Abstract
OBJECTIVE
To examine the association between early retinal arteriolar abnormalities and diabetic peripheral neuropathy (DPN).
RESEARCH DESIGN AND METHODS
Data from 608 people (aged 40–80 years) with diabetes from the population-based Singapore Malay Eye Study were analyzed. Participants underwent binocular two-field digital retinal photography and quantitative sensory testing. DPN was defined as an abnormal response to a monofilament or neurothesiometer test. Quantitative changes of retinal vascular caliber and arteriolar bifurcation geometry were measured using a computer-based program. Qualitative retinal signs of retinopathy and retinal arteriolar wall signs were graded by standardized methods.
RESULTS
DPN was present in 155 people (25.5%). After adjusting for age, sex, diabetes duration, HbA1c, cardiovascular risk factors, antihypertensive medication use, and peripheral arterial disease, people with suboptimal arteriolar caliber (odds ratio 1.94 [95% CI 1.22–3.10]), larger arteriolar branching coefficient (1.58 [1.03–2.42]), diabetic retinopathy (1.82 [1.20–2.75]), and focal arteriolar narrowing (2.92 [1.48–5.76]) were more likely to have DPN. Participants with a greater number of retinal microvascular signs were more likely to have DPN than those without retinal changes (6.11 [2.11–17.71] for two or more signs and 3.47 [1.18–10.21] for one sign compared with none).
CONCLUSIONS
Individuals with diabetes with early retinal arteriolar abnormalities are more likely to have DPN, independent of hyperglycemia and major vascular risk factors. These data support the hypothesis that early microvascular dysfunction, evident in the retina, is an independent risk factor for DPN.
Diabetic peripheral neuropathy (DPN) is one of the most common long-term complications of diabetes and is the major predisposing factor for foot ulceration, lower extremity amputation, and death (1). Despite extensive research, the exact pathophysiologic mechanisms of DPN remain unclear. DPN has been widely classified as a “microvascular complication” of diabetes (2), and microvascular damage, followed by impaired blood supply to the peripheral nerves, may contribute to demyelination and axonal degeneration and eventually lead to signs and symptoms (3). However, few studies have specifically documented a link between microvascular dysfunction and DPN (4).
The retinal microvasculature provides a direct means of visualizing the systemic microcirculation, and retinal vascular changes reflect the deleterious effects of hyperglycemia on the systemic microcirculation (5,6). Previous studies have reported an association between overt diabetic retinopathy (DR) signs (e.g., microaneurysms and hemorrhages) and an increased risk of DPN or lower extremity amputation (6). However, DR signs represent a relatively late manifestation of microvascular disease in the eye.
New quantitative measurements of retinal microvascular structure and geometry, such as retinal vascular caliber and arteriolar bifurcation geometry, have been shown to inform early and preclinical processes in the pathophysiology of diabetes (7,8). As yet, there are scarce data on the association between these quantitatively measured retinal vascular parameters and DPN. In the Wisconsin Epidemiology Study of Diabetes Retinopathy (WESDR), retinal arteriolar caliber narrowing was associated with lower extremity amputation, but neither arteriolar nor venular caliber was associated with self-reported DPN (9). In contrast, retinal arteriolar caliber dilatation was associated with objectively measured DPN in a multiethnic Asian population with diabetes (10).
In view of these inconsistencies, we examined the relationship between a spectrum of retinal arteriolar abnormalities, measured qualitatively and quantitatively, reflecting early microcirculatory dysfunction, and DPN using data from a population-based study.
RESEARCH DESIGN AND METHODS
Study population
The Singapore Malay Eye Study (SiMES) is a population-based cross-sectional study investigating the prevalence and risk factors of eye diseases affecting urban people living in Singapore. Between August 2004 and June 2006, 3,280 Malay men and women, aged 40 to 80 years, were recruited at random, in 10-year age bands, from a computer-generated list provided by the Singapore Ministry of Home Affairs. The response rate was 78.7%. Nonparticipants tended to be older but did not differ by sex, sampling location, or possession of a telephone in their homes. Details of the study procedures and recruitment have been described in detail previously (11). Tenets of the Declaration of Helsinki were followed, the institutional review board granted ethical approval, and written informed consent was obtained from each participant.
