Abstract
OBJECTIVE
Epidemiological studies have repeatedly investigated the association between depression and metabolic syndrome (MetS). However, the results have been inconsistent. This meta-analysis aimed to summarize the current evidence from cross-sectional and prospective cohort studies that evaluated this association.
RESEARCH DESIGN AND METHODS
MEDLINE, EMBASE, and PsycINFO databases were searched for articles published up to January 2012. Cross-sectional and cohort studies that reported an association between the two conditions in adults were included. Data on prevalence, incidence, unadjusted or adjusted odds ratio (OR), and 95% CI were extracted or provided by the authors. The pooled OR was calculated separately for cross-sectional and cohort studies using random-effects models. The I2 statistic was used to assess heterogeneity.
RESULTS
The search yielded 29 cross-sectional studies (n = 155,333): 27 studies reported unadjusted OR with a pooled estimate of 1.42 (95% CI 1.28–1.57; I2 = 55.1%); 11 studies reported adjusted OR with depression as the outcome (1.27 [1.07–1.57]; I2 = 60.9%), and 12 studies reported adjusted OR with MetS as the outcome (1.34 [1.18–1.51]; I2 = 0%). Eleven cohort studies were found (2 studies reported both directions): 9 studies (n = 26,936 with 2,316 new-onset depression case subjects) reported adjusted OR with depression as the outcome (1.49 [1.19–1.87]; I2 = 56.8%), 4 studies (n = 3,834 with 350 MetS case subjects) reported adjusted OR with MetS as the outcome (1.52 [1.20–1.91]; I2 = 0%).
CONCLUSIONS
Our results indicate a bidirectional association between depression and MetS. These results support early detection and management of depression among patients with MetS and vice versa.
Depression is one of the most common psychiatric illnesses affecting adults and is a major public health problem in the U.S. (1). A growing body of evidence shows that depression is related to an increased risk of diabetes (2) and cardiovascular disease (CVD) (3). Metabolic syndrome (MetS) is a cluster of several CVD risk factors, including central obesity, hyperglycemia, elevated blood pressure, hypertriglyceridemia, and decreased HDL cholesterol (4). MetS is also prevalent in the general population (5) and is associated with an increased risk of diabetes and CVD (6). Because both depression and MetS confer significant public health challenges, the association between the two conditions has attracted attention recently.
A number of epidemiological studies have been conducted to investigate this association with inconsistent results reported. In particular, the temporal direction of this association remains unclear. We therefore summarized here the available data from both cross-sectional and prospective cohort studies and performed meta-analyses to investigate the cross-sectional correlation and longitudinal relation between depression and MetS.
RESEARCH DESIGN AND METHODS
Data sources
We conducted a systematic literature search (from the index date of the database up to January 2012) of MEDLINE, EMBASE, and PsycINFO for studies describing the association between depression and MetS. Two search themes were combined using the Boolean operator “and.” The first theme, depression, combined exploded versions of Medical Subject Headings (MeSH in MEDLINE) “depression,” “depressive disorder,” or “antidepressive agents” and corresponding key words in titles and/or abstracts. The second theme, MetS, combined exploded versions of MeSH terms (in MEDLINE) “insulin resistance” or “metabolic syndrome X” and corresponding key words in titles and/or abstracts. Appropriate modifications were used for searches in EMBASE and PsycINFO. No restrictions in the search strategy were inserted. The detailed search strategy is available upon request. In addition, we searched the reference lists of all identified relevant publications and reviews. Experts in this area were also contacted for potential unpublished data.
Study selection
Two authors (A.P. and N.K.) independently assessed literature eligibility, and discrepancies were resolved by consensus or determined by a third author (F.B.H.). Articles were considered for inclusion in the systematic review if 1) the authors reported data from an original, peer-reviewed study (i.e., not case reports, comments, letters, meeting abstracts, or review articles); 2) the study was a cross-sectional or prospective cohort study with a noninstitutional adult population (age >18 years); 3) the authors reported an association between the two conditions (prevalence, incidence, unadjusted or adjusted odds ratio [OR], and its 95% CI); and 4) the study was published in English. We used broad inclusion criteria for studies, including all definitions of MetS (National Cholesterol Education Program Adult Treatment Panel III [NCEP ATP-III], International Diabetes Federation [IDF], and definitions from other organizations or modified versions) (4,7) and depression status (assessed by self-reported symptom scales, physician/clinician diagnosis, or structured clinical diagnostic interview). In the case of multiple publications from the same study, only the most recent paper or article with a longer follow-up was included. We evaluated eligible articles by first screening titles or abstracts followed by a full-text review.
