Abstract
OBJECTIVE
There is an established link between health-related functioning (HRF) and cardiovascular disease (CVD) mortality, and it is known that those with diabetes predominantly die of CVD. However, few studies have determined the combined impact of diabetes and impaired HRF on CVD mortality. We investigated whether this combination carries a higher CVD risk than either component alone.
RESEARCH DESIGN AND METHODS
The Australian Diabetes, Obesity and Lifestyle (AusDiab) study included 11,247 adults aged ≥25 years from 42 randomly selected areas of Australia. At baseline (1999–2000), diabetes status was defined using the World Health Organization criteria and HRF was assessed using the SF-36 questionnaire.
RESULTS
Overall, after 7.4 years of follow-up, 57 persons with diabetes and 105 without diabetes had died from CVD. In individuals with and without diabetes, HRF measures were significant predictors of increased CVD mortality. The CVD mortality risks among those with diabetes or impaired physical health component summary (PCS) alone were similar (diabetes only: hazard ratio 1.4 [95% CI 0.7–2.7]; impaired PCS alone: 1.5 [1.0–2.4]), while those with both diabetes and impaired PCS had a much higher CVD mortality (2.8 [1.6–4.7]) compared with those without diabetes and normal PCS (after adjustment for multiple covariates). Similar results were found for the mental health component summary.
CONCLUSIONS
This study demonstrates that the combination of diabetes and impaired HRF is associated with substantially higher CVD mortality. This suggests that, among those with diabetes, impaired HRF is likely to be important in the identification of individuals at increased risk of CVD mortality.
Cardiovascular disease (CVD) is the leading cause of mortality worldwide, accounting for >20% of all deaths (1). Biological and behavioral variables, such as diabetes, obesity, smoking, and physical inactivity, are robust risk factors for the development of CVD and mortality (2). As well as these established risk factors, subjective health status markers, such as health-related functioning (HRF), have been shown to be associated with an increased risk of cardiovascular mortality (3). It has been shown that psychosocial risk factors exert an association similar in strength to that of biological risk factors for CVD (4), and yet the two types of risk factors are rarely assessed concurrently.
HRF refers to how well an individual functions in their daily life, physically and socially, and their perceived physical and mental well-being (5,6). Most of the literature to date examines HRF as an outcome in patient populations, providing an important indicator of the impact of chronic disease (3,5,7). However, there is increasing evidence to suggest that poor HRF may predict the development of disease, e.g., in type 2 diabetes (T2DM) (8) and CVD (9). Because of the burden of the daily management of T2DM and the development of complications, HRF is particularly important for people with T2DM, with levels of health status shown to be compromised in these populations (5,7,10). Findings from previous studies show that in samples of patients with T2DM, persons reporting low levels of HRF have higher risk of mortality compared with those reporting high functioning (11,12). It is likely, therefore, that exposure to T2DM and poor HRF has an additive, if not synergistic, effect on the risk of cardiovascular mortality; however, the impact of this combined relationship on mortality has not been examined. It is important to understand whether the effects of each disorder simply have an additive impact or whether their combined effects exert a synergistic effect (i.e., greater than the sum of two independent effects) on mortality.
Using longitudinal data from a population-based, national study in Australia, these analyses aimed to examine the combined impact of HRF and T2DM on cardiovascular mortality compared with either risk factor alone.
RESEARCH DESIGN AND METHODS
The population, methods, and response rates of the Australian Diabetes, Obesity and Lifestyle (AusDiab) study have previously been described (13). In brief, AusDiab was a population-based study of 11,247 people aged ≥5 years from 42 randomly selected urban and rural areas of Australia conducted in 1999–2000. At baseline, 55.3% (n = 11,247) of those completing a household questionnaire undertook the full survey. The study was approved by the ethics committee of the International Diabetes Institute. Informed consent for the study was obtained from all participants.
Diabetes classification was based on plasma glucose results, using the 1999 World Health Organization diabetes classification (14). Diabetes was diagnosed on the basis of fasting plasma glucose (FPG) ≥7.0 mmol/L, 2-h plasma glucose ≥11.1 mmol/L, or current treatment with insulin or oral hypoglycemic medication. For these analyses, complete data were available on key variables of interest for 802 participants with T2DM and 9,177 without.