Among the 3,280 SiMES participants, 644 with diabetes underwent quantitative sensory testing. Diabetes was defined as a random glucose value of 11.1 mmol/L or higher, current use of antidiabetic medication, or a physician diagnosis of diabetes (12). No differentiation was made between type 1 and type 2 diabetes; however, more than 98% of people were diagnosed with diabetes after the age of 30 years, and thus, most had type 2 diabetes. We excluded 3 of the 644 participants who did not have retinal photographs and a further 33 with laser photocoagulations, leaving 608 for this analysis. Of these, 605 had gradable retinal photographs of sufficient quality for retinopathy grading, 486 for retinal arteriolar wall signs grading, and 541 for quantitative assessment.
Assessment of DPN
Trained research assistants performed two quantitative sensory tests during a single session while participants were inclined horizontally on a couch. The vibration perception threshold (VPT) was measured with a hand-held neurothesiometer (Horwell Neurothesiometer, Scientific Laboratory Supplies Ltd, Nottingham, U.K.) applied to the apex of both big toes and the medial malleoli of both ankles. After a test procedure on the subject’s palm or forearm, the minimum vibration threshold at which the subject was aware of vibration sensation was recorded to the nearest 0.5 V. The pressure perception threshold was assessed using the 10-g monofilament Touch-test Sensory Evaluator 5.07 (North Coast Medical, U.S.). With the subject’s eyes closed, the nylon monofilament was applied to the plantar surface of the big toe and the third digit, and metatarsophalangeal joints one, three, and five on both feet. The number of sites of the five that the subject was able to sense the fine touch for each foot was recorded. Abnormal vibration sensation was defined as VPT >25 V at any site (13). Insensitivity to the 10-g monofilament was the score of monofilament sensory test of <4 of 5 points on either foot (14). DPN was defined by the presence of either abnormality.
Retinal photographic grading and definitions
After pupil dilatation, two-field retinal photographs, with one centered on the optic disc (field 1) and the other on the macula (field 2), were taken of both eyes at 45° using a digital retinal camera. Photographs were evaluated by trained graders, who were masked to participant characteristics, according to standardized protocols for quantitative and qualitative signs.
Quantitative retinal vascular changes
The Singapore I Vessel Assessment 1.0 (Singapore) computer-assisted program was used to quantify parameters of retinal vascular network geometry from the field 1 image of the right eye of each individual. The left eye was chosen if measurements could not be performed in the right eye. Two trained graders independently applied the computer program and intergrader and intragrader reliability was assessed on a random sample of 50 images, with repeat grading performed after a 2-week interval. Measurements were based on all vessels crossing through a prespecified and standardized area from 0.5 to 2 disc diameters away from the optic disc margin.
Retinal vascular caliber.
Based on a revised Knudtson-Parr-Hubbard equation (15), estimates of retinal vascular caliber from the six largest arterioles and venules, together with the first branch (if larger than 50% of the trunk width) of these vessels, were summarized as the central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE), respectively (16). The intraclass correlation coefficient (ICC) was 0.94 (CRAE) and 0.98 (CRVE) for intergrader reliability and ranged from 0.96 to 0.98 (CRAE) and from 0.98 to 0.99 (CRVE) for the intragrader reliability of graders 1 and 2.
Retinal arterial bifurcation geometry.