Data extraction
Two authors (A.P. and N.K.) independently extracted the following information from each study using a predesigned collection form: study characteristics (study name, authors, publication year and journal, study site, number of participants, and follow-up years for cohort studies), participants’ characteristics (age range or mean age and sex composition), depression and MetS measures, analysis strategy (statistical models and covariates adjusted in the models), and results (prevalence, incidence, unadjusted or adjusted OR, and 95% CI). We evaluated the study quality by allocating 1 score for each of the following aspects: selection bias, standard measures of exposure and outcome, participation rate in cross-sectional studies or follow-up rate in cohort studies, adjustment for important confounding factors (socioeconomic status and lifestyle variables), and generalizability. The scores were summed up and studies were classified as high versus low quality based on the median value.
Data synthesis
Separate meta-analyses were conducted to determine 1) the crude OR of this association in cross-sectional studies (since there is no explicit direction in cross-sectional studies), 2) the adjusted OR with MetS as the independent variable in the original reports of cross-sectional studies, 3) the adjusted OR with depression as the independent variable in the original reports of cross-sectional studies, 4) the OR of baseline MetS status and risk of incident depression in cohort studies, and 5) the OR of baseline depression status and risk of future MetS in cohort studies.
The OR was used as the common measure of association across articles in both cross-sectional and cohort studies. If the study reported effect size other than OR, transformation was performed and the corresponding author was contacted for unpublished data if possible. To be consistent across studies, we used binary variables (yes/no) for both MetS and depression. We did not include studies using depressive scale as a continuous variable because the risk estimates were not comparable with studies using categorized depression measures.
The ORs were pooled using the random-effects model that included between-study heterogeneity, and forest plots were produced. Heterogeneity was evaluated by the I2 statistic, and values of 25, 50, and 75% are considered to represent low, medium, and high heterogeneity, respectively (8). The possibility of publication bias was evaluated using the Begg test and visual inspection of a funnel plot (9). Stratified analyses were performed to evaluate the influences of selected study quality and participant characteristics on study results (10): sex, mean age at baseline, different definitions of MetS, depression measure, continent of origin, and study quality. All statistical analyses were performed with Stata statistical software version 11.0 (StataCorp, College Station, TX). P values were two-sided with a significance level of 0.05.
RESULTS
Literature search and study selection