FPG and 2-h plasma glucose levels were determined by a glucose oxidase method using an Olympus AU600 automated analyzer (Olympus Optical, Tokyo, Japan). Serum triglycerides, total cholesterol, and HDL cholesterol were measured by enzymatic methods. Total HbA1c analysis was performed using high-performance liquid chromatography (Bio-Rad Variant Haemoglobin Testing System; Bio-Rad, Hercules, CA) with standardized conversion to HbA1c values (normal range 4.2–6.3%). Blood pressure was measured using Dinamap or a standard mercury sphygmomanometer. To account for any effect due to differential measurement error, we adjusted manual blood pressure measurements as previously described (15). Hypertension was defined as present if systolic blood pressure (SBP) was ≥140 mmHg and diastolic blood pressure ≥90 mmHg or if the participant reported current treatment for hypertension. Height and weight were measured in light clothing by a trained observer. BMI was calculated as weight in kilograms divided by the square of height in meters. Information on smoking, medication, and history of diabetes was obtained by interview.
Mortality status and underlying and contributory causes of death were determined by linking the AusDiab cohort to the Australian National Death Index (NDI). Name, sex, date of birth, state, date of last contact, and date of death (if available) were used to match participants to the NDI. The accuracy of the NDI to ascertain CVD deaths and vital status has previously been established (16). Only high-level matches were accepted as confirmed deaths, and wherever possible, deaths were confirmed by direct communication with the decedent’s family. People who were not matched to the NDI were assumed to be alive. Deaths were attributed to CVD if the underlying cause of death was coded I10-I25, I46.1, I48, I50-I99, or R96 according to the 2006 ICD-10. In addition, participants with uncomplicated diabetes (ICD-10 codes E109, E119, or E149) or unspecified hyperlipidemia (ICD-10 code E785) as an underlying cause of death on the death certificate were attributed a CVD death (n = 4) if any of the CVD codes (I10-I25, I46.1, I48, I50-I99, or R96) were recorded in the first position on the death certificate.
Health-related functioning was assessed using version 1 of the SF-36 Health Survey (6). The SF-36 (http://www.sf-36.org/tools/sf36.shtml) is a self-administered measure of perceived health status over the preceding week, comprising eight domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Items for each dimension were coded, summed, and translated from worst health (0) to best health (100). Physical functioning refers to the ability to perform activities (walking, climbing stairs, bending and stretching, and lifting and carrying objects) without limitation. Role limitation (physical) refers to the limitations that reduced physical health has on the range and extent of physical activities one is able to perform. Bodily pain refers to the severity of pain and its impact on daily activities. General health is a rating of one’s own health, a comparison with others’ health, and proneness to illness. Vitality refers to how energetic or tired a person feels. Social functioning refers to the impact of physical and emotional health on the ability to perform normal social activities. Role limitation (emotional) refers to limitations that emotional problems put on the range and extent of activities one can perform. Mental health refers to the degree of nervousness or calmness and happiness or sadness.
In addition, the survey established scores for two summary measures: physical health component summary (PCS) and mental health component summary (MCS) scores. Both PCS and MCS are dimensions of health status that have been shown to be useful and valid measures of mental and physical health functioning relative to health profile. Overall, the SF-36 has been widely used in studies of chronic disease in both patient (6) and general population (17) samples.
Statistical methods
Statistical analysis was performed using STATA, version 11.0, for Windows (STATA, College Station, TX). Descriptive information for each of the variables was derived and distribution assessed. The lowest quartile (i.e., poor health-related functioning) of each dimension of the SF-36 scale was used to define impaired HRF (thresholds: PCS ≤70 and MCS ≤68). Categorical variables describing the pattern of mortality by the SF-36 summary scales were created. The categories were as follows (participant numbers given for PCS and MCS, respectively): 1) no T2DM and normal PCS or MCS (n = 7,098 and 6,640), 2) T2DM and normal PCS or MCS (n = 444 and 500), 3) no T2DM and impaired PCS or MCS (n = 2,079 and 2,178), and 4) T2DM and impaired PCS or MCS (n = 358 and 179).
Univariate associations between each dimension of the SF-36 scale and other variables of interest were assessed using ANOVA for metric variables and a χ2 test for categorical variables. Multivariate analysis was performed using Cox proportional hazards models. The first model was adjusted for age and sex. Model 2 adjusted for age, sex, BMI, smoking, history of CVD, SBP, lipid therapy, total cholesterol, and triglycerides. Another model (Model 3) tested the interaction between each domain of SF-36 and HbA1c to assess the synergistic effects of T2DM and impaired HRF on mortality beyond the additive effects of each condition. It should be noted that some of the eight domains were too highly correlated to be included in the same model in order to test their independence from one another. Therefore, these results should not be interpreted as independent of the other domains.