The arterial branching pattern may affect the efficiency of circulation in that an optimal branching arrangement of arteries would facilitate delivery of blood to tissue with a minimum total blood volume and with minimal loss of energy. The geometry of an arterial bifurcation was summarized by bifurcation angle (BA) and branching coefficient (BC). BA was defined as the angle subtended between the two daughter arterioles at the vascular junction. The optimum value for the BA has been proposed to approximate 75° (17). Changes in BA may reflect alterations in blood flow (18), endothelial dysfunction (19,20), and attenuation in oxygen saturation (21). Increased angles have been associated with decreased retinal blood flow (22), whereas decreased angles are associated with aging and hypertension (23). BC measures the changes in the total cross-sectional area across the bifurcation and was calculated using the following formula: branching coefficient = (d12 + d22)/d02, where d0 is the trunk caliber and d1 and d2 are the branch calibers. The theoretic optimum of BC should approximate 1.26 (17). An increased BC represents wider branch vessels, and a decreased branching coefficient indicates narrower branch compared with the trunk vessel. Each may increase energy cost and, hence, reduce the efficiency of circulation and metabolic transport. The ICCs were 0.83 (BA) and 0.78 (BC) for intergrader reliability and ranged from 0.76 to 0.89 and from 0.76 to 0.78 for intragrader reliability.
Qualitative retinal vascular abnormalities.
Intragrader variations and the validity of the grading systems for qualitative signs have been assessed and the results reported previously (24,25). Characteristic lesions of DR were graded according to the scale described by the Early Treatment Diabetic Retinopathy Study (24). For each eye, the maximum grade in either of the two photographic fields was determined for each of the DR lesions, which defined the final retinopathy level, varying from levels 10 (no DR) to 85 (advanced proliferative DR). The retinopathy level for a participant was assigned on the basis of the severity scores of the worse eye. Any DR was defined as present if the level was ≥15; that is, the presence of any of the following lesions: microaneurysms, hemorrhages, hard exudate, cotton wool spots, venous loops/beading, intraretinal microvascular abnormalities, fibrous proliferations, vitreous hemorrhages, and new vessels.
Retinal arteriolar wall signs, including focal arteriolar narrowing (FAN) and arteriovenous nicking (AVN), were recorded in all four quadrants of field 1 and in the arcades of field 2 (25). FAN was defined as present if an arteriole had a constricted area of three-quarters or less the width of distal vessel segment. AVN was graded by comparing with standard photographs and defined as present if there was a decrease in the diameter of the venule on both sides of the arteriole that was crossing it.
Assessment of other risk factors
Information on participants’ sociodemographic characteristics, lifestyle risk factors, medication use, and medical history of diabetes and cardiovascular disease was obtained in a standardized interview. Clinical measurements included a nonfasting blood sample for plasma HbA1c and glucose, serum total and HDL cholesterol, and brachial blood pressures. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or self-reported physician diagnosis of hypertension or current use of antihypertensive medications. Duration of diabetes was estimated by subtracting the year of diagnosis from the date of attendance at the research clinic. Smoking status was categorized as current, former, or never. BMI was calculated as weight in kilograms divided by the square of height in meters. Ankle pressures were measured using a standardized Doppler ultrasound device, and the ankle-brachial pressure index was calculated as the ratio of the higher of the two systolic pressures at the ankle to the average of the right and left brachial artery pressures. Subclinical peripheral arterial disease (PAD) was defined as an ankle-brachial pressure index <0.9.
Statistical analyses
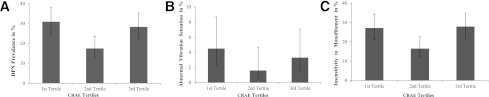
Characteristics of participants by peripheral sensory neuropathy (PSN) status of DPN, abnormal vibration sensation, and insensitivity to monofilament were compared separately by the χ2 test for categoric data and independent sample t test or Mann-Whitney U test for continuous data. Qualitative retinal signs were analyzed as dichotomous variables, and quantitative retinal signs were initially categorized into tertiles according to the distribution of each retinal parameter. We found a U-shaped relationship of CRAE with PSN, where both top and bottom tertiles of CRAE were associated with a higher prevalence rate of PSN (Fig. 1). A similar but nonsignificant trend was also seen for CRVE. Consistent with analysis of qualitative retinal signs as dichotomous variables, we defined suboptimal arteriolar and venular calibers as combined top and bottom tertiles of CRAE and CRVE, respectively.
Figure 1.
Prevalence of DPN (A), abnormal vibration sensation (B), and insensitivity to monofilament (C) according to CRAE tertiles. The error bars indicate 95% CI.