A total of 4,231 articles were found from the three electronic databases. The title and abstract screening based on the aforementioned criteria left us with 422 articles. After examining those articles in full text, 375 articles were excluded (Supplementary Fig. 1). Among the remaining 47 articles, 5 articles used continuous variables for depression scales and 1 article (11) used an extremely low score (Center for Epidemiologic Studies–Depression Scale [CESD]-10 score = 0) to define the reference group (the conventional cutoff is <10). Because the risk estimates might be overestimated in this study, it was not included in the main analysis. However, a sensitivity analysis of including this article did not change the results. Two articles (12,13) used the same samples as the other two studies (14,15), and articles with longer follow-up and more detailed information were retained (14,15). Finally, 39 articles were included (for the complete references of the 39 articles, please see references in Supplementary Data). One cohort study (16) reported the baseline cross-sectional association between depression and MetS and was included in both cross-sectional and cohort analyses. One cross-sectional study (14) and 2 cohort studies (16,17) reported results in both directions. Therefore, 29 cross-sectional studies and 11 cohort studies were included in the meta-analysis. One cohort study (17) is still ongoing, and the authors provided the most recent unpublished results for our meta-analysis.
Cross-sectional studies of the association between depression and MetS
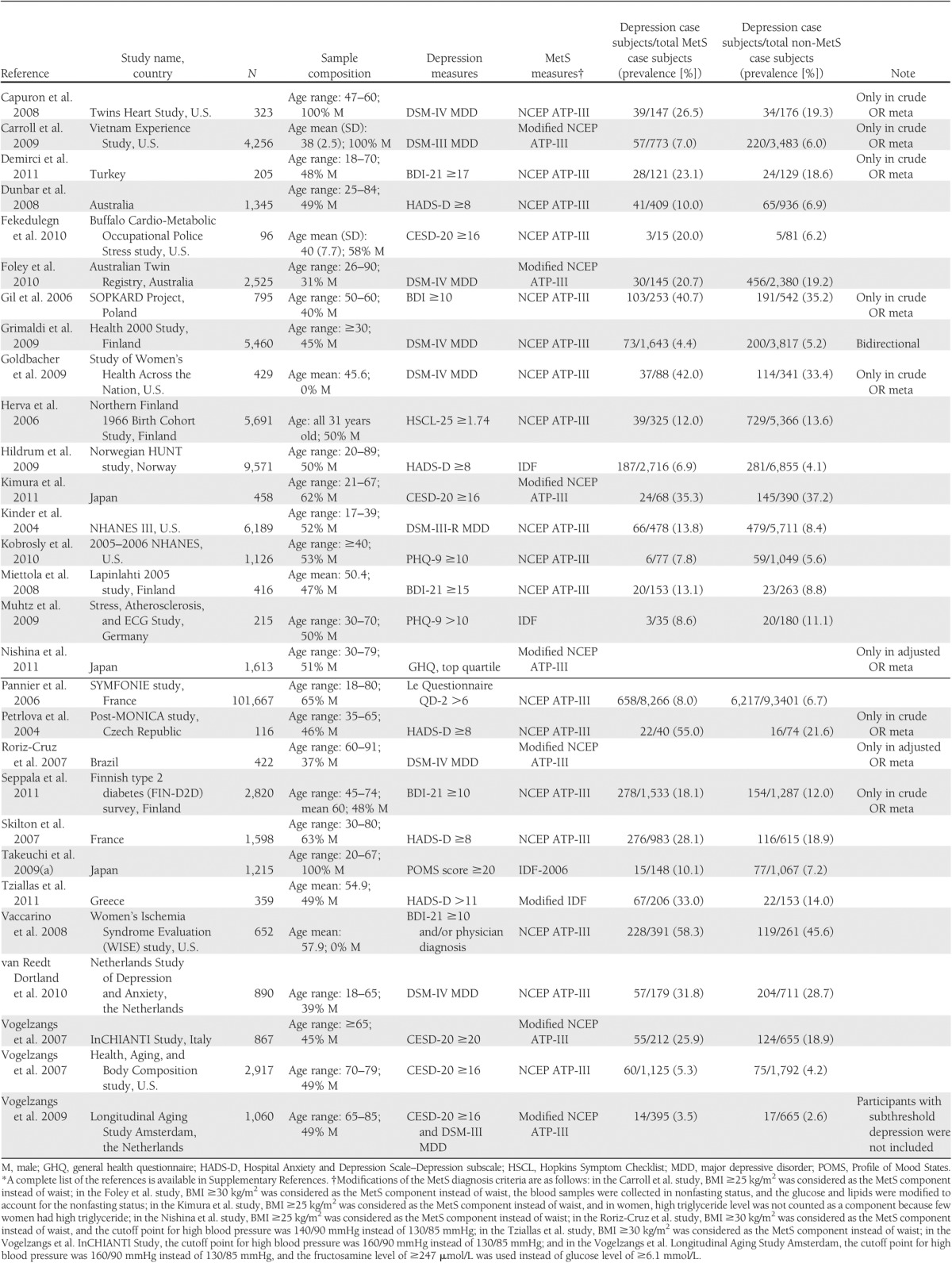
In the 29 cross-sectional studies shown in Table 1, 8 studies used structured or semistructured diagnostic interviews to diagnose major depressive disorder according to the DSM. Nineteen studies assessed depression using self-report symptom scales (e.g., the Beck Depression Inventory [BDI], the CESD, or the Patient Health Questionnaire [PHQ]). Two studies used both measures to identify depression case subjects; however, 1 study required meeting both criteria, and the other study required meeting either criterion. In the studies that used the self-report symptom scales, the threshold score for a depression case subject varied across studies (e.g., BDI score ≥10, ≥15, ≥17, or ≥19 in various studies). MetS was identified based on the NCEP ATP-III or modified versions in most studies, while 4 studies adopted IDF criteria or a modified version. Three studies were conducted in men, 2 in women, and the remaining in both sexes. Most studies were implemented in the U.S. (n = 8) or European countries (n = 14), with 3 in Japan, 2 in Australia, 1 in Brazil, and 1 in Turkey.
Table 1.
Characteristics of cross-sectional studies in the meta-analysis*
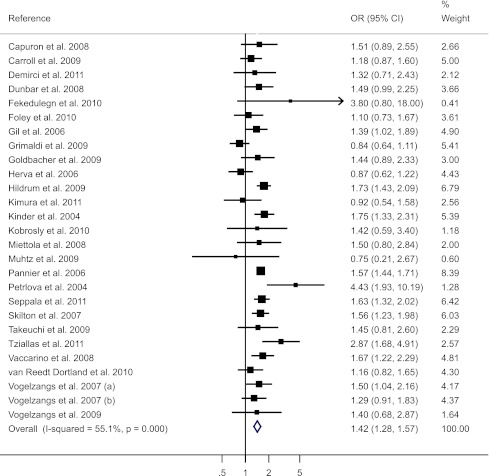
A total of 27 studies (n = 153,298) provided data on the prevalence of depression in adults with and without MetS (another 2 studies provided only adjusted OR and, therefore, were not included here). The pooled crude OR between depression and MetS was 1.42 (95% CI 1.28–1.57) with a moderate heterogeneity detected (I2 = 55.1%) (Fig. 1). No significant publication bias was detected (P = 0.72) (Supplementary Fig. 2A). Subgroup analyses (Supplementary Table 1) showed significant differences by depression measures (P for between-group difference = 0.005) and MetS definitions (P for between-group difference = 0.04). The association was slightly weaker when depression was assessed by a diagnostic interview rather than a self-reported symptom scale (OR = 1.29 vs. 1.51) and was notably weaker when MetS was defined according to the NECP ATP-III criterion compared with other criteria (OR = 1.38 vs. 1.78). No significant between-group difference was found for continent of residence, study quality, age category, and sex.