RESULTS
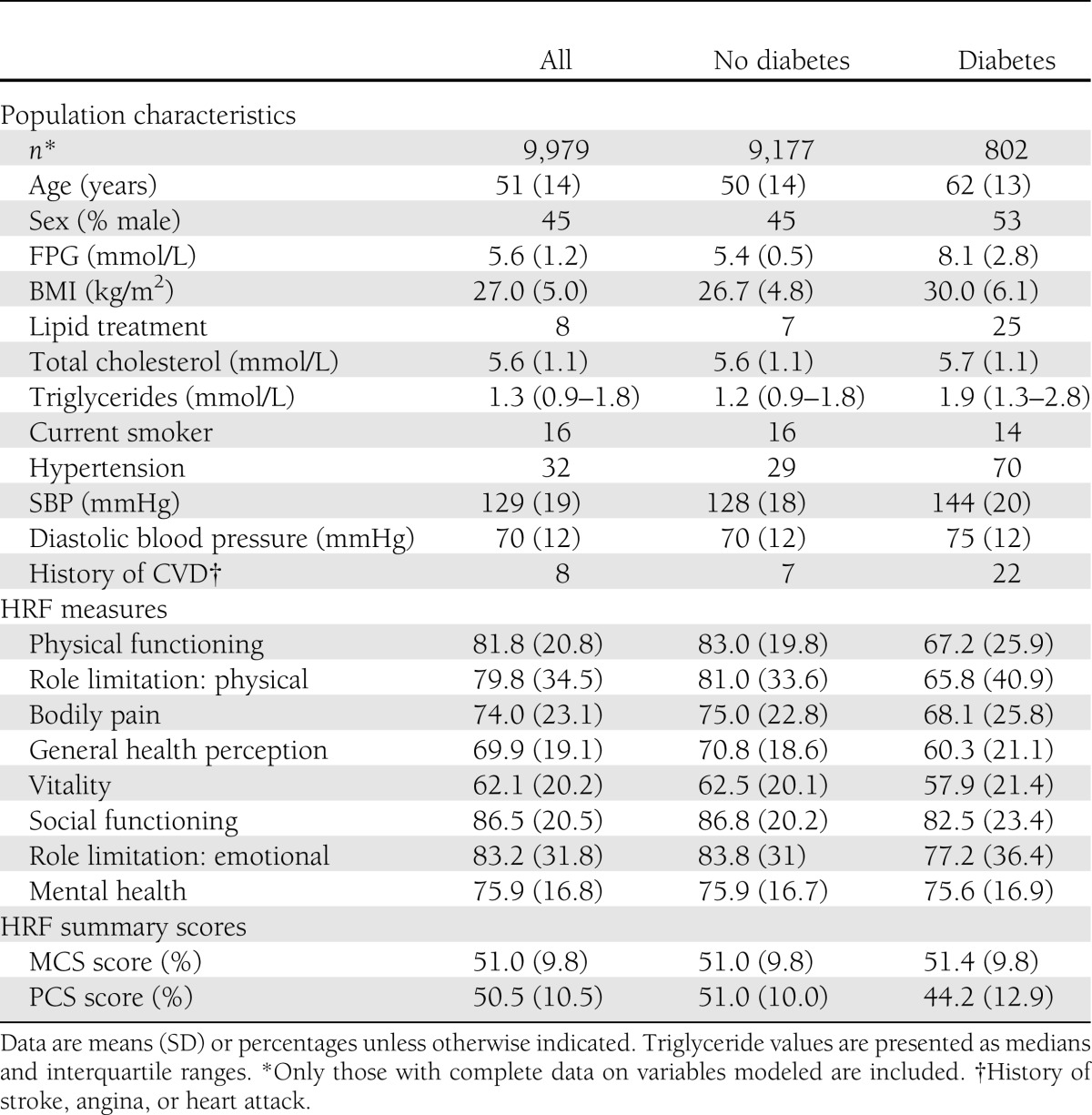
Overall, after 7.4 years of follow-up, 57 of those with T2DM and 105 without had died of CVD. Of these, 66% were male. Complete data for these analyses were available on 9,979 study participants. Table 1 displays the baseline sample characteristics. The mean (SD) age of those without T2DM was 51 years (14) and with T2DM was 62 years (13). Sixteen percent of those without T2DM and 14% of those with T2DM were current smokers. Overall, 7% of those without T2DM and 22% of those with T2DM at baseline had a history of CVD. For each dimension of the SF-36, people with T2DM had lower scores compared with people without T2DM.
Table 1.