Given that the arteriolar branching angle was shown to decrease in participants with PSN in this cohort (Table 1), a smaller BA was considered as “abnormal” and defined as the top tertile of BA to capture the extreme variation. In a similar way, a larger branching coefficient was the bottom tertile of BC relative to other tertiles combined. The association patterns were generally similar in PSN identified by VPT and monofilament, respectively (Table 1 and Fig. 1). However, there were few participants with abnormal VPT (n = 17) and even fewer for retinal vascular signs (e.g., n = 2 for FAN). We therefore combined them, and DPN was the sole outcome in the subsequent analyses.
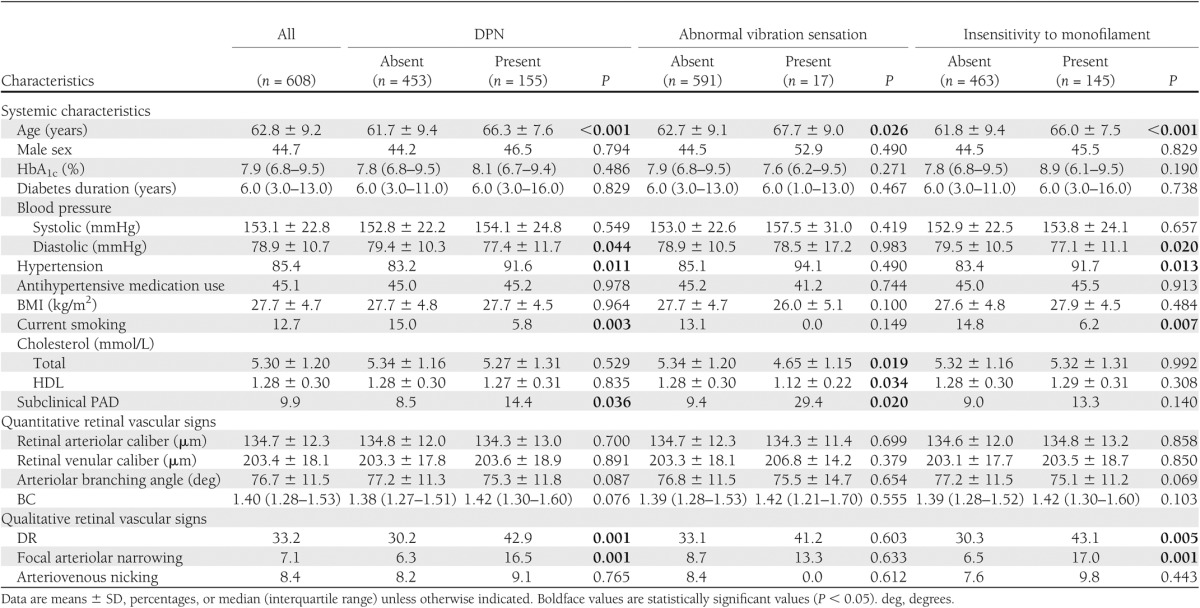
Table 1.
Characteristics of study population according to DPN, abnormal vibration sensation, and insensitivity to 10-g monofilament status
Logistic regression models were constructed to determine the odds ratio (OR) and 95% CI of DPN in association with retinal vascular signs. Age and sex were entered into all models first, followed by additional adjustment for BMI, current smoking, total cholesterol, systolic blood pressure, antihypertensive medication use, subclinical PAD, HbA1c, and duration of diabetes. These variables were selected as potential confounders if, in previous literature, a variable was reported to be associated with both retinal vascular signs and DPN. Interactions between retinal vascular signs and other variables were assessed with respect to effects on DPN. All tests were two-tailed, and a value of P < 0.05 was considered statistically significant. All analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL).
RESULTS
Of the 608 participants with diabetes, the prevalence of DPN was 25.5%. A comparison of characteristics according to DPN status is given in Table 1. People with DPN were older, less likely to be current smokers, and more likely to have subclinical PAD, hypertension, and lower mean diastolic blood pressure.
