher insulin levels; however, BMI has been significantly positively correlated with a decline in serum testosterone in aging men (39), so these data may be reflecting differences in androgen levels. Further studies will be needed to elucidate the clinical characteristics of subjects who may benefit from treatment by metformin and statin. These characteristics will likely differ by diabetes status.
Another limitation of this study is the unavailability of family history of PCa; however, there are no data to suggest that patients with a family history of PCa would be biased toward an antidiabetic medication group. Regarding potential selection bias, a recorded prostate-specific antigen (PSA) score was available for ∼50% of the subjects in this study without bias toward metformin users or sulfonylurea users. This level of PSA screening is consistent with general clinical practice as reported in a study using data from the 2001 Behavioral Risk Factor Surveillance System (BRFSS), an annual cross-sectional, population-based survey conducted by the Centers for Disease Control and Prevention in which 54% of men aged 50–69 years reported an up-to-date PSA screen (40). Therefore, this study sample appears representative of screening procedures for the general U.S. male population. Members of this study cohort were a mean age of ∼70 years at baseline, which is consistent with that of previous observational studies conducted among diabetic men only (21,22). Metformin was introduced into clinical use in the U.K. in 1958 but did not receive acceptance for use in the U.S. until 1995. It will be important to assess the effect on PCa incidence of metformin and statin treatment started at earlier ages as the data begin to accrue.
Medication dose may also impact the effect of metformin and statins on cancer. In our exploratory analyses, we found that a longer proportion of time on metformin daily dose ≥1,000 mg was associated with an increased PCa incidence and that a longer proportion of time on higher statin daily dose was associated with a modest decrease in PCa incidence. We were unable to examine whether differences in dose among those on combination therapy correlated with PCa outcome, however, due to the limited sample size. This is an important issue that requires further investigation.
In summary, in the male type 2 diabetic population with a generally high prevalence of dyslipidemia, treatment with both metformin and statin may have a significantly more favorable effect on PCa incidence than treatment by either medication alone. The independent effect of metformin requires further investigation, including accounting for dose as well as metabolic and hormonal characteristics. Because the number of men with type 2 diabetes who will likely be treated with metformin is increasing rapidly, it is important to determine the effect of metformin on PCa outcome specifically in this population. Our study has provided evidence that the effect may be modified by concomitant statin use, which is common in this population.
Acknowledgments
This work was partly funded by the Cancer Therapy and Research Center of The University of Texas Health Science Center (P30CA054174) and National Institutes of Health Grants K25 DK075092 (C.-P.W.) and DK047482.
No potential conflicts of interest relevant to this article were reported.
D.M.L. and C.-P.W. designed the study, researched the data, and wrote the manuscript. C.L. and J.H. contributed to the discussion and data interpretation and reviewed and edited the manuscript. C.-P.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at an audio poster tour of the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
References
- 1.The National Cancer Institute. SEER Cancer Statistics updated November 10, 2011. Prostate cancer [article online]. Available from http://www.cancer.gov/cancertopics/types/prostate 2011. Accessed 19 January 2012
- 2.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2056–2062 [DOI] [PubMed] [Google Scholar]
- 3.Abdollah F, Briganti A, Suardi N, et al. Does diabetes mellitus increase the risk of high-grade prostate cancer in patients undergoing radical prostatectomy? Prostate Cancer Prostatic Dis 2011;14:74–78 [DOI] [PubMed] [Google Scholar]
- 4.Mitin T, Chen MH, Zhang Y, et al. Diabetes mellitus, race and the odds of high grade cancer in men treated with radiation therapy. J Urol 2011;186:2233–2237 [DOI] [PubMed] [Google Scholar]
- 5.Ben Sahra I, Laurent K, Giuliano S, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells . Cancer Res 2010;70:2465–2475 [DOI] [PubMed] [Google Scholar]
- 6.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461 [DOI] [PubMed] [Google Scholar]
- 7.Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst 2011;103:885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol 2004;22:2388–2394 [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RJ, Freedland SJ. Review of recent evidence in support of a role for statins in the prevention of prostate cancer. Curr Opin Urol 2008;18:333–339 [DOI] [PubMed] [Google Scholar]
- 10.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstraw MA, Amoroso P, Kirby RS. Do statins protect against prostate cancer? BJU Int 2006;97:1147–1148 [DOI] [PubMed] [Google Scholar]
- 12.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med 1997;102:99–110 [DOI] [PubMed] [Google Scholar]
- 15.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008;27:3576–3586 [DOI] [PubMed] [Google Scholar]
- 16.Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol 2011;223:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269–10273 [DOI] [PubMed] [Google Scholar]
- 18.Clements A, Gao B, Yeap SH, Wong MK, Ali SS, Gurney H: Metformin in prostate cancer: two for the price of one. Ann Oncol 2011;22:2556–2560 [DOI] [PubMed] [Google Scholar]
- 19.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol 2008;168:925–931 [DOI] [PubMed] [Google Scholar]
- 20.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control 2009;20:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011;20:337–344 [DOI] [PubMed] [Google Scholar]
- 22.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 23.Management of dyslipidemia in adults with diabetes. American Diabetes Association position statement. Diabetes Care 2003;26:s83\x{2013}s86 [DOI] [PubMed]
- 24.ClinicalTrials.gov. Statin and prostate cancer. http://clinicaltrials.gov/ct2/results?term=statin+and+prostate+cancer Accessed 19 January 2012
- 25.Papadopoulos G, Delakas D, Nakopoulou L, Kassimatis T. Statins and prostate cancer: molecular and clinical aspects. Eur J Cancer 2011;47:819–830 [DOI] [PubMed] [Google Scholar]
- 26.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem 2004;91:54–69 [DOI] [PubMed] [Google Scholar]
- 27.Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends Endocrinol Metab 2008;19:113–121 [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Veterans Affairs. VA Information Resource Center (VIReC). http://www.virec.research.va.gov Accessed 15 December 2011
- 29.Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of A1c. Prim Care Diabetes 2012;6:19–25 [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 31.Mansour M, Schwartz D, Judd R, et al. Thiazolidinediones/PPARγ agonists and fatty acid synthase inhibitors as an experimental combination therapy for prostate cancer. Int J Oncol 2011;38:537–546 [DOI] [PubMed] [Google Scholar]
- 32.Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 2007;25:1476–1481 [DOI] [PubMed] [Google Scholar]
- 33.Hense HW, Kajüter H, Wellmann J, Batzler WU. Cancer incidence in type 2 diabetes patients—first results from a feasibility study of the D2C cohort. Diabetol Metab Syndr 2011;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 2007;26:20–36 [DOI] [PubMed] [Google Scholar]
- 35.Matafome P, Louro T, Rodrigues L, et al. Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes Metab Res Rev 2011;27:54–62 [DOI] [PubMed] [Google Scholar]
- 36.Woodard J, Platanias LC. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem Biophys Res Commun 2010;398:135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stocks T, Lukanova A, Rinaldi S, et al. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int J Cancer 2007;120:2678–2686 [DOI] [PubMed] [Google Scholar]
- 38.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst 2007;99:1793–1800 [DOI] [PubMed] [Google Scholar]
- 39.Rohrmann S, Shiels MS, Lopez DS, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control 2011;22:1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA 2003;289:1414–1420 [DOI] [PubMed] [Google Scholar]