Baseline characteristics of the AusDiab population
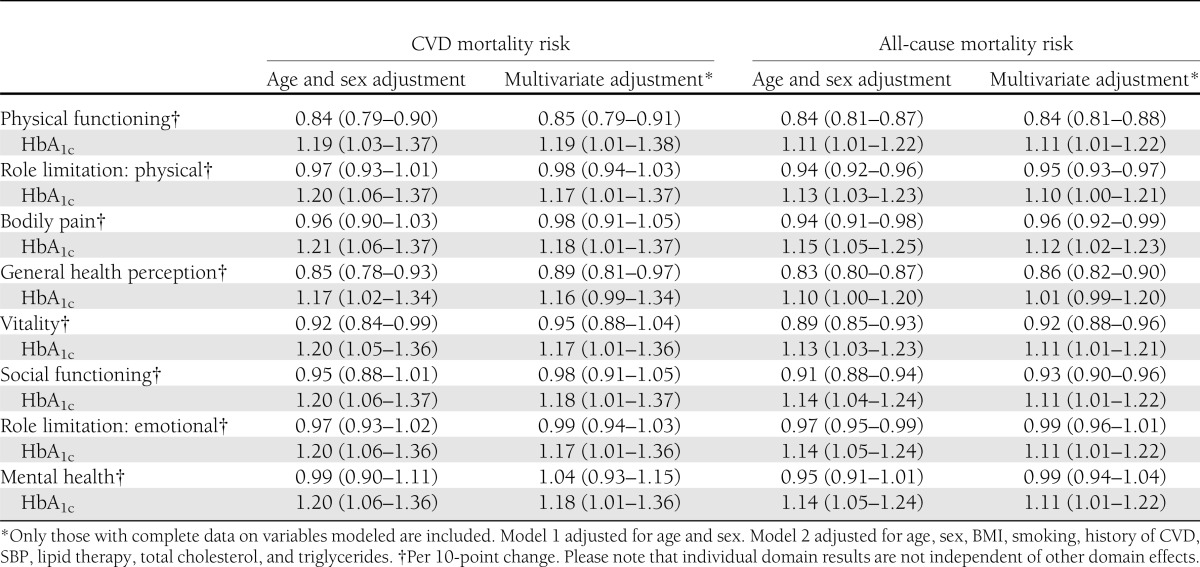
The hazard ratios (HRs) (95% CI) for CVD and all-cause mortality according to domains of HRF and HbA1c are shown in Table 2. Low levels of physical functioning, general health perception, and vitality were associated with higher CVD mortality (adjusted for age and sex). This association was only partially attenuated by further adjustment for BMI, smoking, history of CVD, SBP, lipid therapy, total cholesterol, and triglycerides. Low levels of physical functioning, role limitation (physical), bodily pain, general health perception, vitality, social functioning, and role limitation (emotional) were associated with higher all-cause mortality (after controlling for age and sex). Further adjustment for BMI, smoking, history of CVD, SBP, lipid therapy, total cholesterol, and triglycerides had little impact on the elevated HRs of these domains on all-cause mortality. As stated previously, due to strong correlation between certain domains, it was not possible to identify whether the impact of each domain was independent of the effects of the other domains.
Table 2.
HRs (95% CI) for all-cause and CVD mortality according to dimensions of HRF and HbA1c
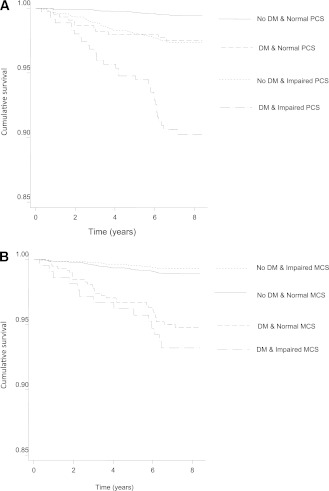
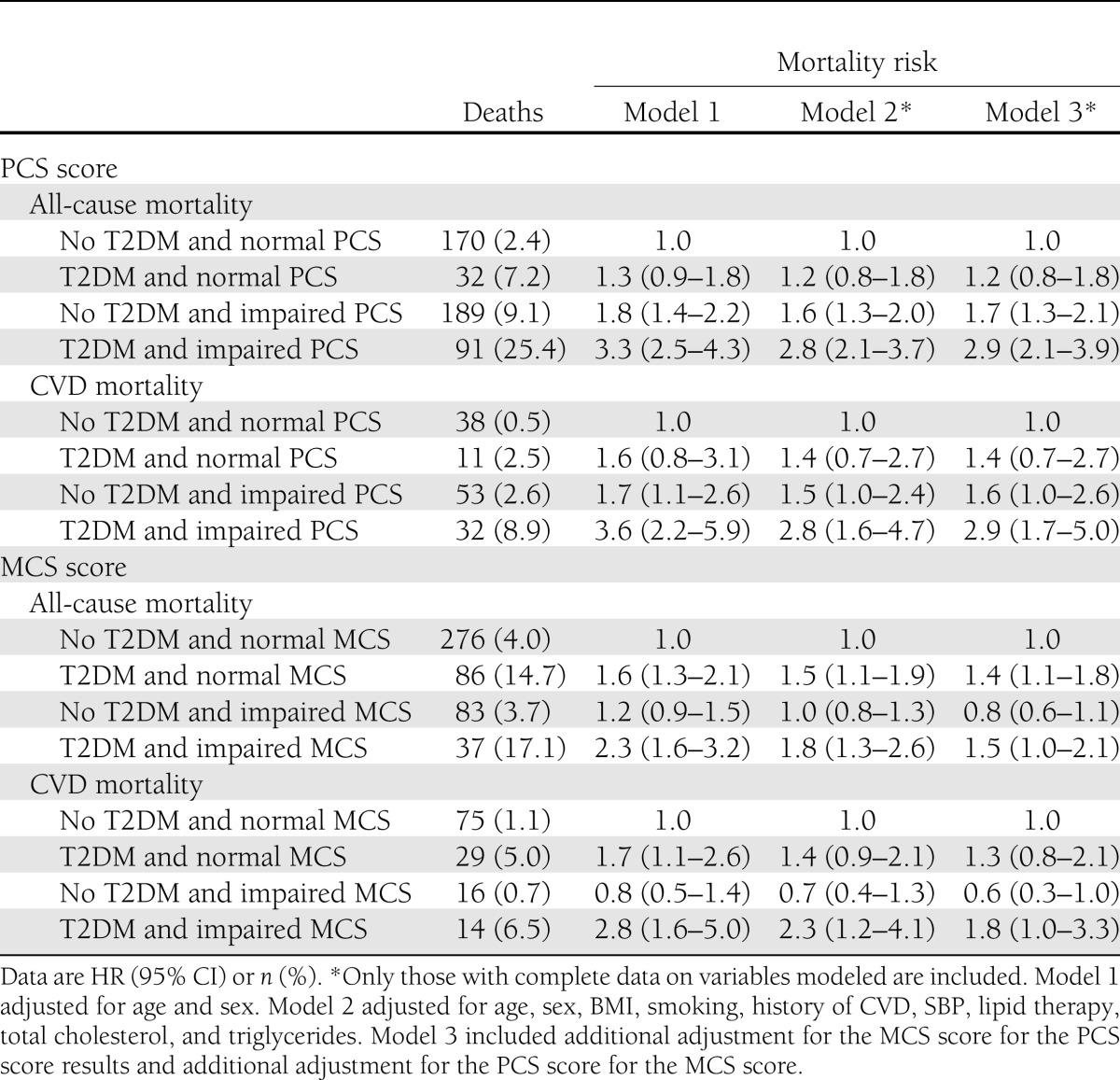