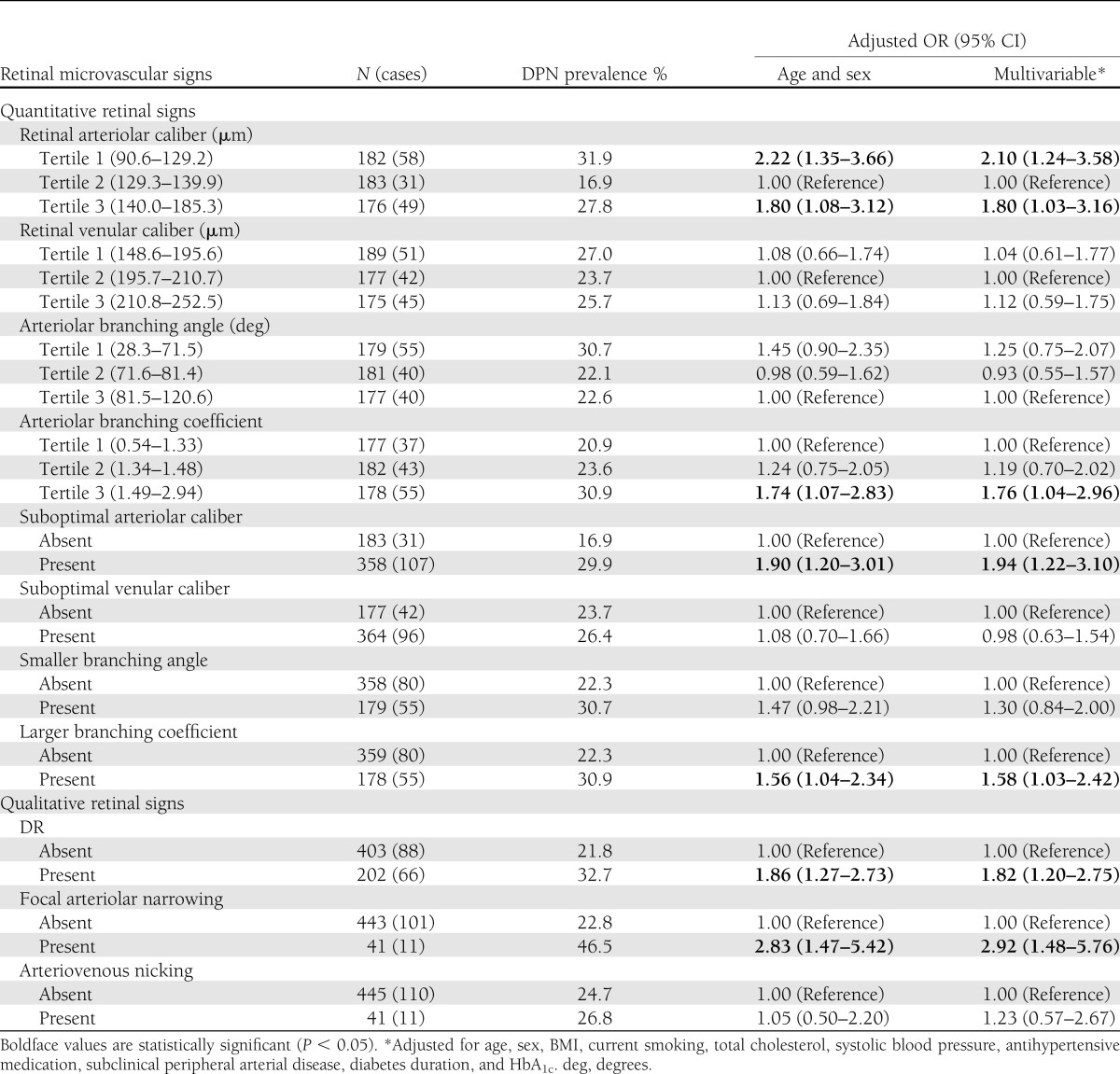
Table 2 reports the associations between retinal microvascular abnormalities and DPN. Taking the middle tertile of CRAE as the reference group, the top (OR 2.22 [95% CI 1.35–3.66]) and bottom (1.80 [1.08–3.12]) tertiles of CRAE were significantly associated with increased odds of DPN. When quantitative signs were analyzed dichotomously, after adjusting for age and sex, people with suboptimal CRAE (1.90 [1.20–3.01]) or larger arteriolar BC (1.56 [1.04–2.34]) were more likely to have DPN than those without the individual signs. For qualitative assessment, people with DR (1.86 [1.27–2.73]) and FAN (2.83 [1.47–5.42]) were more likely to have DPN. After additional adjustment for other vascular risk factors, subclinical PAD, diabetes duration, and HbA1c, the associations with DPN persisted. No statistically significant association was found for suboptimal CRVE, smaller BA, or AVN in either model. There was no significant interaction of retinal signs and any of other variables.