Figure 1.
Forest plot of cross-sectional studies of the crude association between depression and MetS.
Most of the studies performed multivariate logistic regression to adjust for potential confounders (Supplementary Table 2). A total of 11 studies (12 reports because 1 study reported results separately for men and women) ran the regression models using depression as the dependent variable, and the pooled OR was 1.27 (95% CI 1.07–1.51) with a moderate to high heterogeneity detected (I2 = 60.9%) (Supplementary Fig. 3). Twelve studies (14 reports because 2 studies reported results separately for men and women) used MetS as the dependent variable. The pooled OR was 1.34 (1.18–1.51) with no heterogeneity detected (I2 = 0%) (Supplementary Fig. 3).
Three studies were excluded because depressive symptoms score was used as a continuous variable rather than a binary variable. Prescott et al. (18) reported that both men (adjusted OR 1.08 [95% CI 1.05–1.10]) and women (1.04 [1.02–1.07]) had an elevated risk of MetS for 1-unit increase of 17-item Vital Exhaustion sum score. Toker et al. (19) reported that women were at an elevated risk of MetS for 1-unit increase of PHQ-9 (1.94 [1.22–3.07]) but not men (1.19 [0.79–1.80]). Laudisio et al. (20) reported that MetS was associated with the Geriatric Depression Scale score in a multivariate linear regression analysis in women (β = 2.14 [95% CI 0.14–4.14]) but not in men (β = –0.84 [−3.17 to 1.49]). Therefore, even if these studies were included, the significant association between depression and MetS would not change.
Cohort studies of MetS predicting depression risk
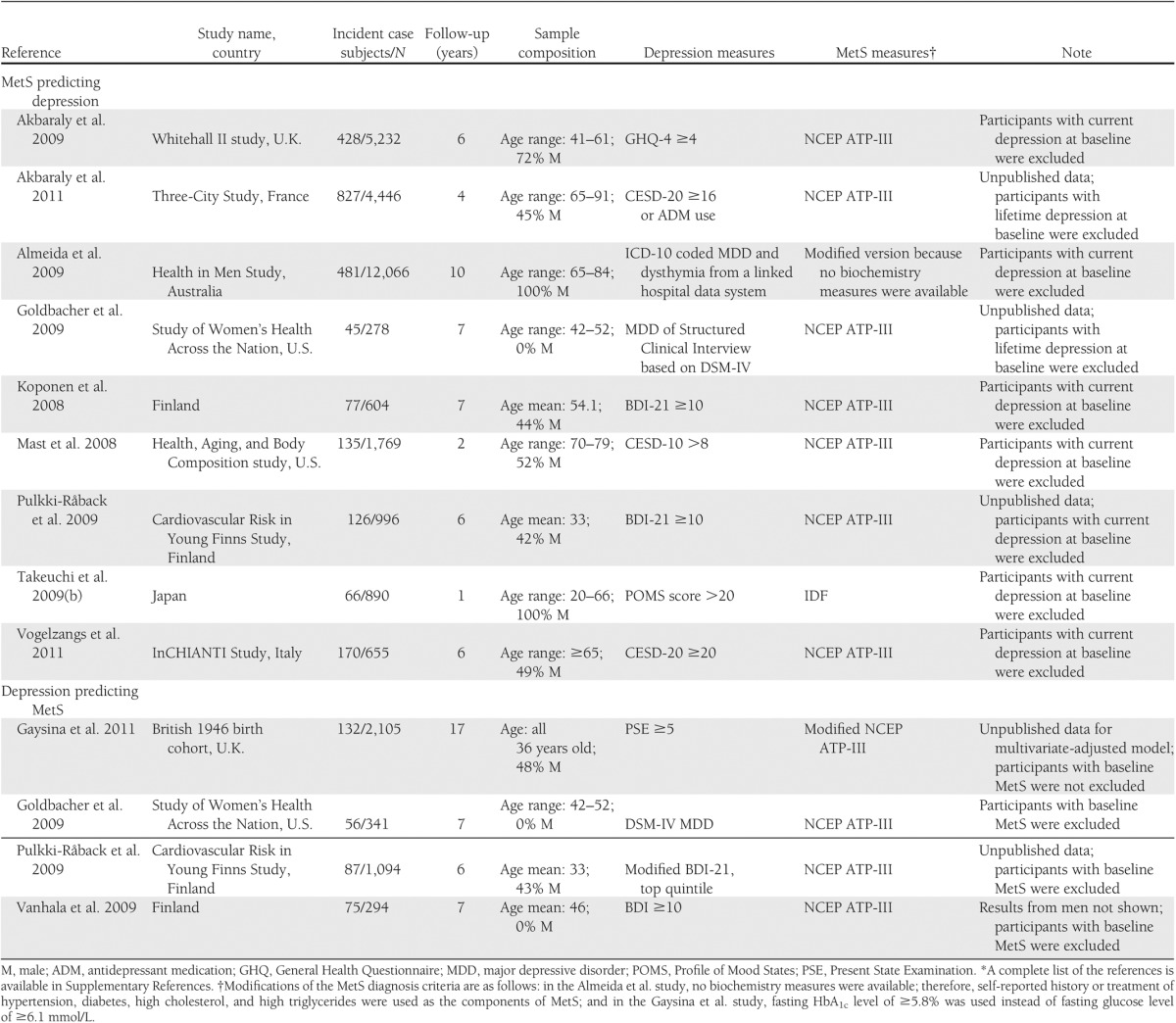
Nine cohort studies investigated the association between baseline MetS status and incident depression with a total sample size of 26,936 and 2,316 depression case subjects. Characteristics of the studies are shown in Table 2. Of the nine studies, MetS was identified by the NECP ATP-III criteria in seven studies, by the IDF criteria in one study, and by the modified NECP ATP-III criteria in one study as a result of the unavailability of biomarker data. In defining depression, six studies used a self-reported symptom scale, two studies used clinical diagnosis–based indicators for depression (one study used a physician diagnosis from ICD-10 codes and one used a structured clinical diagnostic interview), and one study used a self-reported symptom scale and/or antidepressant medication use. Participants with depression at baseline were excluded in all nine studies, with two studies excluding lifetime depression case subjects and the other seven studies excluding the current depression case subjects. Two studies were conducted exclusively in men, one study in women, and six studies were in both sexes with one study reporting results separately for men and women. Four studies enrolled participants aged >65 years; the other five studies enrolled young to middle-aged groups. Five studies were implemented in European countries, two in the U.S., one in Japan, and one in Australia. The follow-up ranged from 1 to 10 years. The statistical models, adjusted covariates, and results from each study are shown in Supplementary Table 3.