The unadjusted combined survival for CVD mortality by categories of T2DM and physical and mental health–related functioning is outlined in Fig. 1. The HRs (95% CI) for all-cause and CVD mortality according to categories of HRF and diabetes status are shown in Table 3. The HRs for CVD mortality among those with T2DM or impaired PCS alone were similar, while those with both T2DM and impaired PCS had a much higher CVD mortality (T2DM only 1.4 [95% CI 0.7–2.7], impaired PCS alone 1.5 [1.0–2.4], and both T2DM and impaired PCS 2.8 [1.6–4.7]) compared with the persons without T2DM and with normal PCS (after adjustment for age, sex, BMI, smoking, history of CVD, SBP, lipid therapy, total cholesterol, and triglycerides). Adjustment for MCS (Model 3) did not appreciably affect the impact of PCS, either alone or in combination with T2DM, on all-cause or CVD mortality. For MCS, the HR for CVD mortality among those with T2DM only was 1.4 (95% CI 0.9–2.1) and for impaired MCS alone was 0.7 (0.4–1.3), while the HR for CVD mortality in people with both T2DM and impaired MCS was 2.3 (1.2–4.1). Inclusion of PCS in Model 3 did not substantially affect these results; the individual impact of MCS and its combined impact with diabetes on mortality were independent of PCS.
Figure 1.
A: Unadjusted survival for CVD mortality according to diabetes and PCS score categories: the AusDiab study. B: Unadjusted survival for CVD mortality according to diabetes and MCS score categories: the AusDiab study.