Table 2.
Associations of quantitative and qualitative retinal microvascular signs with DPN
Additional analyses were performed by modeling deviation from the optimum BA and BC (calculated as the absolute difference from 75° and 1.26, respectively), and the results were similar. Because CRAE and CRVE were correlated (r = 0.53, P < 0.01), the fellow vessel caliber variable was added to the multivariate model, including the other as a covariable, to examine whether both were independently associated with DPN. The association between suboptimal CRAE and DPN was essentially unchanged after additionally controlling for suboptimal CRVE (data not shown).
Because DR may confound the associations of DPN with other retinal signs, analyses were repeated after further adjusting for DR and generated similar results (data not shown). When analyses were restricted to participants without any DR (n = 403), the associations with suboptimal CRAE (OR 2.27 [95% CI 1.18–4.36]) and FAN (2.85 [1.15–7.02]) remained significant, whereas the association with larger BC (1.32 [0.74–2.37]) became nonsignificant. Analyses were also repeated after excluding individuals who were newly diagnosed with diabetes by a random plasma glucose test alone (n = 21) and those diagnosed before age 30 years who were on insulin treatment (n = 4). None of the associations reported were altered (data not shown).
Finally, we examined the burden of retinal arteriolar signs, which was summarized as the total number (0, 1, 2 or greater) of the following signs: DR, FAN, suboptimal CRAE, and larger BC. A significant trend (P < 0.001) was observed with a greater number of these retinal signs for higher risk of DPN after multivariate adjustment. The OR was 6.11 (95% CI 2.11–17.71) in people who had two or more retinal arteriolar signs and 3.47 (1.18–10.21) in those with one sign compared with those without any of the retinal microvascular changes.
CONCLUSIONS
In this study, we showed that a range of retinal microvascular abnormalities, measured quantitatively and qualitatively, were associated with DPN, as defined from quantitative sensory testing. Individuals with a suboptimal arteriolar caliber and larger arteriolar branching coefficient, as well as DR and focal arteriolar narrowing, were more likely to have DPN than those without these signs. Persons with two or more signs were six times more likely to have DPN than those without any signs. More important, we showed that the association with these retinal changes was present even in people without traditional signs of DR, suggesting that the relationship of small artery changes and DPN was present before the onset of clinically detectable microvascular disease.
To our knowledge, this is the first study to demonstrate an association of DPN with a number of quantitatively measured retinal arteriolar parameters, including larger arteriolar BC (evidence of impaired arteriolar bifurcation optimality) and suboptimal arteriolar caliber.
Given that retinal arteriolar abnormalities are markers for early microvascular damage, we suggest that DPN may arise, at least in part, from generalized systemic microvascular disease leading to damage of peripheral nerves. An increased arteriolar BC and greater deviation from the optimum indicate impaired arteriolar bifurcation optimality that may reflect endothelial dysfunction and is also associated with altered shear stress across that network, which may further compound the effects on the vascular endothelium, leading to endothelial inflammation (26).