Table 2.
Characteristics of cohort studies included in the meta-analysis*
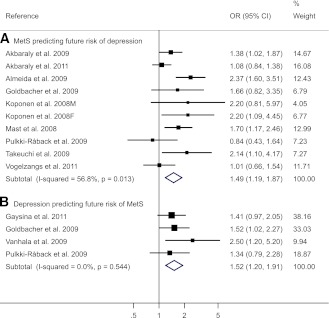
One study reported the results stratified by sex; therefore, there were 10 reports from nine studies. A moderate heterogeneity was detected (I2 = 56.8%), and the pooled adjusted OR was 1.49 (95% CI 1.19–1.89) (Fig. 2). No publication bias was detected (P = 0.25) (Supplementary Fig. 2B). Furthermore, when the diagnostic components of MetS were analyzed separately (Supplementary Table 4), significant positive association was found between central obesity (1.20 [1.07–1.35]), hypertriglyceridemia (1.20 [1.05–1.38]), and low HDL concentrations (1.39 [1.19–1.62]) with risk of depression but not for hyperglycemia (1.05 [0.78–1.42]) and high blood pressure (0.96 [0.72–1.29]) with risk of depression.
Figure 2.
Forest plot of prospective studies of the adjusted OR between depression and MetS: baseline MetS predicting incident depression and baseline depression predicting incident MetS.
The subgroup analyses are shown in Supplementary Table 4. We found that the association was more pronounced in men (OR = 2.15 vs. 1.66), in non-European residents (1.69 vs. 1.25), in studies using diagnostic interview to diagnose depression (2.18 vs. 1.36), and in studies not using NECP ATP-III criteria for MetS definition (2.31 vs. 1.28) compared with their counterparts. However, because of the limited numbers of studies within several subgroups, the results should be interpreted cautiously.
Cohort studies of depression predicting MetS risk
Four cohort studies investigated the association between baseline depression and future risk of MetS with a total sample size of 3,834 and 350 MetS case subjects. In one study, MetS at baseline was not assessed, but the results did not change when baseline obesity or diabetic case subjects were excluded as specified in that study. The characteristics of the studies are shown in Table 2. Of the four studies, depression was defined by a self-reported symptom scale in three studies and by diagnostic interview in one study. All of the four studies used the NECP ATP-III criterion or its modified version to determine MetS status. Two studies were conducted in women and two in both sexes with total and sex-specific results reported. All four studies enrolled participants aged <60 years. Three studies were implemented in European countries and one in the U.S. The follow-up ranged from 6 to 17 years.