Table 3.
HRs (95% CI) for all-cause and CVD mortality according to categories of HRF and diabetes status
To test the nature of the relationship between diabetes and HRF on mortality, i.e., whether effects of the combined disorders on mortality were additive or synergistic, interactions were tested between each domain of the SF-36 and HbA1c, and where significant, these terms were added to the final model for each dimension. For CVD mortality, there was a significant interaction between bodily pain and HbA1c (P = 0.015). For all-cause mortality, there were significant interactions between physical functioning and HbA1c (P = 0.035) and between bodily pain and HbA1c (P = 0.033). There was no significant interaction between HbA1c and mental HRF.
CONCLUSIONS
This is the first study to show that the combined exposure to type 2 diabetes and impaired HRF is associated with an increased risk of cardiovascular and all-cause mortality. Physical and mental health functioning both demonstrated this additive relationship with diabetes on increased mortality, and T2DM and components of impaired physical HRF (the domains of bodily pain and physical functioning) showed a synergistic impact on mortality. These interaction results indicated that greater bodily pain and poorer physical functioning were more strongly related to mortality among people with higher HbA1c compared with those with lower HbA1c. This may suggest that people with diabetes who experience high levels of pain or impaired physical functioning are less likely to participate in behaviors that could improve glycemic control and reduce the development of complications, therefore increasing their mortality risk. It is also possible that these specific HRF impairments are markers of other processes that might increase mortality risk. Owing to strong correlations between certain HRF domains, it was not possible to identify whether bodily pain and physical functioning exerted effects on mortality independent of one another or independent of other domains of HRF.
Previous research in T2DM has largely focused on assessing and modifying conventional risk factors, such as lowering blood glucose and lipids, and increasing physical activity. This approach does not, however, look beyond clinical parameters and fails to take into account the role of subjective health status markers, which can reflect other physical and psychological symptom burden as well as the individual’s perception of their health (5). A number of studies have now begun to recognize and advocate the use of HRF measures in risk assessment and stratification for CVD (3,9) and diabetes (11).
Our findings indicate that HRF is related to life expectancy and that among people with T2DM, impaired HRF significantly increases CVD and all-cause mortality. In populations with type 2 diabetes, a few studies have shown an independent inverse relationship between HRF and all-cause and cardiovascular mortality, using both the SF-36 and the EuroQoL (EQ-5D) scales (11,12). Both instruments are generic measures of HRF widely used among patient samples (12) and the general population (17). The EQ-5D, developed by the EuroQol group, measures an individual’s health state through five dimensions: mobility, self-care, usual activity, pain/discomfort, anxiety, and depression (18). Previous studies have not been able to demonstrate the combined impact of T2DM and impaired HRF on mortality because their samples included only people with diabetes (11,12). Research has shown that people with T2DM are more likely to experience impaired HRF compared with populations without diabetes (5,7,10). It is also well established that hyperglycemia promotes CVD risk through various mechanisms, including inflammation and endothelial dysfunction (19), which consequently increase atherosclerosis and plaque rupture. Therefore, our findings support previous work demonstrating separate associations between HRF and T2DM and between T2DM and mortality. Furthermore, this study extends existing literature, providing data indicating the synergistic impact of T2DM and impaired HRF on mortality, and highlights the significance of HRF as a CVD risk factor by showing that those with impaired HRF without diabetes have a risk of CVD mortality similar to that of people with normal HRF and diabetes.
We have shown that both the physical and mental components of HRF independently contribute to the risk of mortality. This supports previous research from a community sample in Taiwan, which demonstrated a relationship between both the physical and mental components of HRF and mortality, even after adjustment for a range of covariates (20). However, a number of studies have failed to find an association between MCS and mortality. In the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC), physical but not mental health functioning was related to mortality in participants with T2DM (11), and a similar finding was shown in a cardiac patient sample (3). Despite T2DM and impaired MCS significantly increasing mortality in this study, our results indicate that the physical component of HRF did have a stronger effect on mortality than the mental component.
The SF-36 incorporates an assessment of self-rated health, for which there is significant literature supporting its role as a predictor of mortality (21). Self-rated health provides an overall evaluation of current mental and physical health, as well as the trajectory of health. Explanations for the strength of this relationship suggest that self-rated health may influence behaviors that will affect future health but also that such subjective measures are more inclusive and accurate measurements of health than individual clinical variables (22).