We also found a U-shaped relationship between retinal arteriolar caliber and DPN, with “suboptimal” arteriolar caliber (narrower or wider tertiles) associated with the higher prevalence of DPN. This finding concurs with previous studies that reported associations between extreme distributions of retinal arteriolar caliber and the risk of DPN (9,10). More important, a similar U-shaped pattern has also been reported between suboptimal retinal arteriolar caliber and urine albuminuria excretion in diabetes (27). This may be explained as follows: Initially, retinal arteriolar caliber decreases in response to elevated blood pressure, which in diabetes leads to ischemia and tissue hypoxia due to the limited vasodilatory capacity of the vessels. At later stage, oxidative stress induced by hyperglycemia, together with the formation of advanced glycosylated products and overreaction of protein kinase C, may lead to dysfunction in endothelium and arteriolar smooth muscle cells that results in arteriolar autoregulatory dysfunction and persistent retinal arteriolar dilatation (28). According to the laws of Starling and Laplace, retinal arteriolar dilatation may cause a rise in capillary pressure and lead to capillary wall dilatation (microaneurysms), leakage (edema), and rupture (hemorrhage), which are all classical signs of DR (29). Other signs, such as FAN, are thought to be transient markers for concurrent hypertension, whereas suboptimal arteriolar caliber may reflect arteriolar damages from long-term exposure to both hypertension and hyperglycemia.
More recently, functional studies have demonstrated that DR and DPN are both closely linked with impaired microvascular reactivity, as evidenced in the skin, that reflects a combined reduction in responses to endothelial-dependent and -independent (e.g., decreased production of NO or smooth cell responsiveness) vasodilatation or microvascular sclerosis (i.e., a more physical loss of vasodilator reserve) (30,31). These structural and functional microvascular abnormalities both point to a more complex mechanism in the pathogenesis of DPN than pure endothelial dysfunction alone.
The strengths of this study include the use of quantitative methods to measure geometry of retinal microvasculature as well as the assessment of DR and arteriolar wall signs by standardized grading protocols, and the application of sensitive sensory tests to quantify large nerve fiber function. We also minimized residual confounding by PAD by controlling for the ankle-brachial pressure index rather than by relying on subjectively palpating foot pulses.
Limitations need to be discussed. First, the current study was based on cross-sectional data, limiting our ability to determine the temporal sequence of associations. Second, the proportion of participants with ungradable images for the different retinal abnormalities ranged from 0.5 to 20%. The retinal arteriolar wall signs of FAN and AVN were most frequently ungradable due to the prevalence of cataract and other medial opacities. However, the diabetes risk profile and prevalence of DPN were largely similar in participants with and without gradable images, suggesting a limited role for selection bias. Finally, there might be some measurement error in the quantitative signs assessment, particularly in retinal images of poor quality, given that the computer software needs some manual intervention. These nondifferential random errors, however, will tend to dilute associations toward the null.
In summary, we demonstrate that early retinal arteriolar abnormalities, including quantitative measures, were independently associated with DPN in people with diabetes. These findings support a long-standing but unproven hypothesis that early microvascular dysfunction is present in diabetic patients with DPN.
Acknowledgments
This study was supported by grants from the National Medical Research Council and Singapore Bioimaging Consortium.
No potential conflicts of interest relevant to this article were reported.