The pooled adjusted OR was 1.52 (95% CI 1.20–1.91) (Fig. 2) with no heterogeneity detected (I2 = 0%). No publication bias was detected (P = 0.50) (Supplementary Fig. 2C). For the subgroup analyses (Supplementary Table 5), the association was stronger in women (1.72 [1.33–2.23]) but not significant in men (1.03 [0.62–1.69]). No significant differences were found for other stratified variables. Two studies reported results for each component of MetS, and the OR by baseline depression was marginally significant only for central obesity (1.31 [0.99–1.73]) and hypertriglyceridemia (1.28 [0.98–1.67]).
Of note, two articles (21,22) from the same cohort study were excluded from the meta-analysis because depressive symptoms score (BDI score) was used as a continuous variable. In the article with longer follow-up (22), the authors reported that 1-SD increment in the BDI score was associated with 29% increased odds of MetS (OR 1.29 [95% CI 1.04–1.60]). Thus, our results would not change if we included this study.
CONCLUSIONS
To the best of our knowledge, this is the first meta-analysis that examines the association between depression and MetS, using data from both cross-sectional and cohort studies. We found that depression and MetS were significantly correlated in cross-sectional studies, and a bidirectional association was observed in prospective cohort studies.
Most of the cross-sectional studies reported a higher prevalence of depression in participants with MetS compared with those without. However, the prevalence varied significantly by sex, study design, subject sources, and assessment methods of depression and MetS. Therefore, we did not pool the prevalence; instead, pooled the OR, a measure of association that was more consistent across studies. Our estimated crude OR was 1.42 (95% CI 1.28–1.57), suggesting that MetS and depression are significantly related. The effect size remained significant in the pooled ORs of studies adjusting for potential confounders, such as sociodemographic factors and lifestyle factors: the pooled adjusted OR of depression by MetS status was 1.27 (1.07–1.51), and the pooled adjusted OR of MetS by depression status was 1.34 (1.18–1.51).
We found that the association was somewhat stronger in cross-sectional studies that identified depression using a self-reported symptom scale rather than a structured clinical diagnostic interview or clinician diagnosis. One possible explanation is that estimates may differ depending on the use of dimensionally versus categorically based depression assessment tools (23). Categorically based tools—particularly structured psychiatric interviews—would explicitly exclude individuals with subsyndromal depressive symptoms from case status. By contrast, use of self-reported symptom cutoff scores would allow inclusion of many people with clinically significant depressive symptoms who would not meet formal criteria for DSM diagnosis, yet abundant evidence indicates that subsyndromal depressive symptoms, like clinical syndromes, are significantly associated with morbidity, adverse functional outcomes, and excess health care use (24). Thus, inclusion of people with subsyndromal depression in the reference category may have weakened estimates of studies using categorically based depression definitions. However, this is in opposition to cohort studies of MetS predicting depression: participants with MetS were more likely to develop clinical diagnosed depression than self-reported symptoms (OR = 2.18 vs. 1.36). Nevertheless, only two studies used clinical diagnosed depression (16,25), and the definition of MetS in the Almeida et al. (25) study was stringent (meeting all four criteria of high waist circumference and self-reported treatment of dyslipidemia, diabetes, and hypertension). Thus, this result should be interpreted cautiously. We also found that the association was stronger in studies that defined MetS using the IDF criterion instead of the NCEP ATP-III criterion. The major distinction between the two criteria is that the IDF criterion specifies an obligatory component of central obesity, which is optional in the NCEP ATP-III criterion. Depression was significantly associated with central obesity (26), which might explain why the association was stronger when the IDF definition was used.
Cross-sectional studies do not provide the temporal relationship between depression and MetS. We thus conducted a further meta-analysis to investigate the association between depression and MetS in prospective cohort studies. This observed bidirectional association between depression and MetS was consistent with results from the cross-sectional studies and also in agreement with two recent meta-analyses that show a reciprocal association between depression and diabetes (2) and between depression and obesity (27).
The interplay between depression and MetS is likely to be mediated through multiple mechanisms. First, depression has been positively associated with central obesity (26), chronic inflammation (28), and insulin resistance (29), which are underlying etiological mechanisms for MetS (2). Second, depression has known neuroendocrine effects (e.g., dysregulation of the hypothalamic-pituitary-adrenocortical axis and sympathetic nervous system activation) (3), which could influence MetS risk by affecting abdominal fat accumulation, glucose metabolism, and blood pressure regulation (30). Third, depressed individuals tend to have poor diet and sleep disturbance and engage in less physical activity (31), and these behaviors are known risk factors for the development of MetS. Fourth, conventional medication treatment for depression may exert direct effects on various components of MetS and partially explain the observed association (32). In the opposite direction, individuals with MetS have increased levels of inflammatory cytokines (e.g., C-reactive protein and interleukin 6) (5) and leptin resistance (33), which may also be involved in depressive mood (34,35). Other metabolic disturbances, such as insulin-glucose homeostasis and mitochondrial respiration, are also indicated in the pathophysiology of depression (36). Another potential explanation is that vascular damage in the brain might predispose to depression in the elderly according to the vascular depression hypothesis (37). MetS, as a cluster of vascular risk factors, could lead to subclinical vascular damage (38), which in turn may produce depressive symptoms. Furthermore, MetS is associated with a sedentary lifestyle and a negative self-perception due to stigmatization of obesity (a component of MetS), which can lead to an increased risk of depression (27,39). Taken together, the potential mechanisms are complex and may involve several shared physiological pathways, such as obesity and inflammation. Certainly, more studies are needed to explore the mechanisms underlying this reciprocal relation, which will be crucial for the prevention and treatment of both conditions.