There is strong evidence to suggest that diabetes and HRF are associated with psychosocial risk factors, such as depression (7,23). In a sample of patients with T2DM, both the PCS and MCS of SF-36 were associated with depression, anxiety, and illness perceptions (24). Therefore, T2DM and impaired HRF may promote psychosocial ill health, demonstrating a pathway through which CVD mortality risk may be elevated. The mediating effect of psychosocial adversity may act directly on physiological processes that promote CVD risk. For example, a psychological intervention for cancer sufferers showed that, by improving depressive symptoms, inflammatory markers were reduced (25). Another mechanism through which the cumulative effects of T2DM and impaired HRF increase mortality may be through behaviors like poor diet, diabetes self-care, and medical adherence (23). There is a clear link between behaviors such as diet and exercise and HRF (5); however, the literature is more limited regarding the self-management behaviors of adherence and compliance to medical routines. Hanestad and Albrektsen (26) showed that, in a sample of patients with type 1 diabetes, HRF was associated with greater perceived ease of regimen adherence. Results from another cross-sectional study indicated that higher HRF was related to good compliance (27). Therefore, ameliorating HRF levels through psychological and behavioral interventions may benefit health outcomes by improving psychological health and health care practices of individuals with T2DM.
There are limitations to be considered. There is some debate about the use of SF-36 as a measurement tool for quality of life among people with diabetes (28); however, it is generally accepted that, in population samples, it accurately assesses health status or HRF (17). It is possible that impaired HRF may reflect some unmeasured variables; therefore, some of the variance attributed to impaired HRF may be explained by other factors. Complications relating to diabetes are likely to explain some of the increased mortality observed among people with diabetes. Although complete data on all diabetes-related complications were not available, the inclusion of albumin-to-creatinine ratio as a covariate did not appreciably affect the findings (i.e., PCS remained a significant independent predictor of mortality, and the combination of both summary components of HRF with T2DM significantly increased the risk of mortality). Data on depression were not available to explore its role as a mediating variable. The moderate response rates at baseline mean that the findings may be subject to response and selection biases and therefore may not be generalizable across all populations. However, the large national sample and quality of the glucose tolerance data give this work considerable weight.
This study demonstrates the combined impact of diabetes and impaired HRF on cardiovascular and all-cause mortality. These findings indicate that, among people with T2DM, impaired HRF is likely to be important in the identification of individuals at increased risk of CVD mortality.
Acknowledgments
E.D.W. is supported by a Diabetes UK Fellowship (09/0003833). J.E.S. is supported by a National Health and Medical Research Council Fellowship (586623). R.J.T. is supported by the Lions Foundation. The AusDiab study, cocoordinated by the Baker IDI Heart and Diabetes Institute, was supported in part by the Victorian Government’s Operational Infrastructure Support Program, the National Health and Medical Research Council (NHMRC Grant 233200), the Australian Government Department of Health and Ageing, City Health Centre–Diabetes Service–Canberra, the Department of Health and Community Services–Northern Territory, the Department of Health and Human Services–Tasmania, the Department of Health–New South Wales, the Department of Health–Western Australia, the Department of Health–South Australia, the Department of Human Services–Victoria, Diabetes Australia, Diabetes Australia Northern Territory, Estate of the Late Edward Wilson, the Jack Brockhoff Foundation, Kidney Health Australia, Marian & FH Flack Trust, Menzies Research Institute, and the Pratt Foundation.
The AusDiab study was also supported by Abbott Australasia Pty Ltd, Alphapharm Pty Ltd, AstraZeneca, Bristol-Myers Squibb, Eli Lilly Australia, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk Pharmaceuticals, Pfizer Pty Ltd, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital (Sydney, New South Wales, Australia), sanofi-aventis, and Sanofi-Synthelabo. No other potential conflicts of interest relevant to this article were reported.
E.D.W. wrote the manuscript. L.R. contributed to the analyses and reviewed and edited the manuscript. B.F.O. and C.R. reviewed and edited the manuscript. J.E.S. contributed to discussion and reviewed and edited the manuscript. R.J.T. contributed to discussion, analyses, and manuscript writing and reviewed and edited the manuscript. E.D.W. and R.J.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2011.
The authors are enormously grateful for the invaluable contribution to the set-up and field activities of AusDiab, to the Baker IDI Heart and Diabetes Institute staff at the time of data collection: B. Atkins (also of Monash Medical Centre), A. Bonney, M. Dalton, M. de Courten (now of University of Copenhagen), D. Dunstan, H. Jahangir, A. Meehan, N. Meinig, S. Murray, A. Stewart, R. Tapp (now of National Vision Research Institute), T. Whalen, F. Wilson, and P. Zimmet, as well as A. Allman and C. Reid (HITECH Pathology), S. Bennett (Australian Institute of Health & Welfare), S. Chadban (University of Sydney), T. Dwyer (Menzies Centre for Population Research), D. Jolley (Monash University), D. McCarty (Centre for Eye Research Australia), K. O’Dea (Menzies School of Health Research), K. Polkinghorne (Monash Medical Centre), P. Phillips (Queen Elizabeth Hospital), and H. Taylor (Centre for Eye Research Australia).