J.D. researched data, contributed to discussion, and wrote the manuscript. C.Y.C., M.K.I., Y.-F.Z., C.-Y.C., E.L.L., E.S.T., and T.S. contributed to discussion and reviewed and edited the manuscript. T.Y.W. set up and supervised the project, contributed to discussion, and reviewed and edited the manuscript. T.Y.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Boulton AJ, Vinik AI, Arezzo JC, et al. American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 2.Malik RA. Diabetic peripheral neuropathy: linking microvascular etiology to potential treatments. Adv Stud Med 2005;5:S144–S149 [Google Scholar]
- 3.Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: triggers and mechanisms. World J Gastroenterol 2007;13:175–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girach A, Vignati L. Diabetic microvascular complications—can the presence of one predict the development of another? J Diabetes Complications 2006;20:228–237 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care 2007;30:2708–2715 [DOI] [PubMed] [Google Scholar]
- 6.Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res 2008;27:161–176 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TT, Wong TY. Retinal vascular changes and diabetic retinopathy. Curr Diab Rep 2009;9:277–283 [DOI] [PubMed] [Google Scholar]
- 8.Sasongko MB, Wang JJ, Donaghue KC, et al. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care 2010;33:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 2007;114:1884–1892 [DOI] [PubMed] [Google Scholar]
- 10.Sabanayagam C, Tai ES, Lee J, Lim SC, Wong TY. Retinal vessel caliber and peripheral neuropathy in diabetic participants. Microcirculation 2010;17:297–302 [DOI] [PubMed] [Google Scholar]
- 11.Foong AW, Saw SM, Loo JL, et al. Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic Epidemiol 2007;14:25–35 [DOI] [PubMed] [Google Scholar]
- 12.Su DH, Wong TY, Wong WL, et al. Singapore Malay Eye Study Group Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology 2008;115:964–968, e1 [DOI] [PubMed] [Google Scholar]
- 13.Young MJ, Every N, Boulton AJ. A comparison of the neurothesiometer and biothesiometer for measuring vibration perception in diabetic patients. Diabetes Res Clin Pract 1993;20:129–131 [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Fernando DJ, Veves A, Knowles EA, Young MJ, Boulton AJ. Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res Clin Pract 1991;13:63–67 [DOI] [PubMed] [Google Scholar]
- 15.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003;27:143–149 [DOI] [PubMed] [Google Scholar]
- 16.Cheung CY, Hsu W, Lee ML, et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation 2010;17:495–503 [DOI] [PubMed] [Google Scholar]
- 17.Murray CD. The physiological principle of minimum work applied to the angle of branching of arteries. J Gen Physiol 1926;9:835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frame MD, Sarelius IH. Arteriolar bifurcation angles vary with position and when flow is changed. Microvasc Res 1993;46:190–205 [DOI] [PubMed] [Google Scholar]
- 19.Griffith TM, Edwards DH. Basal EDRF activity helps to keep the geometrical configuration of arterial bifurcations close to the Murray optimum. J Theor Biol 1990;146:545–573 [DOI] [PubMed] [Google Scholar]
- 20.Griffith TM, Edwards DH, Randall MD. Blood flow and optimal vascular topography: role of the endothelium. Basic Res Cardiol 1991;86(Suppl. 2):89–96 [DOI] [PubMed] [Google Scholar]
- 21.Chapman N, Haimes G, Stanton AV, Thom SA, Hughes AD. Acute effects of oxygen and carbon dioxide on retinal vascular network geometry in hypertensive and normotensive subjects. Clin Sci (Lond) 2000;99:483–488 [PubMed] [Google Scholar]
- 22.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res 2003;314:107–117 [DOI] [PubMed] [Google Scholar]
- 23.Stanton AV, Wasan B, Cerutti A, et al. Vascular network changes in the retina with age and hypertension. J Hypertens 1995;13:1724–1728 [PubMed] [Google Scholar]
- 24.Wong TY, Cheung N, Tay WT, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology 2008;115:1869–1875 [DOI] [PubMed] [Google Scholar]
- 25.Wang JJ, Mitchell P, Leung H, Rochtchina E, Wong TY, Klein R. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension 2003;42:534–541 [DOI] [PubMed] [Google Scholar]
- 26.Patton N, Pattie A, MacGillivray T, et al. The association between retinal vascular network geometry and cognitive ability in an elderly population. Invest Ophthalmol Vis Sci 2007;48:1995–2000 [DOI] [PubMed] [Google Scholar]
- 27.Awua-Larbi S, Wong TY, Cotch MF, et al. Retinal arteriolar caliber and urine albumin excretion: the Multi-Ethnic Study of Atherosclerosis. Nephrol Dial Transplant 2011;28:3523–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation 2007;14:25–38 [DOI] [PubMed] [Google Scholar]
- 29.Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care 2008;31:1842–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TT, Shaw JE, Robinson C, et al. Diabetic retinopathy is related to both endothelium-dependent and -independent responses of skin microvascular flow. Diabetes Care 2011;34:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 2009;94:2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]