This meta-analysis has strengths and limitations. The primary strength is that this is the first meta-analysis that explicitly examines the bidirectionality of the depression-MetS relationship on the basis of a comprehensive literature search. We contacted authors for unpublished data and found no indication of publication bias in all the analyses. However, the meta-analysis was limited to English-language publications, and we may have missed some articles of other languages. We also observed robust and consistent associations across different subgroups via sensitivity analyses and subgroup analyses. Yet as a major limitation, there was evidence of heterogeneity across the studies used for the analysis of association between MetS and risk of depression in both study designs. This heterogeneity may be attributable to the differences in study design, sample size, analysis strategies, participants’ characteristics, and diagnostic criteria of depression and MetS definition criteria. To account for the heterogeneity, we chose random-effects models for the meta-analyses, but the results were not materially changed when we used fixed-effect models. Furthermore, few cohort studies examine the association between baseline depression and future risk of MetS and, thus, more investigations along this line are needed.
In spite of these limitations, our results have significant implications for both clinical care and public health. Mounting evidence suggests that depression is associated with increased risks of diabetes (2) and CVD (3). MetS is regarded as an intermediate condition that frequently proceeds to the clinical manifestations of diabetes and CVD, although MetS is not usually diagnosed in clinical settings. Our results suggest that the association between depression and diabetes/CVD might start at an early stage before individuals meet the diagnostic criteria of diabetes or CVD. Therefore, we argue that in patients with depression, the cardiometabolic risk factors and MetS status should be carefully monitored, and proper treatment and lifestyle changes could be advised if the patients are at a higher risk of diabetes/CVD. On the other hand, for people with MetS who are already susceptible to diabetes/CVD, early detection of depression may inform appropriate preventive strategies. Collaborative care for patients with depression and diabetes/CVD recently has been demonstrated to be effective in control of both depression and comorbidities (40). Certainly, more studies are still needed to evaluate whether early screening and collaborative care for patients with depression and MetS (or its components) could reduce the future risk of diabetes and vascular diseases.
Acknowledgments
O.I.O. was supported by NIH Career Development Award K08-AG-029813 from the National Institute on Aging and R01-MH-091448 from the National Institute of Mental Health. Q.S. was supported by Career Development Award K99-HL-098459 from the National Heart, Lung, and Blood Institute. M.K. was supported by the National Institutes of Health (NIH) Grants R01-HL-036310 and R01-AG-034454, the Academy of Finland, and the BUPA Foundation, U.K. F.B.H. was supported by NIH Grant DK-58845. The funding sources were not involved in data collection and analysis, writing, or publication of this article.
No potential conflicts of interest relevant to this article were reported.
A.P. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. N.K. researched data, contributed to discussion, and reviewed the manuscript. O.I.O., Q.S., M.K., and R.R.R. contributed to discussion and reviewed the manuscript. F.B.H. researched data, contributed to discussion, and reviewed and edited the manuscript. All authors contributed substantially to the conception and design or analysis and interpretation of data and the drafting or critical revision of the manuscript for important intellectual content. A.P. and F.B.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to Drs. Laura Pulkki-Råback, University of Helsinki, Finland; Frédérique Thomas, Centre d’Investigations Préventives et Cliniques, France; James Dunbar, Greater Green Triangle University Department of Rural Health, Australia; Edie Goldbacher, La Salle University, U.S.; Tasnime Akbaraly, Institut National de la Santé et de la Recherche Médicale, France; Timo Partonen, National Institute for Health and Welfare, Finland; Takeaki Takeuchi, Teikyo University School of Medicine, Japan; Yasumi Kimura, Fukuyama University, Japan; Hiroyasu Iso, Osaka University, Japan; Darya Gaysina, University College London, U.K.; Debra Foley, University of Melbourne, Australia; Desta Fekedulegn, National Institute for Occupational Safety and Health, U.S.; and Christoph Muhtz, University Hospital Hamburg-Eppendorf, Germany, for providing their unpublished data or clarifying inquiry of their papers.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2055/-/DC1.