References
- 1.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, World Health Organization, 2008 [Google Scholar]
- 2.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–157 [DOI] [PubMed] [Google Scholar]
- 3.Rumsfeld JS, MaWhinney S, McCarthy M, Jr, et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA 1999;281:1298–1303 [DOI] [PubMed] [Google Scholar]
- 4.Rosengren A, Hawken S, Ôunpuu S, et al. INTERHEART investigators Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:953–962 [DOI] [PubMed] [Google Scholar]
- 5.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev 1999;15:205–218 [DOI] [PubMed] [Google Scholar]
- 6.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 7.Wexler DJ, Grant RW, Wittenberg E, et al. Correlates of health-related quality of life in type 2 diabetes. Diabetologia 2006;49:1489–1497 [DOI] [PubMed] [Google Scholar]
- 8.Tapp RJ, O’Neil A, Shaw JE, Zimmet PZ, Oldenburg BF, AusDiab Study Group Is there a link between components of health-related functioning and incident impaired glucose metabolism and type 2 diabetes? The Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 2010;33:757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation 2002;106:43–49 [DOI] [PubMed] [Google Scholar]
- 10.Al-Shehri AH, Taha AZ, Bahnassy AA, Salah M. Health-related quality of life in type 2 diabetic patients. Ann Saudi Med 2008;28:352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleefstra N, Landman GW, Houweling ST, et al. Prediction of mortality in type 2 diabetes from health-related quality of life (ZODIAC-4). Diabetes Care 2008;31:932–933 [DOI] [PubMed] [Google Scholar]
- 12.Landman GW, van Hateren KJ, Kleefstra N, Groenier KH, Gans RO, Bilo HJ. Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabetes (ZODIAC-18). Diabetes Care 2010;33:2378–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunstan DW, Zimmet PZ, Welborn TA, et al. Australian Diabetes, Obesity and Lifestyle Study (AusDiab) The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract 2002;57:119–129 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Organization, 1999 [Google Scholar]
- 15.Briganti EM, Shaw JE, Chadban SJ, et al. Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Untreated hypertension among Australian adults: the 1999-2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003;179:135–139 [DOI] [PubMed] [Google Scholar]
- 16.Magliano D, Liew D, Pater H, et al. Accuracy of the Australian National Death Index: comparison with adjudicated fatal outcomes among Australian participants in the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Aust N Z J Public Health 2003;27:649–653 [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res 1994;3:7–12 [DOI] [PubMed] [Google Scholar]
- 18.EuroQol G EuroQol—a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199–208 [DOI] [PubMed] [Google Scholar]
- 19.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880 [DOI] [PubMed] [Google Scholar]
- 20.Tsai SY, Chi LY, Lee CH, Chou P. Health-related quality of life as a predictor of mortality among community-dwelling older persons. Eur J Epidemiol 2007;22:19–26 [DOI] [PubMed] [Google Scholar]
- 21.Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med 2009;69:307–316 [DOI] [PubMed] [Google Scholar]
- 22.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav 1997;38:21–37 [PubMed] [Google Scholar]
- 23.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care 2004;27:1066–1070 [DOI] [PubMed] [Google Scholar]
- 24.Paschalides C, Wearden AJ, Dunkerley R, Bundy C, Davies R, Dickens CM. The associations of anxiety, depression and personal illness representations with glycaemic control and health-related quality of life in patients with type 2 diabetes mellitus. J Psychosom Res 2004;57:557–564 [DOI] [PubMed] [Google Scholar]
- 25.Thornton LM, Andersen BL, Schuler TA, Carson WE., 3rd A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med 2009;71:715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanestad BR, Albrektsen G. Quality of life, perceived difficulties in adherence to a diabetes regimen, and blood glucose control. Diabet Med 1991;8:759–764 [DOI] [PubMed] [Google Scholar]
- 27.Chaveepojnkamjorn W, Pichainarong N, Schelp FP, Mahaweerawat U. Quality of life and compliance among type 2 diabetic patients. Southeast Asian J Trop Med Public Health 2008;39:328–334 [PubMed] [Google Scholar]
- 28.Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med 2009;26:315–327 [DOI] [PubMed] [Google Scholar]