References
- 1.Reeves WC, Strine TW, Pratt LA, et al. Mental illness surveillance among adults in the United States. MMWR Surveill Summ 2011;60(Suppl. 3):1–29 [PubMed] [Google Scholar]
- 2.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998;55:580–592 [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005;28:1769–1778 [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101 [PubMed] [Google Scholar]
- 10.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–1573 [DOI] [PubMed] [Google Scholar]
- 11.East C, Willis BL, Barlow CE, et al. Depressive symptoms and metabolic syndrome in preventive healthcare: the Cooper Center longitudinal study. Metab Syndr Relat Disord 2010;8:451–457 [DOI] [PubMed] [Google Scholar]
- 12.Rintamäki R, Grimaldi S, Englund A, et al. Seasonal changes in mood and behavior are linked to metabolic syndrome. PLoS ONE 2008;3:e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luppino FS, van Reedt Dortland AKB, Wardenaar KJ, et al. Symptom dimensions of depression and anxiety and the metabolic syndrome. Psychosom Med 2011;73:257–264 [DOI] [PubMed] [Google Scholar]
- 14.Grimaldi S, Englund A, Partonen T, et al. Experienced poor lighting contributes to the seasonal fluctuations in weight and appetite that relate to the metabolic syndrome. J Environ Public Health 2009;2009:165013 [DOI] [PMC free article] [PubMed]
- 15.van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand 2010;122:30–39 [DOI] [PubMed] [Google Scholar]
- 16.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med 2009;71:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulkki-Råback L, Elovainio M, Kivimäki M, et al. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychol 2009;28:108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott E, Godtfredsen N, Osler M, Schnohr P, Barefoot J. Social gradient in the metabolic syndrome not explained by psychosocial and behavioural factors: evidence from the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil 2007;14:405–412 [DOI] [PubMed] [Google Scholar]
- 19.Toker S, Shirom A, Melamed S. Depression and the metabolic syndrome: gender-dependent associations. Depress Anxiety 2008;25:661–669 [DOI] [PubMed] [Google Scholar]
- 20.Laudisio A, Marzetti E, Pagano F, Pozzi G, Bernabei R, Zuccalà G. Depressive symptoms and metabolic syndrome: selective association in older women. J Geriatr Psychiatry Neurol 2009;22:215–222 [DOI] [PubMed] [Google Scholar]
- 21.Räikkönen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism 2002;51:1573–1577 [DOI] [PubMed] [Google Scholar]
- 22.Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care 2007;30:872–877 [DOI] [PubMed] [Google Scholar]
- 23.Kessler RC. The categorical versus dimensional assessment controversy in the sociology of mental illness. J Health Soc Behav 2002;43:171–188 [PubMed] [Google Scholar]
- 24.Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord 2011;129:126–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida OP, Calver J, Jamrozik K, Hankey GJ, Flicker L. Obesity and metabolic syndrome increase the risk of incident depression in older men: the health in men study. Am J Geriatr Psychiatry 2009;17:889–898 [DOI] [PubMed] [Google Scholar]
- 26.Xu Q, Anderson D, Lurie-Beck J. The relationship between abdominal obesity and depression in the general population: a systematic review and meta-analysis. Obes Res Clin Pract 2011;5:e267–e278 [DOI] [PubMed] [Google Scholar]
- 27.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–229 [DOI] [PubMed] [Google Scholar]
- 28.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009;71:171–186 [DOI] [PubMed] [Google Scholar]
- 29.Pan A, Ye X, Franco OH, et al. Insulin resistance and depressive symptoms in middle-aged and elderly Chinese: findings from the Nutrition and Health of Aging Population in China Study. J Affect Disord 2008;109:75–82 [DOI] [PubMed] [Google Scholar]
- 30.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 2009;94:2692–2701 [DOI] [PubMed] [Google Scholar]
- 31.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry 2008;30:127–137 [DOI] [PubMed] [Google Scholar]
- 32.McIntyre RS, Park KY, Law CW, et al. The association between conventional antidepressants and the metabolic syndrome: a review of the evidence and clinical implications. CNS Drugs 2010;24:741–753 [DOI] [PubMed] [Google Scholar]
- 33.Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertens Rep 2008;10:131–137 [DOI] [PubMed] [Google Scholar]
- 34.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:201–217 [DOI] [PubMed] [Google Scholar]
- 35.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 2007;7:648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIntyre RS, Soczynska JK, Konarski JZ, et al. Should depressive syndromes be reclassified as “Metabolic Syndrome Type II”? Ann Clin Psychiatry 2007;19:257–264 [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–922 [DOI] [PubMed] [Google Scholar]
- 38.Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg 2011;54:819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatzenbuehler ML, Keyes KM, Hasin DS. Associations between perceived weight discrimination and the prevalence of psychiatric disorders in the general population. Obesity (Silver Spring) 2009;17:2033–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]